Abstract
Statement of the Problem:
The bond strength of composites with different adhesive systems with dentin is an important factor in long term durability of composite restorations. The effect of titanium tetrafluoride (TiF4) as anti caries agent and sodium hypochlorite (NaOCl) as disinfectant on the shear bond of nanofilled and silorane based composite resins have not been investigated in previous studies.
Purpose:
This study was conducted to determine bond strength between dentin and two composite systems, by means of shear bond test using TiF4 and NaOCl.
Materials and Method:
Middle dentin of 60 intact extracted maxillary premolar teeth were exposed by sectioning the crowns at a depth of 2mm from central groove and parallel to the occlusal surface. Standardized smear layer was created using a 600-grit silicon carbide paper and then samples were embedded in acrylic resin blocks. Then the samples were randomly divided into 6 \groups summarized as Group I: Z350, Group II: Z350+ NaOCl, Group III: Z350+ TiF4, Group IV: P90, Group V: P90+ NaOCl, Group VI: P90+ TiF4 according to manufacturer’s instruction. Then samples were subjected to shear bond strength (SBS) test using universal testing machine and data were analyzed using ANOVA and Tukey tests (p< 0.05).
Results:
Application of 5% NaOCl caused a significant decrease in SBS of nanofilled composite resin (p= 0.004), and also silorane based composite resin (p= 0.006). Application of 4% TiF4 caused a significant increase in SBS of silorane based composite resin (p= 0.001). The effect of TiF4 on nanofilled composite was not statistically significant.
Conclusion:
Using TiF4 has a positive effect on increasing the shear bond while NaOCl has negative effect on bond strength.
Keywords: TiF4, NaOCl , Shear Bond Strength , Nanofilled composite , Silorane composite
Introduction
Materials that contain fluoride release different levels of fluoride that can prevent caries production. Topical fluorides such as metal fluorides, especially titanium tetrafluoride, have become popular in the recent dental studies due to their unique interaction with dental hard tissue.[1-3] While commonly used fluorides such as sodium fluoride (NaF), Tin (II) fluoride (SnF2), acidulated phosphate fluoride (APF) may not be considered as long term beneficial materials, titanium tetrafluoride (TiF4) has been shown to offer greater protection against caries and tooth erosion. The unique interaction of TiF4 with tooth structure leads to the formation of a resistant tenacious coating, referred as glaze-like layer, on the tooth surface,[4] and it has a significant rapid uptake of fluoride in enamel, dentin, and root surface.[5-6] The advantage has been related to the titanium group present in the compound which synergizes the effect of fluoride.[6-7]
Different studies that used TiF4 or sodium hypochlorite (NaOCl) as pretreatment have reported different results.[1] Devabhaktuni and Manjunath concluded that application of TiF4 on dentin did not significantly affect the bond strength of the composite resin.[8] Irrigation solutions and medications used during dental treatment may lead to changes in the chemical and physical properties of dentin.[9-10] One of the most commonly used irrigation solution and intracanal dressing are 0.5% sodium hypochlorite.[11] This alkaline material reacts with organic tissue, changing the chemical structure and affecting the mechanical properties of dentin.[12-13] In untreated dentin, tensile strength varies with location and orientation of dentinal tubules.[14] NaOCl has been used on dentin as a deproteinizing agent.[15-16]
Recently, a new category of resin composites, named nanofilled composites, were developed.[17-18] It has been proven that composites with more filler content such as nanofilled composites have better mechanical properties.[19]
In the new class of low shrinking composites based on silorane technology, the silorane resin replaces the conventionally used methacrylate resin matrix within conventional dental composites; thereby, providing lower polymerization shrinkage as well as better hydrolytic stability.[20-21]
Little information is available about the effect of TiF4 4% and NaOCl 5% on shear bond strength of nanofilled and silorane based composite resins. The aim of this study was to determine the in-vitro effect of TiF4 and NaOCl application on the shear bond strength of these two composite resins to dentin.
Materials and Method
In this experimental study, sixty intact extracted human maxillary premolars were used. They were without any caries, cracks, or fractures and extracted for orthodontic reasons. The teeth were hand-scaled and stored in solution of 0.5 % chloramine T for 24 hours and then stored in distilled water until to the time of use. Middle dentin was exposed by sectioning the crowns at a depth of 2mm from central groove and parallel to the occlusal surface using a diamond disk (4138, KG soren son, Barueri, SP, Brazil) under water cooling.[22] The root of the sectioned teeth were embedded in cylindrical aluminium blocks of 2cm diameter and 2.5cm height and stabilized in acrylic resin, 1.0 mm below the CEJ. Then, the occlusal surfaces of the teeth were ground to produce a flat and smooth dentin surface with 600-grit silicon carbide abrasive paper under constant water spray.[23-24] In this study, a halogen light polymerizing unit (optilux501, sybronkerr, Danbury, CT. USA) with light intensity of 600mW/cm 2 was used for curing the adhesive and resin composites.
TiF4 (Sigma Aldrich, USA) was dissolved in deionized distilled water to achieve a concentration of 4% (wt/v; PH 1.2).[25] The teeth were randomly assigned to six groups of ten teeth (n=10). Plastic cylinders with an inner diameter of 3mm and a height of 2mm were used to produce composite blocks. The composite resins applied over central part of prepared dentin, considering the manufacturersʹ recommendations as follows:
Group I in which the procedure was etching for15 seconds with 37% phosphoric acid gel (3M, ESPE, USA), rinsing for 15 seconds with water, gentle air drying plus applying two consecutive coats of single bond (3M, ESPE, USA), drying gently for 2-5 seconds, light-curing for 10 seconds, applying nanofilled composite resin (Z350) (3M, ESPE, USA), finally light polymerization for 40 seconds.
The procedures in group II were similar to those in group I except that in this group prior to acid application, the exposed dentin surfaces were produced by the application of 5% NaOCl (Merck, Germany) by drip pan for 2 minutes. Then the NaOCl-treated surfaces were rinsed thoroughly for 60 seconds.[24]
The procedures in group III were similar to those in group I; however, after acid application and before the application of single bond, the exposed dentin surfaces were produced by the active application of 4% TiF4 by microbrush for 60 seconds; the TiF4 treated surface was then rinsed thoroughly for 30 seconds.
In group IV, the procedures were consequently the application of one coat of the silorane system primer for 15 seconds with gentle agitation using fully saturated applicator, gentle air drying to evaporate solvent, light polymerization for 10 seconds, application of silorane bonding to the entire preparation and gently air drying until the bonding was spread to an even film and light polymerization for 10 seconds. Then, application of silorane based composite resin (P90) (3M, ESPE, USA) with light polymerization for 40 seconds was performed respectively.
The procedures in group V were similar to those in group IV except that in this group before self-etch primer application, the exposed dentin surfaces were produced by the application of 5% NaOCl by drip pan for 2 minutes, and the NaOCl-treated surfaces were then rinsed thoroughly for 60 seconds.[24]
The procedures in group VI were similar to those in group IV; however, in this group before self-etch primer application, the exposed dentin surfaces were produced by the active application of 4% TiF4 by microbrush for 60 seconds, and the TiF4 treated surface was then rinsed thoroughly for 30 seconds.[25]
The test specimens were then stored in distilled water at 37°c for one day before being subjected to the shear test.[23] Shear bond strength (SBS) testing was performed using the universal testing machine (ZwickRoell; Z250, and Germany).
Statistical analysis
Mean values of the different study groups were compared using two-way ANOVA, followed by Student t- test and one-way ANOVA followed by Tukey HSD, and were processed using SPSS version 17. P-value of ≤ 0.05 was considered significant.
Results
The mean and standard deviation of 6 study groups shear bond strength is summarized in Table 1.
Table 1.
The mean shear bond strength of two composites and pretreatment materials (a = p≤ 0.05, b = p≤ 0.01, c = p≤ 0.001)
| Groups | Mean±SD | Significant Differences | |
|---|---|---|---|
| 1 | Z350 (I) | 21.43±2.03 | I vs. II (b),IV (c),V (c) |
| 2 | Z350+NaOCl (II) | 18.59±2.29 | II vs. I (b), III (a), V (b), VI (c) |
| 3 | Z350 +TiF4 (III) | 21.1±0.64 | III vs. II (a), IV (c), V (c) |
| 4 | P90 (IV) | 18.1±1.79 | IV vs. I (c), III (b), V (b), VI (c) |
| 5 | P90 + NaOCl (V) | 15.9±1.22 | V vs. I(c), II (b), III(a), IV(b), VI(c) |
| 6 | P90 + TiF4 (VI) | 20.7±1.19 | VI vs. II (c), IV (c), V (c) |
Figure 1 shows the mean bond strength of two composites after application of TiF4 and NaOCl pretreatments. Two-way ANOVA test showed that composite pretreatment-materials (NaOCl and TiF4) had significant interactions. Student t-test was used to evaluate the effect of composite type on the shear bond strength and showed that without any pretreatment, the shear bond strength of Z350 is significantly higher than P90 (p= 0.001). At the time of NaOCl application, the shear bond strength of Z350 (group II) is significantly higher than P90 (group V) (p= 0.005). When using TiF4 solution there was, no significant differences between Z350 (group III) and P90 (group VI) (p= 0.363).
Figure 1.
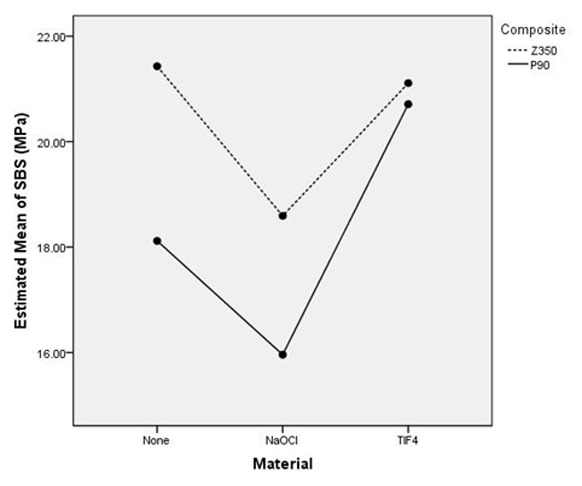
The mean shear bond strength of two composites. The vertical axis has shown the mean bond strength (MPa) and horizontal axis has shown pretreatment material type.
Finally, to evaluate the effect of TiF4 and NaOCl pretreatments on the shear bond strength of each composite, one- way ANOVA and Tukey HSD tests showed that application of NaOCl on the dentin surface before etching lead to significant reduction of bond strength in Z350 (p= 0.004) while using this pretreatment before priming dentin surface led to significant reduction in bond strength of P90 (p= 0.006).Using TiF4 on dentin surface after etching has no significant effect on bond strength (p= 0.918) while application of TiF4 before priming dentin surface caused significant increase in bond strength of P90 (p= 0.001).
Discussion
Exposure to high levels of fluoride, during tooth formation and also after it, can play an important role in caries prevention. Fluoride releasing materials may be classified into different groups such as sodium fluoride, potassium fluoride, titanium tetrafluoride, and so on.[26] From those groups, TiF4 solution has been considered as a smear layer modifying agent in recent years.[19] Bridi et al.[25] observed similar results in penetration of different adhesive systems to dentin with and without the use of TiF4, so the same resin tags in number and length could explain the result obtained in the group I and group III of our experimental groups.
The shear bond strength of group VI is significantly higher than that of the control group. This statistically significant difference may be due to the fact that silorane primer with the pH of 2.4 is considered relatively mild.[27] This leads to dentin decalcification being resumed to a few hundredths of nanometers contributed to shorter resin tag formation while using 4% TiF4. Cho et al. showed that the application of phosphoric acid etching before silorane system adhesive caused higher bond strength to dentin;[22] hence, the wear caused by active mode application of 4% TiF4 and rinsing the TiF4 and smear layer in group VI may be responsible for the higher bond strength in this group compared to the control group.
Wettability is one of the most important physicochemical surface properties and NaOCl is a non-specific proteolytic material which can remove organic materials and magnesium and carbonate ions.[23] Previous studies have shown that after NaOCl treatment of dentin, an increase in wettability is expected because collagen removal produces a hydrophilic surface. The complete removal of organic components of the demineralized collagen matrix by NaOCl also increases the porosity of the intact dentin. The present study showed that the use of 5% NaOCl significantly decreased shear bond strength of single bond and silorane adhesive to dentin (p< 0.05). This is in accordance with the findings of the previous studies.[23-24,28]
Current dentin priming monomers are usually dissolved in acetone and/or ethanol which can displace water from the dentin surface and from the moist collagen network, thus promoting the infiltration of the monomers through the nano spaces of the dens collagen web and enhancing bond strength. The resin replaces the water within the pores between the collagen fibers. Single bond and silorane adhesive are alcohol based and the weak water chasing capability of the volatile resin solvent appears not to displace the water effectively from the intertubular network, resulting in less resin infiltration through the collagen network, so single bond and silorane adhesive diffuses very slowly to the intertubular network. This short dwell time is insufficient to permit a full diffusion of the monomer into the substrate. In this way, nanometric porosities of intertubular dentin created by the NaOCl treatment were not reached by monomer, leaving an adhesive interface with voids. This may explain the lowering of bond strength in the using of NaOCl.[24] Ebrahimi et al.[23] showed that the decrease of bond strength as a result of the use of NaOCl can also be attributed to damages to the organic matrix of dentin, especially to collagen fibers. Approximately, 22% wt of dentin is composed of organic materials, which predominantly consist of type I collagens that have an important role in the mechanical properties of dentin. NaOCl reacts with amino acids of dentin proteins and breaks down peptide chains; therefore, it may change the mechanical properties of dentin by destroying the organic content of dentin. They showed that NaOCl damages the organic component of dentin; therefore, organic monomers do not sufficiently penetrate into the demineralized dentin, resulting in a lack of proper bond strength.[23] Owing to the similarities between the findings of the present study and those of Ebrahimi et al.,[23] it can be suggested that the application of 5% NaOCl offers a credible explanation for the decrease of bond strength in nanofilled and silorane resin composites.
A part of this study was designed to evaluate the bonding capacity of self-etching adhesives against the established total-etch products. Self-etching adhesives are newer compositions, developed to simplify and reduce technique sensitivity of dentin bonding systems.[29] In this study, the self-etch adhesive resulted in lower bond strength to dentin than total-etch adhesive (p< 0.05). The adhesive system of silorane based restorative system is a self-etching adhesive and is exclusively for use with silorane based composite.[30] It is known that the silorane system adhesive is composed of an acidic hydrophilic one step primer of the self-etching type, with pH of 2.7, which is considered relatively mild.[27] Mine demonstrated that mild pH of silorane primer leads to dentin decalcification being resumed to a few hundreds of nanometers.[31] It is stated that a hydrophobic resin adhesive (silorane bond) must be applied and light activated independently.[32] The relatively high amount of HEMA keeps this resin solution homogenous and prevents separation of the primer phases, but makes it vulnerable to water sorption. Pucci et al. pointed that the silorane-based primer may be considered a one-step adhesive system, as the primer is light cured.[33] This may justify the low bond strength values of silorane-based primer compared to the two-step adhesive system.
Conclusion
Within the limits of the present study, it can be concluded that nanofilled composite resin has higher shear bond strength than silorane based composite resin. The use of 4% TiF4, before silorane based composite system, can increase the shear bond strength but using 5% NaOCl reduced the bond strength of both composite resins to dentin.
Acknowledgement
The authors thank the Vice-Chancellery of Shiraz University of Medical Science for financial support of this research (Grant#6133) and Dr. Zarangiz Movahhedi for preparing testing solutions. This article is based on the thesis by Dr. Nader Razazan No.1567. The authors also thank Dr. Mehrdad Vossoughi of Dental Research Development center, of the school of dentistry for the statistical analysis and Dr. Ehya Amalsaleh for improving and editing the English manuscript.
Conflict of Interest:The authors deny any conflict of interest that could influence their work.
References
- 1.Wahengbam P, Tikku AP, Lee WB. Role of titanium tetrafluoride (TiF(4)) in conservative dentistry: A systematic review. J Conserv Dent. 2011; 14: 98–102. doi: 10.4103/0972-0707.82598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves RD, Souza TM, Lima KC. Titanium tetrafluoride and dental caries: a systematic review. J Appl Oral Sci. 2005; 13: 325–328. doi: 10.1590/s1678-77572005000400002. [DOI] [PubMed] [Google Scholar]
- 3.Magalhães AC, Rios D, Honório HM, Delbem AC, Buzalaf MA. Effect of 4% titanium tetrafluoride solution on the erosion of permanent and deciduous human enamel: an in situ/ex vivo study. J Appl Oral Sci. 2009; 17: 56–60. doi: 10.1590/S1678-77572009000100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Büyükyilmaz T, Ogaard B, Rølla G. The resistance of titanium tetrafluoride-treated human enamel to strong hydrochloric acid. Eur J Oral Sci. 1997; 105: 473–477. doi: 10.1111/j.1600-0722.1997.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 5.Hals E, Tveit AB, Tötdal B, Isrenn R. Effect of NaF, TiF4 and APF solutions on root surfaces in vitro, with specialreference to uptake of F. Caries Res. 1981; 15: 468– 476. doi: 10.1159/000260554. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Harless JD. The effect of topical fluoride agents on fluoride uptake and surface morphology. J Dent Res. 1981; 60: 1842–1848. doi: 10.1177/00220345810600110301. [DOI] [PubMed] [Google Scholar]
- 7.Tezel H, Ergücü Z, Onal B. Effects of topical fluoride agents on artificial enamel lesion formation in vitro. Quintessence Int. 2002; 33: 347–352. [PubMed] [Google Scholar]
- 8.Devabhaktuni S, Manjunath M. Effect of 4% titanium tetrafluoride application on shear bond strength of composite resin: An in vitro study. J Conserv Dent. 2011; 14: 43–45. doi: 10.4103/0972-0707.80741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slutzky-Goldberg I, Maree M, Liberman R, Heling I. Effect of sodium hypochlorite on dentin microhardness. J Endod. 2004; 30: 880–882. doi: 10.1097/01.don.0000128748.05148.1e. [DOI] [PubMed] [Google Scholar]
- 10.Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001; 34: 120–132. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Spanberg LZ. Instruments, materials, and devices. In: Cohen SS, Burns RC, eds. Pathways of the pulp. 8th ed. St Louis: Mosby; 2002. pp. 521–572. [Google Scholar]
- 12.Chng HK, Palamara JE, Messer HH. Effect of hydrogen peroxide and sodium perborate on biomechanical properties of human dentin. J Endod. 2002; 28: 62–67. doi: 10.1097/00004770-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Doğan H, Qalt S. Effects of chelating agents and sodium hypochlorite on mineral content of root dentin. J Endod. 2001; 27: 578–580. doi: 10.1097/00004770-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Takahashi H, Nishimura F. Anisotropy of tensile strengths of bovine dentin regarding dentinal tubuleorientation and location. Dent Mater J. 2002; 21: 32–43. doi: 10.4012/dmj.21.32. [DOI] [PubMed] [Google Scholar]
- 15.Perdigão J, Lopes M, Geraldeli S, Lopes GC, García-Godoy F. Effect of a sodium hypo-chlorite gel on dentin bonding. Dent Mater. 2000; 16: 311–323. doi: 10.1016/s0109-5641(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 16.Toledano M, Perdigão J, Osorio E, Osorio R. Influence of NaOCl deproteinization on shear bond strength in function of dentindepth. Am J Dent. 2002; 15: 252–255. [PubMed] [Google Scholar]
- 17.Da Silva EM, Poskus LT, Guimarães JG. Influence of light-polymerization modes on the degree of conversion andmechanical properties of resin composites: a comparative analysis between a hybrid and a nanofilled composite. Oper Dent. 2008; 33: 287–293. doi: 10.2341/07-81. [DOI] [PubMed] [Google Scholar]
- 18.Dresch W, Volpato S, Gomes JC, Ribeiro NR, Reis A, Loguercio AD. Clinical evaluation of a nanofilled composite in posterior teeth: 12-month results. Oper Dent. 2006; 31: 409–417. doi: 10.2341/05-103. [DOI] [PubMed] [Google Scholar]
- 19.Alavi SAA, Koohpeima F, Motamedi M, Heidari S. The Effect of Different Light Curing Units and Composite Thicknesses on the Shear Bond Strength of Composite to Dentin. J Islam Dent Assoc IRAN (JIDA) 2012; 24: 251–255. [Google Scholar]
- 20.Usha H, Kumari A, Mehta D, Kaiwar A, Jain N. Comparing microleakage and layering methods of silorane-based resin composite in class V cavities using confocal microscopy: An in vitro study. J Conserv Dent. 2011; 14: 164–168. doi: 10.4103/0972-0707.82624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuti WY, Chaves FO, Di Hipólito V, Rodrigues FP, D'Alpino PH. Fracture resistance of teeth restored with different resin-based restorative systems. Braz Oral Res. 2012; 26: 275–281. doi: 10.1590/s1806-83242012005000004. [DOI] [PubMed] [Google Scholar]
- 22.Cho SY, Kang HY, Kim KA, Yu MK, Lee KW. Effect of adhesive hydrophobicity on microtensile bond strength of low-shrinkage silorane resin to dentin. J Korean Acad Conserv Dent. 2011; 36:280–289. [Google Scholar]
- 23.Ebrahimi Chaharom ME, Kahnamoii MA, Kimyai S, Hajirahiminejad Moghaddam MR. Effect of sodium hypochlorite on the shear bond strength of fifth- and seventh-generation adhesives to coronal dentin. Africa J Biotechno. 2011; 10: 12697–12701. [Google Scholar]
- 24.Mathai1 V, Christaine Angelo1, M Jayakumar, K Sarath Babu, K Effect of sodium hypochlorite on shear bond strength using three different adhesive systems: an in-vitro study. Int J Bioassays. 2013; 2: 637–640. [Google Scholar]
- 25.Bridi EC, Amaral FL, França FM, Turssi CP, Basting RT. Influence of dentin pretreatment with titanium tetrafluoride and self-etchingadhesive systems on microtensile bond strength. Am J Dent. 2013; 26: 121–126. [PubMed] [Google Scholar]
- 26.Cury JA, de Oliveira BH, dos Santos AP, Tenuta LM. Are fluoride releasing dental materials clinically effective on caries control? . Dent Mater. 2016;32:323–333. doi: 10.1016/j.dental.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Isaac SZ, Bergamin AC, Turssi CP, Amaral FL, Basting RT, França FM. Evaluation of bond strength of silorane and methacrylate based restorative systems to dentin using different cavity models. J Appl Oral Sci. 2013; 21: 452–459. doi: 10.1590/1679-775720130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manimaran VS, Srinivasulu S, Rajesh Ebenezar A, Mahalaxmi S, Srinivasan N. Application of a proan-thocyanidin agent to improve the bond strength of root dentin treated with sodium hypochlorite. J Conserv Dent. 2011; 14: 306–308. doi: 10.4103/0972-0707.85822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perdigão J, Gomes G, Duarte S Jr, Lopes MM. Enamel bond strengths of pairs of adhesives from the same manufacturer. Oper Dent. 2005; 30: 492–499. [PubMed] [Google Scholar]
- 30.Ivanovas S, Hickel R, Ilie N. How to repair fillings made by silorane-based composites. Clin Oral Investig. 2011; 15: 915–922. doi: 10.1007/s00784-010-0473-z. [DOI] [PubMed] [Google Scholar]
- 31.Mine A, De Munck J, Van Ende A, Cardoso MV, Kuboki T, Yoshida Y, et al. TEM characterization of a silorane composite bonded to enamel/dentin. Dent Mater. 2010; 26: 524–532. doi: 10.1016/j.dental.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Sauro S, Pashley DH, Mannocci F, Tay FR, Pilecki P, Sherriff M, et al. Micropermeability of current self-etching and etch-and-rinse adhesives bonded to deep dentine: a comparison study using a double-staining/ confocal microscopy technique. Eur J Oral Sci. 2008; 116: 184–193. doi: 10.1111/j.1600-0722.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 33.Pucci CR, de Oliveira RS, Caneppele TM, Torres CR, Borges AB, Tay FR. Effects of surface treatment, hydration and application method on the bondstrength of a silorane adhesive and resin system to dentine. J Dent. 2013; 41: 278–286. doi: 10.1016/j.jdent.2012.11.016. [DOI] [PubMed] [Google Scholar]


