Abstract
Purpose
The incidence of human papillomavirus (HPV) associated head and neck cancers (HNCs) have been increasing in Peru. However, the burden of oral HPV infection in Peru has not been assessed. The objective of this cross-sectional study was to estimate the prevalence and correlates of oral HPV infection in a population-based sample from males and females from Lima, Peru.
Methods
Between January 2010 and June 2011, a population-based sample of 1099 individuals between the ages of 10 and 85 from a low-income neighbourhood in Lima, Peru was identified through random household sampling. Information on demographic, sexual behaviours, reproductive factors and oral hygiene were collected using interviewer-administered questionnaires. Oral rinse specimens were collected from each participant, and these specimens were genotyped using the Roche Linear Array assay. ORs were used to assess differences in the prevalence of any oral HPV and any high-risk oral HPV infection by demographic factors, sexual practices and oral hygiene among individuals 15+ years of age.
Results
The prevalence of any HPV and any high-risk HPV (HR-HPV) was 6.8% and 2.0%, respectively. The three most common types were HPV 55 (3.4%), HPV 6 (1.5%) and HPV 16 (1.1%). Male sex (aOR, 2.21; 95% CI 1.22 to 4.03) was associated with any HPV infection after adjustment.
Conclusions
The prevalence of oral HPV in this study was similar to estimates observed in the USA. Higher prevalence of oral infections in males was consistent with a male predominance of HPV-associated HNCs and may signal a sex-specific aetiology in the natural history of infection.
INTRODUCTION
The sixth most common types of cancer worldwide are head and neck squamous cell carcinomas, including cancers of the oral cavity, oropharynx and larynx; 633 000 cases and 355 000 deaths are reported annually.1 Human papillomavirus (HPV) infection has recently been established as a major etiologic cause of a subset of head and neck squamous cell carcinomas cancers, which affect the oropharynx, specifically the base of tongue and tonsils. Other risk factors include conventional environmental indicators, such as tobacco and alcohol use.2 The incidence of HPV-associated head and neck cancers (HNCs) has increased significantly over the last two decades in both the USA and countries in the developing world including Peru.3–6 Unlike in the USA and countries in Europe, the burden of oral HPV infection has not been extensively investigated in the developing world.
A large cross-sectional study of oral HPV infection conducted in the USA determined the prevalence of oral HPV infection to be 6.9% among males and females of 14–69 years of age.6,7 The most prevalent type was HPV 16, found in 1.0% of the study population. A recent meta-analysis of studies conducted in mostly the USA and countries in Europe observed that oral HPV prevalence was 4.5%, while the prevalence of the most common type, HPV 16, was 1.3%. Only 4 of these 18 studies were conducted in low-income and middle-income nations.8
The objective of this study was to estimate the prevalence of oral HPV infection as well as to characterise demographic and behavioural correlates of infection in healthy males and females residing in a low-income neighbourhood in Peru. Given the observed increase in HPV-associated HNC in this setting and the largely unknown burden in the developing world, a better understanding of the epidemiology of oral HPV is crucial to improve disease surveillance.
MATERIALS AND METHODS
Subject participation and study design
This study is composed of a population-based sample of 1435 healthy males and females between the ages of 10 and 85 recruited from Las Pampas in the district of San Juan de Miraflores in Lima, Peru between January 2010 and June 2011. Recent census data collected in this neighbourhood was initially used to identify potential eligible individuals for this study. This census information was used to assign trained study staff to groups of randomly selected households to assess interest and evaluate eligibility for study enrolment. Overall, healthy individuals aged between 10 and 85 years of age were considered eligible for enrolment in the study. Eligibility of individuals <18 years of age was assessed through discussion with their parents or guardians.
Information on demographics, sexual history, oral health/awareness and other risk behaviours was collected using an interview-administered questionnaire. Demographic information included sex, age, marital status, the highest education level attained and employment status. Oral health/awareness information included yearly visits to a dentist, and general oral health represented by the number of dental problems in the past year. Information on other high-risk behaviours was collected, including cigarette use and alcohol use, as well as when an individual’s last drink was consumed. Sexual risk behaviours included age at first sexual intercourse, number of lifetime sexual partners (including vaginal, anal and oral sex), oral sex with most recent partner and condom use with most recent sexual partner. Sexual risk factors were obtained from all participants, with ‘no sex’ being selected if the participant was not sexually active. An oral rinse specimen was collected from each study participant at the time the questionnaire was administered. Written informed consent was obtained from all participants, with parental permission obtained in the case of minors less than 18 years of age. All study protocols were approved by the Institutional Review Board at Universidad Peruana Cayetano Heredia, Lima, Peru. The Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland approved the analysis of the dataset.
Specimen collection and processing
Oral rinse specimens were collected by a 30 s rinse and gargle with 10 mL of Scope mouthwash (Procter & Gamble, Cincinnati, Ohio, USA) for assessment of oral HPV status.9,10 Specimens were processed within 4 h of collection per standard procedure.10 Oral rinse specimens were centrifuged at 2000 g for 10 min to concentrate cells present in the specimen. Following this centrifugation, the supernatant was poured off and re-suspended in phosphate buffered saline (PBS) to wash the cell pellet. Following this wash step, the cell pellet was centrifuged again and re-suspended for storage and DNA extraction. Processed specimens were then stored in PBS at −80°C; HPV detection and genotyping were performed within 2 weeks of sample collection.11,12 A random, convenience sample from the larger study group was selected for HPV detection and genotyping analysis (n=1114/1435; 77.8% of total). In order to assure representativeness of the subset selected for HPVanalysis, a comparison of demographic sexual and other behavioural factors was performed between individuals selected versus non-selected for the HPV analyses (data not shown). This comparison showed no differences between the two groups for any variable assessed.
HPV detection and genotyping
All DNA extraction and HPV testing of oral rinse specimens were performed at the Johns Hopkins Bloomberg School of Public Health. The oral exfoliated cells collected through the oral rinse procedure were separated by centrifugation, washed and re-suspended in cell lysis solution, and treated with proteinase K overnight. After incubation, DNA was extracted using Qiagen MDX Biorobot using a modified media extraction protocol.12 Positive and negative control, SiHA (HPV 16 positive) and K562 (HPV-negative) cells, were included at defined concentrations in every extraction procedure. Following extraction, the presence of HPV DNA was measured in all samples using the Roche Linear Array assay.13 This assay detects and discriminates up to 37 different oncogenic and non-oncogenic genotypes of HPV using a combination of consensus PCR and reverse line-blot hybridisation methodologies. This assay has been shown to have a high analytic sensitivity and specificity for the detection of HPV in oral rinse specimens.13,14 HPV types defined as high risk (HR-HPV) in this study were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73 and 82.15–17
Statistical analysis
The prevalence of any HPV and HR-HPV types were estimated overall, by age group and sex for the entire study population among individuals with single and multiple infections. Differences in the prevalence by sex and age group were compared using a χ2 test. A p value of <0.05 was considered statistically significant.
Logistic regression analysis was used to ascertain the correlates of prevalent infection of any HPV and any HR-HPV infection. This analysis was conducted among individuals 15+ years of age due to the limited reliability and potential source of bias among responses on employment and sexual behaviour among children 10–14 years of age. Odds Ratio (OR) and 95% CIs were estimated to measure the strength of association. Separate univariate models were fitted for each demographic, behavioural and dental health variables. Missing information for each variable was treated as a response in the univariate analyses. Factors that showed a p value of <0.2 in univariate analysis were retained in the multivariate model. A p value of <0.05 was considered statistically significant in the final multivariate model. The same final model was constructed for any HR-HPV infections. All analyses were performed using STATAV.12.1.18
RESULTS
Of the total 1435 individuals originally sampled, a random, convenience sample of 1114 individuals were selected for HPV DNA detection. For assessment of factors correlated with HPV prevalence, an additional 15 participants were excluded from analysis due to missing age or gender characteristics.
The overall prevalence of any HPV and any HR-HPV was 7.35% and 2.24%, respectively. The most prevalent HPV type was HPV 55 (3.57%) followed by HPV 6 (1.53%), HPV 16 (1.22%), HPV 62 (0.71%) and HPV 81 (0.61%) (figure 1). Coinfection with at least two HPV types was found in 1.63% of the study population (n=16) and 22.22% of all HPV-positive individuals. The prevalence of any HPV infection overall was higher among males as compared with females in each age group, although this result was not statistically significant (figure 2). Similar patterns in age-specific prevalence were observed for HR-HPV infections. The prevalence of any HR-HPV was significantly higher among males aged 19–27 years as compared with females of the same age group (5.63% vs 1.08%; p<0.05).
Figure 1.
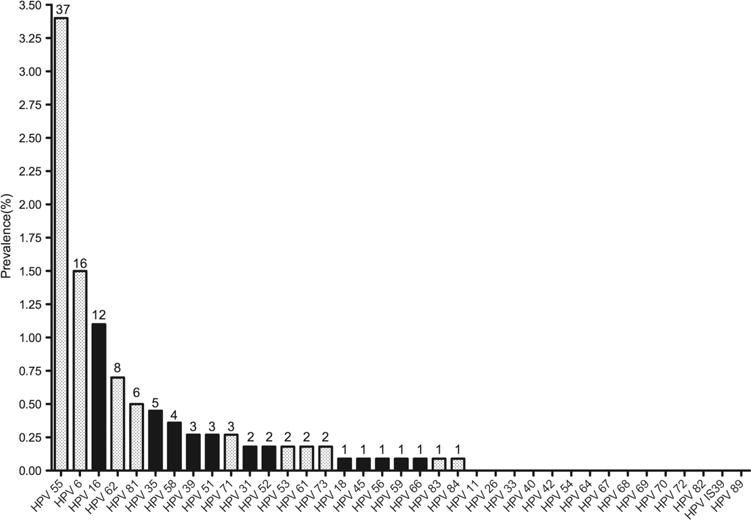
Prevalence and distribution of human papillomavirus (HPV) genotypes among individuals 10–84 years of age. (Numbers at the top of each bar are the absolute number of individuals with detectable HPV genotype).
Figure 2.
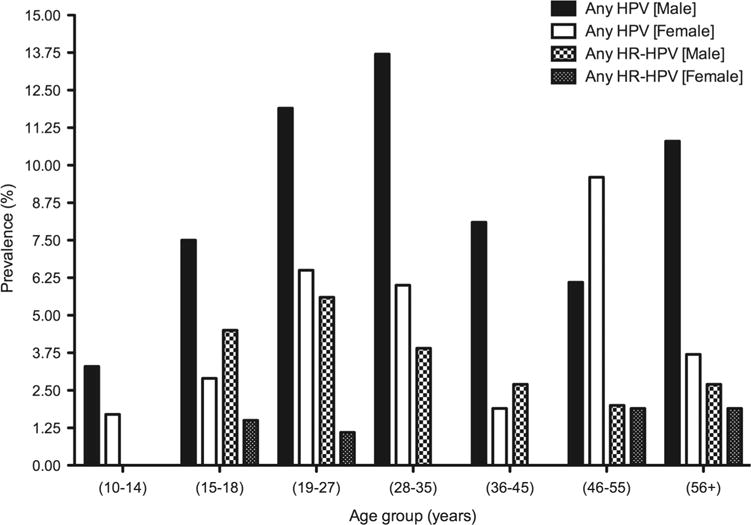
Age-specific prevalence of any and high-risk human papillomavirus (HPV) genotypes among men and women.
Nine hundred and eighty individuals (n=980) aged 15 years or older were included in the correlate analysis of HPV prevalence consisting of 383 males and 597 females with a median age of 28 years (IQR: 21–45). Univariate analyses of demographic, clinical and behaviour characteristics are detailed in table 1. Overall, increasing age, male sex, employment status, education level, lifetime number of sexual partners, age at first sex, engaging in oral sex, number of dental issues reported in the last year, smoking and alcohol use had a p value of <0.02 in univariate analysis and were therefore retained in the multivariate analysis.
Table 1.
Baseline demographic and clinical characteristics
| Variable | Total (n= 980) | Any HPV (n=72)
|
High risk HPV (n=22)
|
||
|---|---|---|---|---|---|
| No. (%) with infection | Unadjusted OR | No. (%) with infection | Unadjusted OR | ||
| HPV prevalence (%) | 7.35% | 2.24% | |||
| Sex | |||||
| Female | 597 (60.9) | 33 (5.5) | 1.00 (Reference) | 6 (1.0) | 1.00 (Reference) |
| Male | 383 (39.1) | 39 (10.2) | 1.94 (1.20 to 3.14) | 16 (4.2) | 4.29 (1.67 to 11.07) |
| Age | |||||
| (15–18) | 135 (13.8) | 7 (5.1) | 1.00 (Reference) | 4 (2.9) | 1.00 (Reference) |
| (19–27) | 327 (33.4) | 29 (8.8) | 1.78 (0.76 to 4.17) | 10 (3.0) | 1.03 (0.32 to 3.35) |
| (28–35) | 134 (13.7) | 12 (8.9) | 1.80 (0.68 to 4.71) | 2 (1.5) | 0.49 (0.09 to 2.75) |
| (36–45) | 140 (14.2) | 5 (3.6) | 0.67 (0.21 to 2.19) | 1 (0.7) | 0.23 (0.02 to 2.13) |
| (46–55) | 153 (15.6) | 13 (8.4) | 1.70 (0.66 to 4.39) | 3 (2.0) | 0.65 (0.14 to 2.98) |
| (56+) | 91 (9.3) | 6 (6.5) | 1.29 (0.42 to 3.97) | 2 (2.2) | 0.73 (0.13 to 4.10) |
| Marital status | |||||
| Married | 245 (25.0) | 15 (6.1) | 1.00 (Reference) | 7 (2.9) | 1.00 (Reference) |
| Single | 430 (43.9) | 35 (8.1) | 1.35 (0.72 to 2.54) | 11 (2.6) | 0.89 (0.34 to 2.33) |
| Cohabitating | 245 (25.0) | 17 (6.9) | 1.14 (0.55 to 2.34) | 2 (0.8) | 0.27 (0.57 to 1.36) |
| Divorced/separated/widowed | 56 (5.7) | 5 (8.9) | 1.50 (0.52 to 4.32) | 2 (3.6) | 1.26 (0.25 to 6.23) |
| Missing | 4 (0.4) | 0 (0) | – | 0 (0) | – |
| Education | |||||
| Primary or less | 311 (31.8) | 17 (5.5) | 1.00 (Reference) | 6 (1.9) | 1.00 (Reference) |
| Secondary | 443 (45.2) | 38 (8.4) | 1.62 (0.90 to 2.93) | 13 (2.9) | 1.53 (0.58 to 4.09) |
| Technical/university | 212 (21.6) | 17 (8.0) | 1.50 (0.75 to 3.02) | 3 (1.4) | 0.72 (0.18 to 2.95) |
| Missing | 14 (1.4) | 0 (0) | – | 0 (0) | – |
| Employment | |||||
| Not employed | 417 (42.5) | 33 (7.9) | 1.00 (Reference) | 9 (2.1) | 1.00 (Reference) |
| Work for someone | 298 (30.4) | 20 (6.7) | 0.84 (0.47 to 1.49) | 9 (3.0) | 1.54 (0.58 to 4.09) |
| Self-employed or retired | 261 (26.6) | 18 (6.9) | 0.86 (0.47 to 1.56) | 4 (1.5) | 0.73 (0.18 to 2.95) |
| Missing | 4 (0.5) | 1 (25.0) | – | 0 (0) | – |
| Lifetime number of partners | |||||
| Zero | 192 (19.6) | 12 (6.2) | 1.00 (Reference) | 3 (1.5) | 1.00 (Reference) |
| One | 338 (34.5) | 20 (5.9) | 0.94 (0.45 to 1.97) | 5 (1.5) | 0.94 (0.22 to 4.00) |
| Two | 209 (21.3) | 18 (8.6) | 1.41 (0.66 to 3.02) | 6 (2.9) | 1.86 (0.46 to 7.55) |
| Three or more | 234 (23.9) | 22 (9.4) | 1.55 (0.75 to 3.23) | 8 (3.4) | 2.23 (0.58 to 8.52) |
| Missing | 7 (0.7) | 0 (0) | – | 0 (0) | |
| Age at first sex | |||||
| 10–16 | 193 (19.6) | 19 (9.8) | 1.00 (Reference) | 9 (4.6) | 1.00 (Reference) |
| 17–22 | 499 (50.9) | 37 (7.4) | 0.73 (0.41 to 1.31) | 9 (1.8) | 0.37 (0.14 to 0.96) |
| 23+ | 96 (9.8) | 4 (4.2) | 0.39 (0.13 to 1.21) | 1 (1.0) | 0.22 (0.03 to 1.72) |
| Never had sex | 192 (19.7) | 12 (6.3) | 0.61 (0.29 to 1.29) | 3 (1.6) | 0.32 (0.08 to 1.22) |
| Condom use | |||||
| Yes | 556 (56.7) | 46 (8.2) | 1.57 (0.81 to 3.02) | 14 (2.5) | 1.11 (0.40 to 3.14) |
| No sex | 192 (19.6) | 12 (6.3) | 1.16 (0.51 to 2.65) | 3 (1.6) | 0.68 (0.16 to 2.90) |
| Missing | 11 (1.1) | 2 (18.2) | – | 0 (0) | – |
| Oral sex | |||||
| Yes | 196 (20.0) | 19 (9.6) | 1.48 (0.85 to 2.56) | 8 (4.0) | 2.34 (0.97 to 5.66) |
| Smoking status | |||||
| Never | 708 (72.2) | 47 (6.6) | 1.00 (Reference) | 12 (1.7) | 1.00 (Reference) |
| Former | 189 (19.3) | 17 (9.0) | 1.39 (0.77 to 2.48) | 6 (3.2) | 1.90 (0.70 to 5.13) |
| Current | 83 (8.5) | 8 (10.8) | 1.50 (0.68 to 3.29) | 4 (4.8) | 2.93 (0.92 to 9.32) |
| Alcohol use | |||||
| Never | 255 (26.0) | 22 (8.6) | 1.00 (Reference) | 7 (2.8) | 1.00 (Reference) |
| Ever | 729 (73.4) | 49 (6.7) | 0.77 (0.46 to 1.30) | 14 (1.9) | 0.70 (0.28 to 1.76) |
| Missing | 6 (0.6) | 1 (16.7) | – | 1 (16.7) | – |
| Yearly dental visits | |||||
| Yes | 382 (38.9) | 24 (6.3) | 0.78 (0.47 to 1.30) | 6 (1.6) | 0.58 (0.22 to 1.49) |
| Missing | 2 (0.2) | 1 (50.0) | – | 0 (0) | – |
| Number of dental problems in last year | |||||
| No issues reported | 404 (41.2) | 29 (7.2) | 1.00 (Reference) | 11 (2.7) | 1.00 (Reference) |
| One issue reported | 281 (28.6) | 21 (7.5) | 1.04 (0.58 to 1.87) | 5 (1.8) | 0.48 (0.22 to 1.88) |
| Two issues reported | 194 (19.8) | 14 (7.2) | 1.00 (0.52 to 1.95) | 4 (2.1) | 0.75 (0.24 to 2.40) |
| Three or more issues reported | 101 (10.3) | 8 (7.9) | 1.11 (0.49 to 2.51) | 2 (2.0) | 0.72 (0.16 to 3.31) |
HPV, human papillomavirus.
In multivariate analysis, the prevalence of any oral HPV infection was higher in males as compared with females (adjusted OR (aOR), 2.31 (95% CI 1.22 to 4.02)) (table 2). Similarly, males had significantly higher prevalence of HR-HPV as compared with females (aOR, 4.29 (95% CI 1.36 to 13.50)). Individuals with two (aOR, 6.97 (95% CI 1.12 to 43.2)) lifetime partners compared with no sexual partners had a higher prevalence of HR-HPV.
Table 2.
Multivariate analyses of determinants of oral HPV infection
| Variable | Any HPV Adjusted OR |
High risk HPV Adjusted OR |
|---|---|---|
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 2.21 (1.22 to 4.02) | 4.29 (1.36 to 13.50) |
| Age | ||
| (15–18) | 1.00 | 1.00 |
| (19–27) | 2.22 (0.82 to 6.03) | 0.95 (0.22 to 4.18) |
| (28–35) | 2.44 (0.76 to 7.83) | 0.48 (0.06 to 3.72) |
| (36–45) | 1.05 (0.25 to 4.49) | 0.40 (0.03 to 4.96) |
| (46–55) | 2.75 (0.83 to 9.07) | 1.08 (0.15 to 7.40) |
| (56+) | 1.66 (0.42 to 6.50) | 0.40 (0.03 to 4.73) |
| Employed | ||
| Not employed | 1.00 | 1.00 |
| Work for someone | 0.56 (0.29 to 1.08) | 0.94 (0.31 to 2.87) |
| Self-employed or retired | 0.56 (0.28 to 1.15) | 0.31 (0.07 to 1.45) |
| Education | ||
| Primary school or less | 1.00 | 1.00 |
| Secondary school | 1.60 (0.80 to 3.19) | 1.32 (0.39 to 4.45) |
| University/technical | 1.55 (0.67 to 3.59) | 0.68 (0.13 to 3.60) |
| Lifetime number of partners | ||
| Zero | 1.00 | 1.00 |
| One | 1.54 (0.57 to 4.16) | 4.74 (0.80 to 28.0) |
| Two | 2.02 (0.73 to 5.62) | 6.97 (1.12 to 43.2)* |
| Three or more | 1.50 (0.54 to 4.12) | 3.99 (0.69 to 23.0) |
| Age at first sex | ||
| 10–16 | 1.00 | 1.00 |
| 17–22 | 0.64 (0.34 to 1.21) | 0.38 (0.14 to 1.07) |
| 23+ | 0.30 (0.08 to 1.11) | – |
| Oral sex | ||
| No | 1.00 | 1.00 |
| Yes | 1.16 (0.61 to 2.20) | 1.68 (0.56 to 5.03) |
| Number of dental problems in last year | ||
| No issues reported | 1.00 | 1.00 |
| One issue reported | 1.04 (0.56 to 1.91) | 0.74 (0.24 to 2.25) |
| Two issues reported | 0.98 (0.48 to 1.98) | 0.62 (0.16 to 2.38) |
| Three or more issues reported | 1.49 (0.62 to 3.55) | 1.22 (0.23 to 6.27) |
| Smoking | ||
| Never | 1.00 | 1.00 |
| Former | 1.10 (0.55 to 2.21) | 1.34 (0.39 to 4.53) |
| Current | 1.35 (0.58 to 3.16) | 2.86 (0.77 to 10.59) |
| Ever alcohol use | ||
| No | 1.00 | 1.00 |
| Yes | 0.58 (0.31 to 1.02) | 0.40 (0.14 to 1.20) |
HPV, human papillomavirus.
DISCUSSION
HNCs are the sixth most common cancers worldwide and the incidence of HPV-associated subsets of these cancers have shown significant increases in both developed and low-income and middle-income countries, such as Peru, over the last 20 years.5,6 Our population-based study, the largest performed in Peru to date, demonstrates an oral HPV prevalence similar to that observed in large population-based studies conducted in the USA. The prevalence of any oral HPV infection was significantly higher in males.
The prevalence of HR-HPV infections, including HPV 16, in our study was lower than what has been observed in both the USA and other developed countries.7,8 This result supports the lower incidence of HPV-associated HNC reported in Peru compared with the USA (1.0/100 000 persons vs 2.6/100 000 persons, respectively). The unexpectedly high prevalence of HPV 55, a non-oncogenic type, likely reflects local genotype abundance. However, further prevalence surveys are required to confirm this result and determine its relevance to clinical outcome.
This study demonstrated that males have a higher prevalence of any and HR-HPV compared with females. The higher prevalence of HPV infection in males is consistent with the nearly threefold higher incidence of HNCs detected in males as compared with females in other studies.6,19,20 The difference in prevalence by gender was sustained even after adjustment for age and sexual behaviours, which suggests that the higher prevalence of oral HPV in males is potentially driven by biological differences between genders as compared with differences in behaviours that may lead to increased likelihood of exposure among men to oral HPV types. However, insufficient sample sizes and potential misclassification of measurement of sexual factors caution the interpretation of these conclusions.
Previous studies have demonstrated an association of alcohol use and smoking with oral HPV infection and certain subsets of HNCs.21–23 We observed a lower prevalence of oral HPV infection among individuals who reported ever using alcohol while individuals who reported current smoking had a near threefold higher prevalence of oral HPV in adjusted analyses. Alcohol use has been identified as a risk factor for the development of HPV-negative oropharyngeal cancers and therefore would not necessarily be a direct risk factor for oral HPV infection, particularly after taking into account differences in smoking status and sexual behaviour. The lack of measurement of the intensity and cumulative use of alcohol among individuals in this study makes it difficult to explore this association. On the other hand, the higher prevalence of oral HPV among current smokers in this study, particularly after adjustment for sexual risk factors, suggests a possible independent role of cigarette smoking increasing the likelihood of oral HPV acquisition, possibly through modulation of the local immune response of the oral cavity. As with alcohol use, the lack of more detailed measurement of smoking exposure combined with the relatively low overall frequency of cigarette use limits the analysis.
This study has several limitations. First, this study had a relatively small sample size limiting our ability to observe subtle difference in oral HPV prevalence more finely across ages and by different behavioural and demographic factors. However, the direction and significance of the association of certain factors with HPV prevalence in this study is similar to prior work performed in other settings.7,18,21,22 Second, this study may not be fully generalisable to the wider population of Lima or Peru. Third, given the cross-sectional design of the current study, it is difficult to ascertain the temporal relationship of many of the behavioural factors with acquisition of oral HPV infection. We did not collect information on the household from which individuals were recruited which limited our ability to control for the clustering of HPV infection and certain risk factors. However, all individuals were recruited from a single neighbourhood in Lima, which may minimise inter-household differences. Risk factor information was collected based on participant self-report. Certain stigmatised behaviours, such as risky sexual behaviours and alcohol use, may therefore be misreported overall or differentially by specific subgroup (ie, sex, age), which would result in biased estimates of the association with HPV infection.
We describe the prevalence and correlates of oral HPV infection in Lima, Peru. The incidence of HPV-associated HNCs has increased significantly in both countries of the developed and developing world; however, data on oral HPV prevalence are relatively limited, particularly in low-income and middle-income countries. These findings provide background information that may help explain the changing trends of HNCs as well as confirmatory data to show that the epidemiology of oral HPV infection is similar across geographic and socioeconomic regions. Furthermore, these findings provide additional information that may help explain the higher incidence of HPV-associated HNCs among males as compared with females. The higher prevalence of oral HPV infection among males as compared with females, independent of sexual risk factors, suggests a sex-specific bias in the natural history of oral HPV infection and carcinogenesis.
Key messages.
-
▸
The prevalence of any and high-risk oral human papillomavirus (HPV) infection in men and women aged 10–85 years in Lima, Peru is 6.8% and 2.0%, respectively.
-
▸
The most common HPV and high-risk HPV type detected in this population-based cross-sectional study was HPV 55 (3.4%) and HPV 16 (1.1%).
-
▸
Men had a higher prevalence of HPV infection as compared with women overall and across multiple ages after adjusting for sexual and demographic factors.
-
▸
The pattern of age-specific and sex-specific prevalence of oral HPV infection in this study was similar to population-based studies of conducted in the US.
Acknowledgments
Funding This work was supported by the National Institutes of Health Fogarty Scholars Program (5R24 TW007988); the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp (38523), as well as the National Institute of Dental and Craniofacial Research.
Footnotes
Handling editor Jackie A Cassell
Contributors LW, RHG, PEG and MAM developed the study design and were involved in data collection along with LC. BJR performed the statistical analysis. MAM facilitated the plan for data analysis and assisted in the interpretation of results. Contributors BJR and MAM collaborated in the writing of the manuscript. BJR, LW, RHG, PEG and MAM revised the manuscript before submission.
Competing interests MAM is an employee of Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Patient consent Obtained.
Ethics approval Institutional Review Board of PRISMA; Ethics review board of the Institution of National Health of Peru (ethics committee approval number CE1006.009/036-2013-CI-INS).
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363:1488–9. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 3.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 4.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29:1488–94. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 5.Walter L, Vidaurre T, Gilman RH, et al. Trends in head and neck cancers in Peru between 1987–2008: experience from a large public cancer hospital in Lima. Head Neck. 2013;36:729–34. doi: 10.1002/hed.23369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–19. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreimer AR, Bhatia RK, Messeguer AL, et al. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010;37:386–91. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 9.Heath EM, Morken NW, Campbell KA, et al. Use of buccal cells collected in mouthwash as a source of DNA for clinical testing. Arch Pathol Lab Med. 2001;125:127–33. doi: 10.5858/2001-125-0127-UOBCCI. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Closas M, Egan KM, Abruzzo JA, et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10:687–96. [PubMed] [Google Scholar]
- 11.D’Souza G, Sugar E, Ruby W, et al. Analysis of the effect of DNA purification on detection of human papillomavirus in oral rinse samples by PCR. J Clin Microbiol. 2005;43:5526–35. doi: 10.1128/JCM.43.11.5526-5535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawton G, Thomas S, Schonrock J, et al. Human papillomaviruses in normal oral mucosa: a comparison of methods for sample collection. J Oral Pathol Med. 1992;21:265–9. doi: 10.1111/j.1600-0714.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 13.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravitt PE, Peyton CL, Apple RJ, et al. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 17.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 18.StataCorp. Stata statistical sofware: release 12. TX: StataCorp LP, College Station; 2011. [Google Scholar]
- 19.Blomberg M, Nielsen A, Munk C, et al. Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 2011;129:733–41. doi: 10.1002/ijc.25699. [DOI] [PubMed] [Google Scholar]
- 20.Joseph AW, D’Souza G. Epidemiology of human papillomavirus-related head and neck cancer. Otolaryngol Clin North Am. 2012;45:739–64. doi: 10.1016/j.otc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31:20–8. [PubMed] [Google Scholar]
- 22.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and hman papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 23.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]


