Abstract
In this work, we report a study of long-chain zwitterionic poly(sulfobetaine methacrylate) (pSBMA) surfaces grafted via atom transfer radical polymerization (ATRP) for their resistance to bacterial adhesion and biofilm formation. Previously, we demonstrated that p(SBMA) is highly resistant to nonspecific protein adsorption. Poly(oligo(ethylene glycol) methyl ether methacrylate) (pOEGMA) grafted surfaces were also studied for comparison. Furthermore, we quantify how surface grafting methods will affect the long-term biological performance of the surface coatings. Thus, self-assembled monolayers (SAMs) of alkanethiols with shorter-chain oligo(ethylene glycol) (OEG) and mixed SO3−/N+(CH3)3 terminated groups were prepared on gold surfaces. The short-term adhesion (3 h) and the long-term accumulation (24 h or 48 h) of two bacterial species (Gram-positive Staphylococcus epidermidis and Gram-negative Pseudomonas aeruginosa) on these surfaces were studied using a laminar flow chamber. Methyl (CH3) SAM on gold and a bare glass were chosen as references. p(SBMA) reduced short-term adhesion of S. epidermidis and P. aeruginosa relative to glass by 92% and 96%, respectively. For long-term biofilm formation, qualitative images showed that p(SBMA) dramatically reduced biofilm formation of S. epidermidis and P. aeruginosa as compared to glass.
Introduction
Microbial adhesion onto surfaces and subsequent formation of biofilm are critical issues for many biomedical and engineering applications. Generally, the initial step in biofilm formation is a nonspecific, reversible attachment of bacteria to substratum surfaces. Once permanently attached, bacteria start to synthesize insoluble exopolysaccharides (EPS) that encase the adherent bacteria in a three-dimensional matrix [1]. With the accumulation of EPS and the reproduction of bacteria, colonies develop into mature biofilm. The EPS matrix not only helps the cell to adhere to a substratum and trap nutrients from the water due to charged and neutral polysaccharide groups, but the EPS matrix also protects bacteria from immune host responses, predators, and antimicrobial agents [1]. Therefore, one of the most effective methods to prevent the formation of biofilm is to avoid or reduce the initial adhesion of bacteria to a surface. Although the initial stage of nonspecific attachment of bacteria onto a surface is a key determinant in the subsequent formation of biofilm, the molecular basis of nonspecific bacterial adhesion to artificial surfaces is not yet fully understood. In order to suppress the attachment of microorganisms onto the surfaces, the most commonly used method is to graft surfaces with hydrophilic polymers, such as poly(ethylene glycol) (PEG) derivatives and more recently phosphorylcholine (PC)-based polymers.
It has been shown in several previous studies that PEG-coated surfaces can reduce bacterial adhesion as compared to their control samples over a short period of time (e.g., 3 hours), such as Streptococcus mutans on PEG-coated polystyrene by Harris et al [2], P. aeruginosa on a set of plasma deposited PEG-like film by Johnston et al [3], S. epidermidis, S. aureus, S. salivarius, E. coli, and P. aeruginosa on PEG-brush coated glass by Roosjen et al [4], Pseudomonas sp. on PEG-grafted surfaces by Kingshott et al [5], S. aureus on oligo(ethylene glycol) (OEG) self-assembled monolayers (SAMs) by Cooper and Tegoulia [6], and for S. aureus and S. epidermidis on OEG SAMs by Ostuni et al [7]. Recently, various factors influencing microbial adhesion to PEG brushes (i.e., PEG chain length and adsorbed protein) were systematically studied. Roosjen et al. observed that long-chain PEGs were more effective than short-chain OEG at resisting adhesion of P. aeruginosa, although the length of PEG brush only slightly influenced the adhesion of S. epidermidis [4]. PEG was also observed to suppress the adhesion of S. epidermidis for 24 h in PBS, for 48 h in urine, and for only 4 hours in saliva due to various proteins from the complex mediums used [8]. It is often assumed that a surface resistant to nonspecific protein adsorption will also resist bacterial adhesion/biofilm formation. However, Kingshott et al [9] reported that stainless steel modified with PEG resists protein adsorption, but not bacterial adhesion. Whitesides and coworkers [7] observed that the ability to reduce protein adsorption at a surface did not correlate with the ability to reduce bacterial adhesion. Although PEG-coated materials can effectively resist nonspecific protein adsorption and short-term bacterial adhesion, PEG coatings have limited success in preventing long-term biofilm formation [4]. Furthermore, PEG is a polyether that autoxidizes relatively rapidly, especially in the presence of oxygen and transition metal ions [10]. Most biochemically relevant solutions contain transition metal ions. It was reported that PEG decomposed after exposed to air for one week at 45°C and after one month at 20°C. In vivo the hydroxyl groups of PEG can be oxidized enzymatically to aldehydes and acids, allowing proteins and cells to attach [10]. The susceptibility of PEGs to oxidation damage reduces their utility for applications that require long-term material stability.
The lipid components that constitute the cytoplasm membrane are mainly zwitterionic phospholipids and are believed to be nonthrombogenic [11, 12]. In early studies by Whitesides and coworkers, both phosphorylcholine and sulfobetaine SAMs greatly reduced nonspecific protein adsorption, but could not proportionally suppress the attachment of S. aureus and S. epidermidis under static conditions [7]. Lewis showed that surfaces coated with a MPC-co-DMA copolymer reduced E. coli adhesion by 90% compared to uncoated latex, silicone, polyurethane (PU) and polyvinyl chloride (PVC) surfaces after 18 h incubation [13]. Hirota et al observed that MPC coated surfaces repressed S. aureus, Streptococcus mutans, P. aeruginosa and Candida albicans attachment by at least 95% compared to uncoated surfaces, and the dramatically reduced bacterial attachment was attributed to the strong hydration capacity of MPC-based surfaces [14]. Our recent studies demonstrated for the first time that zwitterionic pSBMA [15, 16] and poly(carboxybetaine methacrylate) (pCBMA) [17] surfaces are highly resistant to nonspecific protein adsorption (i.e., fibrinogen adsorption of < 0.3 ng/cm2). However, the resistance of p(SBMA) to bacterial adhesion and biofilm formation has not been investigated.
The objectives of this work are to (1) determine the capacity of zwitterionic pSBMA coated surfaces resisting nonspecific attachment and biofilm formation of two bacterial strains – Gram-positive S. epidermidis and Gram-negative P. aeruginosa, and (2) explore the relationship between bacterial attachment/biofilm formation and the thickness of the surfaces. Long-chain pSBMA and pOEGMA were prepared via atom transfer radical polymerization (ATRP) while short-chain mixed SO3−/N+(CH3)3 (SA/TMA) and OEG alkanethiols were self-assembled onto gold surfaces. Previously, it was shown [7, 18, 19] that SA/TMA is highly resistant to nonspecific protein adsorption. A methyl SAM on gold and a bare glass slide were used as references. The short-term attachment of two bacteria was determined in PBS buffer for 3 h and the long-term biofilm formation was examined in growth medium for 24 h for P. aeruginosa and 48 h for S. epidermidis. Results showed that pSBMA and pOEGMA not only effectively resist nonspecific protein adsorption, but also dramatically reduce bacterial adhesion and biofilm formation. Although OEG and mixed SA/TMA SAM suppressed bacterial adhesion for three hours, both surfaces failed to prevent biofilm formation after 24 h. Failure of the SAMs in the long-term study is believed to be caused by their lack of stability on gold surfaces.
Materials and methods
Chemicals
Poly(ethylene glycol) methyl ether methacrylate, bromoisobutyryl bromide (BIBB 98%), pyridine (98%), N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (SBMA 97%), 11-mercapto-1-undecanol (2, 97%), 2,2′-bipyridine (BPY 99%), 1-Dodecanethiol, and 0.15M phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (Milwaukee, USA). Human fibrinogen (Fg) was also purchased from Sigma-Aldrich. (1-mercaptoundec-11-yl)tetra(ethylene glycol) (EG4OH), 11-mercapto-N,N,N-trimethylammonium chloride (TMA), and 11-mercaptoundecylsulfonic acid (SA) were purchased from Prochimia (Gdansk, Poland).
Preparation of self assembled monolayers (SAMs)
In this work, three SAMs were prepared: (1) OEG (2) mixed SO3−/N+(CH3)3, and (3) a methyl-terminated (CH3) SAMs. Glass chips were first coated with an adhesion-promoting chromium layer (thickness 2 nm) and a surface plasmon active gold layer (48 nm) by electron beam evaporation under vacuum. Before SAM preparation, the gold-coated glass substrate was cleaned by washing with ethanol and distilled water in sequence, dried with N2, then left in an UV ozone cleaner for 20 min, followed by rinsing with distilled water and ethanol, and dried by N2. To prepare CH3 or OEG SAM, the cleaned chip was immediately soaked in a 0.1 mM ethanol solution of 1-dodecanethiol or hydroxyl-terminated OEG thiols for 24 h to form SAM on a gold surface, and the chip was rinsed in sequence with ethanol and water, and dried in a stream of N2 [20]. To prepare the mixed SA/TMA SAM, the cleaned chip was immediately soaked in a 0.2 mM of TMA and SA in PBS buffer pH 7.4 for 24 h to form a mixed SAM on the gold surface, and then the chip was rinsed in sequence with ethanol and water, and dried in a stream of N2.
Atom transfer radical polymerization (ATRP)
PSBMA and pOEGMA were prepared based on previous reports [16, 21] The CuBr and the gold surface with immobilized initiators were placed in a sealed reaction tube in a dry box under nitrogen atmosphere. Rubber septum stoppers sealing the tube were removed and a degassed solution (pure water and methanol in a 1:1 volume ratio) with SBMA [16] or OEGMA and BPY was then transferred to the tube using syringe under nitrogen. After the reaction, the substratum was removed and rinsed with ethanol and water, and the samples were kept in water overnight. The chip was rinsed with PBS buffer to remove unbound polymers before any experiments.
Measurements of protein adsorption
In this study, a custom-built surface plasmon resonance (SPR) sensor was used to measure protein adsorption on the coated substrata [22, 23]. A SPR chip was attached to the base of the prism and optical contact was established using refractive index matching fluid (Cargille). A dual-channel flow cell with two independent parallel flow channels was used to contain liquid sample during experiments. A peristaltic pump (Ismatec) was utilized to deliver liquid sample to the two channels of the flow cell. Fibrinogen solution of 1.0 mg/mL in PBS (0.15 M, pH 7.4) was delivered to the surfaces at a flow rate of 0.05 mL/min. A surface-sensitive SPR detector was used to monitor protein-surface interactions in real time. In this work, wavelength shift was used to measure the change in surface concentration (mass per unit area) [16].
Bacterial species and culture conditions
The bacterial species, S. epidermidis and P. aeruginosa PAO1 with a GFP expressing plasmid, were used in this work. S. epidermidis and P. aeruginosa were first cultured in separate pure cultures overnight at 37°C on trypticase soy broth (TSB) (BD, USA) [24] agar plates. Cultures on agar plates can be used for two weeks, if kept at 4°C. Several colonies were used to inoculate 25ml of TSB (10g/L) for S. epidermidis and TSB (10g/L) containing 200μg of carbenicillin per ml for P. aeruginosa [24]. These initial cultures were incubated at 37°C with shaking at 100 rpm for 18 hours and were then used to inoculate a second culture of each species in 200 ml of appropriate medium.
Bacterial adhesion assay
A custom-built parallel flow chamber was used to investigate the bacterial attachment and biofilm formation onto the various substrata. Dimensions of the flow chamber were 300 mm (L), 38 mm (W), and 3.5 mm (deep). The flow chamber was sterilized and cleaned using the method reported previously [24]. 12 mm glass cover slips coated with organic or polymer films were attached to removable sample plugs. Sample plugs when placed in the flow chamber were design to sit flush with the inner flow surface.
For a 3-h adhesion study, when the second suspended culture (see above) reached an optical density of 1.0 at 600nm, bacteria were collected by centrifugation at 8,000 × g for 10 min at 4°C,. Cell pellets were washed three times with sterile phosphate buffered saline (PBS, pH7.4) and subsequently suspended in PBS to a final concentration of 108 cells/ml [24]. This cell suspension was then pumped through the flow cell at a flow rate of 6 ml/min (shear stress = 0.1s−1) and the effluent collected in a waste container for proper disposal. After three hours, the flow cell was washed for 15 minutes with sterile PBS at the same flow rate. Since P. aeruginosa strain used in this study carries green fluorescent protein (GFP) gene, samples with P. aeruginosa were observed directly with a CCD-CoolSNAP camera (Roper scientific, Inc., USA) mounted on Nikon Eclipse 80i with 100x oil lens and epifluorescent illumination through a FITC filter. Sample plugs exposed to S. epidermidis were removed after three hours, placed into 3 ml of sterile PBS in a glass scintillation vial, then sonicated for 30s at 40% maximum setting to remove attached bacteria. Bacteria were stained with Live/Dead BacLight™, and bacterial suspension was subsequently filtered through a polycarbonate membrane filter with 0.2 μm pore size (Millipore, USA). The number of live and dead cells was determined, respectively, through FITC and Rhodamine filters with the same microscope described above.
For the 24-h or 48-adhesion study, bacteria from a second suspended culture were washed and subsequently suspended in PBS to a concentration of 108 cells/ml, as above. The cell suspension was pumped through the flow cell at a flow rate of 6 ml/min, and then flow was terminated and bacteria were allowed to adhere to the surfaces under static conditions for 30 min. After 30 min, the appropriate sterile growth medium was pumped into flow cell at a flow rate of 6 ml/min. After 24 h or 48 h, the flow cell was washed for 15 minutes by sterile PBS at the same flow rate. The accumulated P. aeruginosa was directly observed by the CCD-CoolSNAP camera mounted on Nikon Eclipse 80i with 100x oil lens and epifluorescent illumination through a FITC filter. S. epidermidis was stained with Live/Dead BacLight™ and then observed by the same microscope with 100x oil lens and epifluorescent illumination through a FITC filter.
Results and discussion
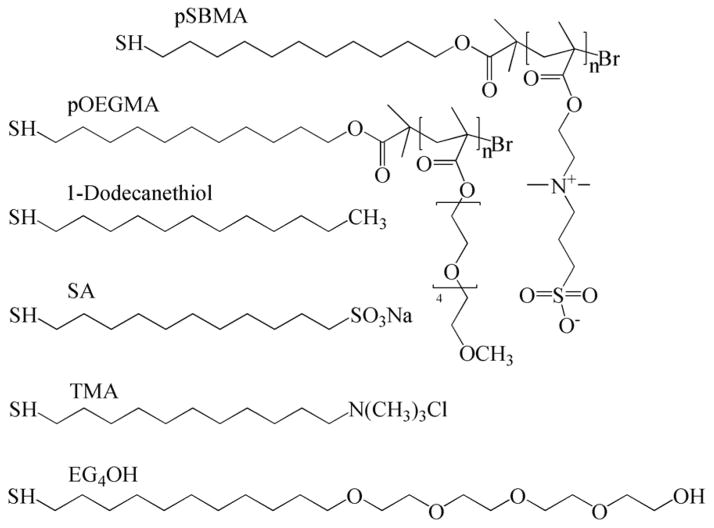
In this study, pSBMA or pOEGMA grafted surfaces via ATRP and SAMs with mixed SA/TMA, OEG or CH3 terminated groups (Scheme 1) were studied for their resistance to bacterial adhesion and biofilm formation. Surfaces were prepared following methods reported previously [16, 19, 21, 25, 26]. It was reported previously that bacterial attachment could be influenced by surface preparation [5] and coating stability [8]. ATRP and self-assembly are two methods to create well-packed surfaces with longer and shorter chains, respectively [16, 27, 28].
Scheme 1.
Chemical structures
Protein adsorption
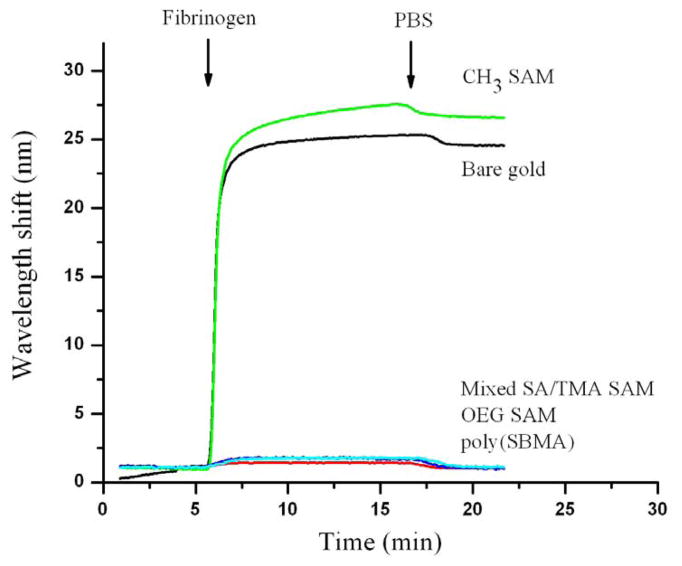
Protein adsorption on the various substrata was measured by SPR. Fibrinogen is used as a model protein since it adsorbs to a variety of surfaces. Figure 1 shows the change in SPR signal as a function of time on different surfaces. Poly(SBMA), mixed SA/TMA SAMs and OEG SAMs are highly resistant to nonspecific fibrinogen adsorption while bare gold and hydrophobic CH3 SAMs show high fibrinogen adsorption. SPR results confirmed previous findings of low protein adsorption on these surfaces [16, 19, 25, 26].
Figure 1.
Adsorption of 1mg/ml fibrinogen in PBS buffer on CH3 SAM, OEG SAM, mixed SA/TMA SAM, a surfaces grafted with poly(SBMA) via ATRP, and a bare gold surface. 1nm wavelength shift in SPR is equivalent to 0.15mg/m2 adsorbed proteins.
Although the initial stage of nonspecific attachment of bacteria onto a surface is a key determinant in the subsequent formation of biofilm, the molecular basis of the initial stage of nonspecific bacterial adhesion to artificial surfaces is not fully understood. In the early studies, it was generally believed that the ability to resist protein adsorption is a prerequisite for a surface to resist bacterial adhesion [29, 30]. This hypothesis has been tested by several research groups. Ostuni et al. (2001) showed that the adhesion of S. aureus and S. epidermidis on SAMs can not be correlated with the adsorption of proteins on surfaces. This hypothesis was also tested in this study.
Short-term bacterial adhesion assays
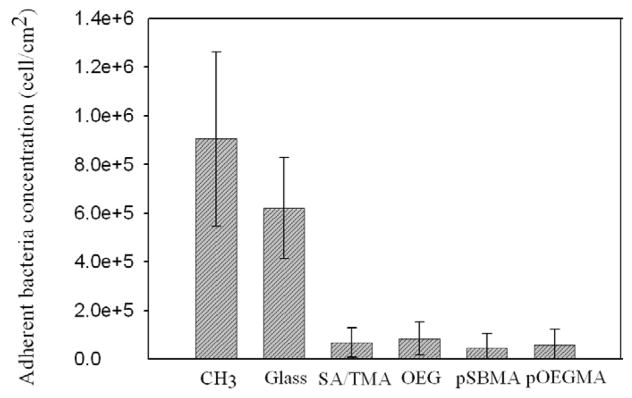
Short-term attachment of S. epidermidis and P. aeruginosa onto pSBMA, pOEGMA, mixed SA/TMA SAMs, and OEG SAMs was characterized using a laminar flow chamber. Glass and CH3 SAMs were used as references. Results for the short-term adhesion of S. epidermidis are reported in Figure 2. Significant decreases in adhesion of S. epidermidis were observed on pSBMA, pOEGMA, mixed SA/TMA SAMs, and OEG SAMs as compared to bare glass and CH3 SAMs. There is no significant difference between these four surfaces to resist bacterial adhesion (p>0.05). Attachment of S. epidermidis on the hydrophobic (CH3 SAM) is 45% higher than that on glass (p<0.05). These results are consistent with those reported previously [6, 31]. Similar to reductions in fibrinogen adsorption, S. epidermidis adhesion is greatly reduced on mixed SA/TMA SAMs, OEG SAMs, pSBMA and pOEGMA. This trend was also reported previously by Cooper and Whitesides [6, 32]. This phenomenon is likely due to the protein contents on the outer membrane of S. epidermidis. Christine Heilmann et al. [33] reported that a mutant of S. epidermidis which lost the expression of four cell surface proteins had lost the ability to adhere to a polystyrene surface. Genetic reconstitution of one of those proteins completely recovered their ability to stick the hydrophobic surface.
Figure 2.
S. epidermidis attachment on various surfaces at 3 h; reported as the mean ± SD (n=10).
As shown in Figure 3, the short-term adhesion of P. aeruginosa was quite different on the surfaces studied. The glass surface showed the highest adsorption of bacteria after three hours. The adhesion of P. aeruginosa on OEG SAMs is significantly reduced by 87% in comparison to the glass surface (p<0.05). Although the attachment of P. aeruginosa on OEG SAMs is 30% higher than that on mixed SA/TMA SAMs, there is no significant difference between these two surfaces (p>0.05). Adhesion of P. aeruginosa on pSBMA and pOEGMA was further reduced by 80% and 75% respectively as compared to OEG SAMs (p<0.05) and no significant difference was observed between pSBMA and pOEGMA (p>0.05). The lower adhesion of P. aeruginosa on pSBMA and pOEGMA than on mixed SA/TMA and OEG SAMs is due to the longer molecular chains of the nonfouling groups, thus the thicker non-fouling films. It was observed in the previous work by Roosjen et al that long-chain PEG is more effective than short-chain OEG at reducing the adhesion of P. aeruginosa, while the length of PEG brush had little impact on the adhesion of S. epidermidis [4]. While pSBMA and pOEGMA have very low bacterial adhesion, their ability to inhibit bacterial adhesion can be related to their hydration ability. In our recent studies, we showed that zwitterions (e.g., pSBMA) and mixed charges (e.g., mixed SA/TMA) bind water molecules via ionic solvation while neutral and hydrophilic polymer (e.g., OEG or pOEGMA) bind water molecules via hydrogen bonding [18, 19, 34, 35].
Figure 3.
P. aeruginosa attachment on various surfaces at 3 h; reported as the mean ± SD (n=10).
It is interesting to observe that the hydrophobic (CH3 SAMs) showed very low attachment of P. aeruginosa, which may be due to a very different mechanism. The reduced adhesion of P. aeruginosa on the hydrophobic surface is likely caused by the low surface energy of the methyl group. The bacteria likely detached from the hydrophobic surface when the surface passed through an air-liquid interface, which has also been shown before [36, 37]. Unlike the stronger interaction force between S. epidermidis and the hydrophobic surface mediated by the surface proteins of S. epidermidis, presumably, the adhesion force between P. aeruginosa and CH3 SAMs is relatively weaker due to polysaccharide contents on the surface of P. aeruginosa.
Long–term bacterial accumulation assays
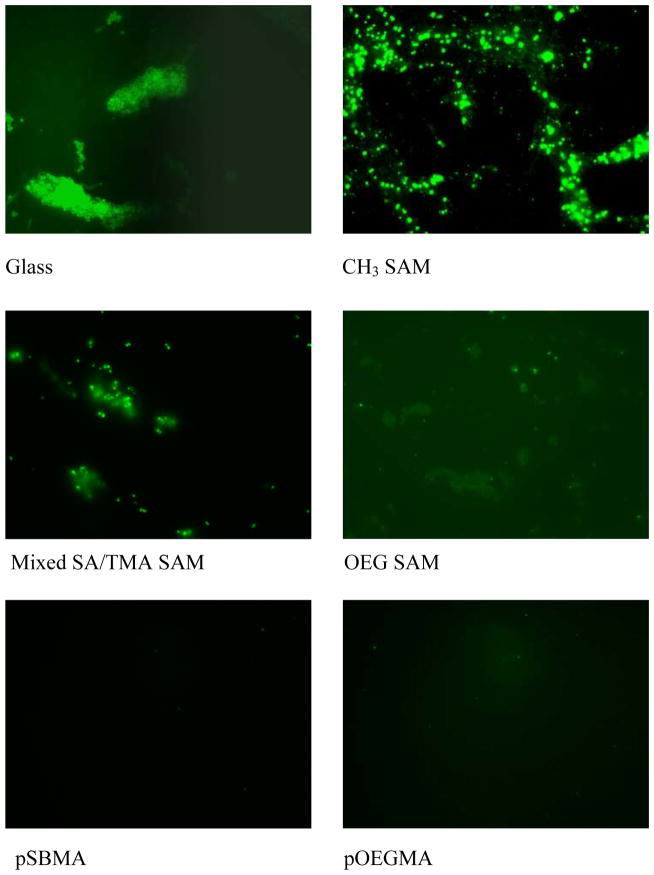
Figure 4 shows a series of representative qualitative images of accumulated S. epidermidis on glass, pSBMA, pOEGMA, mixed SA/TMA SAMs, OEG SAMs, and CH3 SAMs. In these growth mode experiments, less biofilm accumulation was observed qualitatively on pSBMA and pOEGMA surfaces over 48-h period. The accumulation of S. epidermidis on mixed SA/TMA SAMs, OEG SAMs, and glass were slightly different. The hydrophobic CH3 SAMs surface exhibited the highest accumulation of S. epidermidis. The long-term accumulation of S. epidermidis on these surfaces generally corresponds to nonspecific protein adsorption and the short-term adhesion of S. epidermidis.
Figure 4.
Reprehensive fluorescence microscopy graphs of S. epidermidis attachment on various surfaces at 48 h.
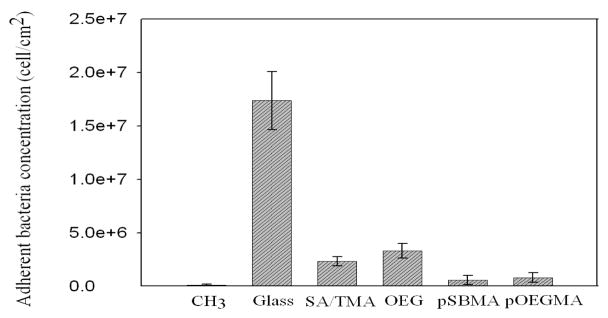
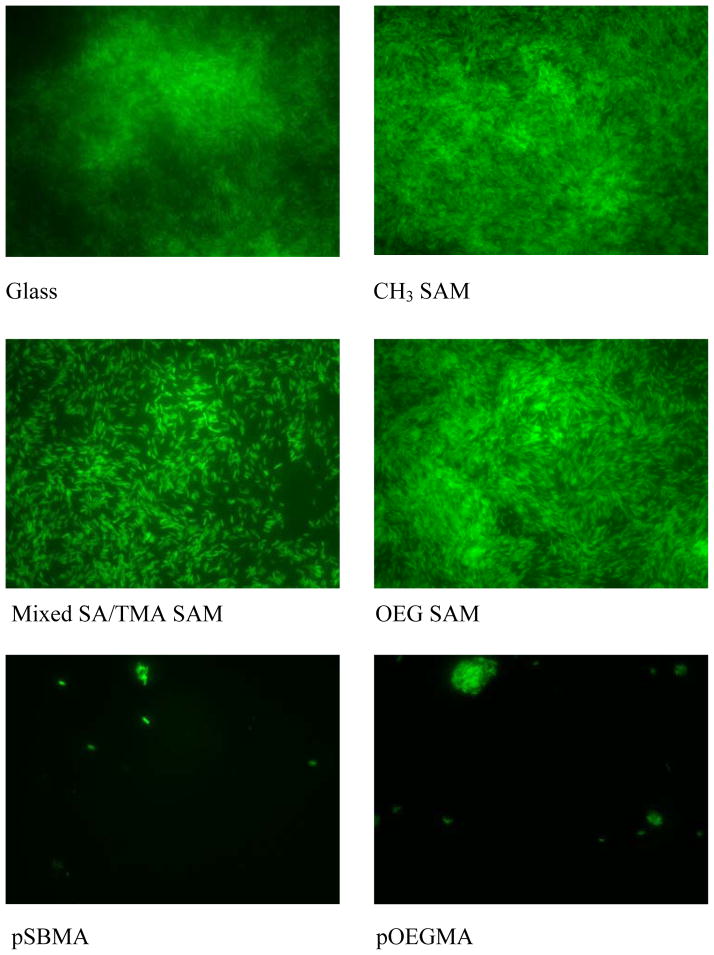
In the long-term accumulation of P. aeruginosa shown as Figure 5, slight differences were observed in the accumulation of bacteria on glass, CH3 SAMs, OEG SAMs and mixed SA/TMA SAMs. The four surfaces were covered by P. aeruginosa after 24 h. The bacterial accumulation on SA/TMA SAMs is qualitatively less than that on the other three surfaces. Poly(SBMA) and pOEGMA exhibited the least accumulation of the bacteria. In the short-term adhesion study, the dramatically reduced adhesion of P. aeruginosa on the hydrophobic surface is anticipated to be caused by the detachment of bacteria when the surface passed through an air-liquid interface. In the long-term accumulation study, the attached bacteria start to synthesize EPS to encase the bacteria. The secreted EPS subsequently strengthens the interactions between bacteria and the surface. Thus, the accumulated bacteria were less disturbed when the sample was taken out from the flow cell and the surface passed through an air-liquid interface.
Figure 5.
Reprehensive fluorescence microscopy graphs of P. aeruginosa attachment on various surfaces at 24 h.
The excellent performance of pSBMA and pOEGMA in dramatically reducing the accumulation of both S. epidermidis and P. aeruginosa is due to their ability to resist nonspecific protein adsorption and bacterial adhesion. The short-term adhesion study showed that fewer P. aeruginosa adhered to pSBMA and pOEGMA surfaces than to SAM surfaces. The reduced bacterial adhesion levels lead to less accumulation of biofilm in the bacterial culture media after 24 hours. pSBMA and pOEGMA surfaces more effectively resist biofilm formation since they are much thicker than the SAMs and have much higher densities of nonfouling groups. Similarly, Ma et al [21] reported that both film thickness and polymer surface density attribute to the protein resistance of pOEGMA. In addition, oxidation of the thiolate groups of the SAMs and subsequent release of monolayers is another possibility even though it is difficult to prove or rule out this possibility in the presence of bacteria. In one of our control experiments, we immersed OEG SAMs in aerated freshwater for 1 or 2 days and then measured protein adsorption. Protein adsorption is still very low. However, this does not mean that OEG SAMs are stable in the presence of bacteria. Once bacteria have attached to the surfaces, they start to replicate and synthesize EPS, which can change the surface properties gradually and eventually lead to the failure of the surfaces. Thus, the inferior performance of mixed SA/TMA SAMs and OEG SAMs, particularly for the long-term accumulation of P. aeruginosa from this work, suggests that the effectiveness of a nonfouling coating is likely due to not only the nature of the nonfouling group, but also the thickness of the nonfouling films.
Conclusions
Both zwitterionic pSBMA and hydrophilic/neutral pOEGMA brushes grafted via ATRP dramatically reduce bacterial adhesion and accumulation. This reduction is due to their intrinsically strong hydration via electrostatic interactions for pSBMA or hydrogen bonding interactions for pOEGMA. Furthermore, this reduction also results from their longer chains and higher densities. Results indicate that the effectiveness of a nonfouling coating is due to not only the nature of the nonfouling group, but also the surface packing of the nonfouling group. Results suggest that zwitterionic pSBMA is an excellent nonfouling material to resist bacterial adhesion and biofilm formation and ATRP is an excellent method to prepare effective nonfouling surfaces. Due to the susceptibility of PEGs to oxidation damage, zwitterionic-based materials are very promising as next-generation nonfouling materials.
Acknowledgments
This work is supported by the Office of Naval Research (N000140410409) and NIH/NIBIB (R21 EB000987-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg K, Bergstrom K, Brink C, Osterberg E, Tiberg F, Harris JM. Effects on protein adsorption, bacterial adhesion and contact-angle of grafting PEG chains to polystyrene. J Adhes Sci and Tech. 1993;7(6):503–517. [Google Scholar]
- 3.Johnston EE, Bryers JD, Ratner BD. Interactions between Pseudomonas aeruginosa and plasma-deposited PEO-like thin films during initial attachment and growth. Polym Prepr (Am Chem Soc, Div Polym Chem) 1997;38(1):1016–1017. [Google Scholar]
- 4.Roosjen A, Van der Mei HC, Busscher HJ, Norde W. Microbial adhesion to poly(ethylene oxide) brushes: influence of polymer chain length and temperature. Langmuir. 2004;20(25):10949–10955. doi: 10.1021/la048469l. [DOI] [PubMed] [Google Scholar]
- 5.Kingshott P, Wei J, Bagge-Ravn D, Gadegaard N, Gram L. Covalent attachment of poly(ethylene glycol) to surfaces, critical for reducing bacterial adhesion. Langmuir. 2003;19(17):6912–6921. [Google Scholar]
- 6.Tegoulia VA, Cooper SL. Staphylococcus aureus adhesion to self-assembled monolayers: effect of surface chemistry and fibrinogen presence. Colloids Surf B-Biointerfaces. 2002;24(3–4):217–228. [Google Scholar]
- 7.Ostuni E, Chapman RG, Liang MN, Meluleni G, Pier G, Ingber DE, Whitesides GM. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir. 2001;17(20):6336–6343. [Google Scholar]
- 8.Roosjen A, de Vries J, Van der Mei HC, Norde W, Busscher HJ. Stability and effectiveness against bacterial adhesion of poly(ethylene oxide) coatings in biological fluids. J Biomed Mater Res Part B. 2005;73B(2):347–354. doi: 10.1002/jbm.b.30227. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Ravn DB, Gram L, Kingshott P. Stainless steel modified with poly(ethylene glycol) can prevent protein adsorption but not bacterial adhesion. Colloids Surf B-Biointerfaces. 2003;32(4):275–291. [Google Scholar]
- 10.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir. 2001;17(18):5605–5620. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 11.Hayward JA, Chapman D. Biomembrane surfaces as models for polymer design - the potential for hemocompatibility. Biomaterials. 1984;5(3):135–142. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara K, Nakabayashi N, Sakakida M, Nishida K, Shichiri M. Biocompatible microdialysis hollow-fiber probes for long-term in vivo glucose monitoring. In: Akmal N, Usmani A, editors. Polymers in Sensors: Theory and practice. ACS symposium series. Vol. 690. Washington, DC: American Chemical Society; 1998. [Google Scholar]
- 13.Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B-Biointerfaces. 2000;18(3–4):261–275. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 14.Hirota K, Murakami K, Nemoto K, Miyake Y. Coating of a surface with 2-methacryloyloxyethyl phosphorylcholine (MPC) co-polymer significantly reduces retention of human pathogenic microorganisms. FEMS Microbiol Lett. 2005;248(1):37–45. doi: 10.1016/j.femsle.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Chen SF, Zhang Z, Jiang SY. Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir. 2006;22(5):2222–2226. doi: 10.1021/la052962v. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Chen SF, Chang Y, Jiang SY. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J Phys Chem B. 2006;110(22):10799–10804. doi: 10.1021/jp057266i. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Chen SF, Jiang SY. Dual-functional biomimetic materials: Nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules. 2006;7(12):3311–3315. doi: 10.1021/bm060750m. [DOI] [PubMed] [Google Scholar]
- 18.Chen SF, Zheng J, Li LY, Jiang SY. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. J Am Chem Soc. 2005;127(41):14473–14478. doi: 10.1021/ja054169u. [DOI] [PubMed] [Google Scholar]
- 19.Chen SF, Yu FC, Yu QM, He Y, Jiang SY. Strong resistance of a thin crystalline layer of balanced charged groups to protein adsorption. Langmuir. 2006;22(19):8186–8191. doi: 10.1021/la061012m. [DOI] [PubMed] [Google Scholar]
- 20.Yu QM, Chen SF, Taylor AD, Homola J, Hock B, Jiang SY. Detection of low-molecular-weight domoic acid using surface plasmon resonance sensor. Sens Actuators, B. 2005;107(1):193–201. [Google Scholar]
- 21.Ma HW, Hyun JH, Stiller P, Chilkoti A. “Non-fouling” oligo(ethylene glycol)-functionalized polymer brushes synthesized by surface-initiated atom transfer radical polymerization. Adv Mater. 2004;16(4):338–341. [Google Scholar]
- 22.Homola J. On the sensitivity of surface plasmon resonance sensors with spectral interrogation. Sens Actuators, B. 1997;41(1–3):207–211. [Google Scholar]
- 23.Homola J, Dostalek J, Chen SF, Rasooly A, Jiang SY, Yee SS. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int J Food Microbiol. 2002;75(1–2):61–69. doi: 10.1016/s0168-1605(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 24.Wagner VE, Koberstein JT, Bryers JD. Protein and bacterial fouling characteristics of peptide and antibody decorated surfaces of PEG-poly(acrylic acid) co-polymers. Biomaterials. 2004;25(12):2247–2263. doi: 10.1016/j.biomaterials.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Li LY, Chen SF, Zheng J, Ratner BD, Jiang SY. Protein adsorption on oligo(ethylene glycol)-terminated alkanethiolate self-assembled monolayers: the molecular basis for nonfouling behavior. J Phys Chem B. 2005;109(7):2934–2941. doi: 10.1021/jp0473321. [DOI] [PubMed] [Google Scholar]
- 26.Chen SF, Li LY, Boozer CL, Jiang SY. Controlled chemical and structural properties of mixed self-assembled monolayers of alkanethiols on Au(111) Langmuir. 2000;16(24):9287–9293. [Google Scholar]
- 27.Mrksich M, Grunwell JR, Whitesides GM. Biospecific adsorption of carbonic-anhydrase to self-assembled monolayers of alkanethiolates that present benzenesulfonamide groups on gold. J Am Chem Soc. 1995;117(48):12009–12010. [Google Scholar]
- 28.Sigal GB, Whitesides GM. Benzenesulfonamide peptide conjugates as probes for secondary binding sites near the active site of carbonic anhydrase. Bioorg Med Chem Lett. 1996;6(5):559–564. [Google Scholar]
- 29.Ratner BD, Hoffmann FJ, Schoen JE, Lemons F. Biomaterials science: an introduction to materials and medicine. New York, NY: Academic Press; 1996. [Google Scholar]
- 30.Chapman RG, Ostuni E, Liang MN, Meluleni G, Kim E, Yan L, Pier G, Warren HS, Whitesides GM. Polymeric thin films that resist the adsorption of proteins and the adhesion of bacteria. Langmuir. 2001;17(4):1225–1233. [Google Scholar]
- 31.Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res Microbiol. 2005;156(4):506–514. doi: 10.1016/j.resmic.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prime KL, Whitesides GM. Self-assembled organic monolayers - model systems for studying adsorption of proteins at surfaces. Science. 1991;252(5009):1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 33.Heilmann C, Gerke C, PerdreauRemington F, Gotz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64(1):277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng J, Li LY, Chen SF, Jiang SY. Molecular simulation study of water interactions with oligo (ethylene glycol)-terminated alkanethiol self-assembled monolayers. Langmuir. 2004;20(20):8931–8938. doi: 10.1021/la036345n. [DOI] [PubMed] [Google Scholar]
- 35.Zheng J, Li LY, Tsao HK, Sheng YJ, Chen SF, Jiang SY. Strong repulsive forces between protein and oligo (ethylene glycol) self-assembled monolayers: a molecular simulation study. Biophys J. 2005;89(1):158–166. doi: 10.1529/biophysj.105.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Suarez C, Busscher HJ, van der Mei HC. Analysis of bacterial detachment from substratum surfaces by the passage of air-liquid interfaces. Appl Environ Microbiol. 2001;67(6):2531–2537. doi: 10.1128/AEM.67.6.2531-2537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakker DP, Huijs FM, de Vries J, Klijnstra JW, Busscher HJ, Van der Mei HC. Bacterial deposition to fluoridated and non-fluoridated polyurethane coatings with different elastic modulus and surface tension in a parallel plate and a stagnation point flow chamber. Colloids Surf B-Biointerfaces. 2003;32(3):179–190. [Google Scholar]