Abstract
Structural brain abnormalities have been amply demonstrated in schizophrenia. These include volume decrements in the perirhinal/entorhinal regions of the ventromedial temporal lobe, which comprise the primary olfactory cortex. Olfactory impairments, which are a hallmark of schizophrenia, precede the onset of illness, distinguish adolescents experiencing prodromal symptoms from healthy youths, and may predict the transition from the prodrome to frank psychosis. We therefore examined temporal lobe regional volumes in a large adolescent sample to determine if structural deficits in ventromedial temporal lobe areas were associated, not only with schizophrenia, but also with a heightened risk for psychosis. Seven temporal lobe regional volumes (amygdala [AM], hippocampus, inferior temporal gyrus, parahippocampal gyrus, superior temporal gyrus, temporal pole, and entorhinal cortex [EC]) were measured in 386 psychosis spectrum adolescents, 521 adolescents with other types of psychopathology, and 359 healthy adolescents from the Philadelphia Neurodevelopment Cohort. Total intracranial and left EC volumes, which were both smallest among the psychosis spectrum, were the only measures that distinguished all 3 groups. Left AM was also smaller in psychosis spectrum compared with healthy subjects. EC volume decrement was strongly correlated with impaired cognition and less robustly associated with heightened negative/disorganized symptoms. AM volume decrement correlated with positive symptoms (persecution/special abilities). Temporal lobe volumes classified psychosis spectrum youths with very high specificity but relatively low sensitivity. These MRI measures may therefore serve as important confirmatory biomarkers denoting a worrisome preclinical trajectory among at-risk youths, and the specific pattern of deficits may predict specific symptom profiles.
Keywords: psychosis risk, MRI, neurodevelopment, negative symptoms, olfaction
Introduction
Early identification of schizophrenia is crucial for effective treatment.1 Reliable measures that denote psychosis vulnerability prior to the emergence of symptoms are therefore of significant interest.2 One promising domain, in this regard, is olfaction. Olfactory deficits are a hallmark of schizophrenia.3 These are evident prior to the onset of illness and may be predictive, among clinical high-risk (prodromal) individuals, of poor functional outcome, including frank psychosis.4–9
Importantly, olfactory deficits are not simply exemplars of diffuse cognitive impairment. They reflect fundamental abnormalities of the primary olfactory sensory apparatus. Structural anomalies are observed in the posterior nasal cavities,10 olfactory bulbs,11,12 and perirhinal and entorhinal cortices, which together comprise the primary olfactory cortex.13–15 Volume reductions in this olfactory cortical region correlate directly with patients’ impairments in odor detection.13 Otherwise healthy individuals with hyposmia similarly exhibit reduced olfactory cortical volumes, further supporting a structural–functional causal linkage.16
Although schizophrenia volume decrements appear diffusely throughout the temporal lobe,17 primary olfactory cortex volume decrements are relatively specific. Anatomically, the perirhinal and entorhinal cortices (often aggregated into “entorhinal” region in MRI studies) sit behind the temporal pole (TP). Comparing these contiguous regions, we observed volume reductions in schizophrenia in perirhinal and entorhinal cortices, but not TP.13 While schizophrenia imaging studies have not focused extensively on these temporal lobe subregions, reports support our finding—entorhinal cortical gray matter volume is consistently reduced,13–15 while the adjacent TP typically,13,18,19 but not always,20 remains intact.
We have hypothesized that this dissociation reflects the fact that these 2 regions are developmentally distinct.13 The entorhinal cortex (EC) is part of the phylogenetically older allocortex,21 which develops early embryologically (late first/early second trimester).22,23 During this critical risk period, developmental insults heighten the subsequent risk for schizophrenia.24 The TP, a neocortical structure that is clearly identifiable only in primates,25 emerges as a distinct substructure in the latter half of pregnancy.26 Consequently, developmental insults during the early critical risk period would likely disrupt the emerging EC, but have little impact on the prenascent TP. This selective regional anomaly may therefore reflect early embryonic perturbations that increase the risk for future psychosis.
Thus, reduced temporal lobe volume should also be apparent, prior to the emergence of overt illness, in younger individuals at risk for developing schizophrenia. Indeed, there is a growing4–9 but imperfect27 body of evidence that prodromal adolescents are impaired in their sense of smell, and this deficit may predict which vulnerable youths will progress to frank psychosis5,6 or otherwise have poor functional outcomes.9,28 These behavioral findings highlight the potential sensitivity of olfactory system impairment as a predictive vulnerability marker among at-risk individuals.
To determine whether a specific EC volume decrement, similar to that observed in schizophrenia, is also present in at-risk youths prior to illness onset, we examined temporal lobe MRI measures in psychosis spectrum youths, healthy youths, and youths with other notable psychopathology. If confirmed, this would support the connection between this structural abnormality and the developmental pathoetiology of schizophrenia. It could also provide a simple neuroanatomic biomarker to facilitate early identification of those at risk for schizophrenia prior to the emergence of clinically significant symptomatology. This is the first study to examine medial temporal lobe structures in a young community-based, non-help seeking, high-risk cohort.
Methods
Participants
Participants were part of the Philadelphia Neurodevelopmental Cohort (PNC),29,30 a community-based sample of 9,498 youths, aged 8–21 years, who underwent comprehensive clinical and cognitive evaluations. A subset (1601) received multimodal neuroimaging.31 Participants provided informed consent or assent plus parental consent, and procedures were approved by University of Pennsylvania and Children’s Hospital of Philadelphia Institutional Review Boards.
Clinical assessment included 3 structured screening tools to assess a broad spectrum of psychosis-relevant experiences and other psychopathology. See Calkins et al29,30 and supplementary material for descriptions and threshold classification criteria. Subjects were classified as “psychosis spectrum” (PS) if they exceeded threshold on the psychosis spectrum screen, regardless of the presence or absence of other psychopathology. Subjects were classified as “other psychopathology” (OP) if they did not meet PS criteria, but exceeded threshold for another psychopathology domain. Subjects were classified as “healthy controls” (HC) if they did not meet criteria for either PS or OP. Subjects were further screened and excluded for significant comorbid medical conditions.31
Of 1601 subjects with neuroimaging data, 190 were excluded based on medical history. Another 145 were excluded for poor MR image quality (supplementary methods). This yielded a final sample of 1266 subjects: 386 PS, 521 OP, and 359 HC (table 1). Excluded subjects were indistinguishable based on clinical or demographic features. Notably, 336 of 386 PS subjects exceeded threshold criteria for at least one OP domain in addition to psychosis spectrum (table 2). A subset of PS (17%) and OP (11%) subjects were taking psychoactive medications, most commonly stimulants (supplementary table S1).
Table 1.
Demographic Characteristics
| Psychosis Spectrum (n = 386) | Healthy Controls (n = 359) | Other Psychopathology (n = 521) | |
|---|---|---|---|
| Sex (% male)a,b | 49.2 | 51.5 | 42.2 |
| Age (years ± SD)b,c | 15.8±3.0 | 15.0±3.9 | 14.9±3.5 |
| Education (years ± SD) | 8.1±2.7 | 7.9±3.8 | 7.9±3.4 |
| Race (%)a,b,c | |||
| Caucasian | 31.3 | 55.7 | 43.6 |
| African American | 55.7 | 32.3 | 44.7 |
| Other | 13.0 | 12.0 | 11.7 |
| Maternal education (years ± SD)a,b,c | 13.8±2.2 | 14.7±2.6 | 14.2±2.5 |
| Global cognition score (z ± SD)b,c | −0.02±0.56 | 0.14±0.61 | 0.08±0.56 |
| Intracranial volume (cm3 ± SD)a,c | 1491±187 | 1556±166 | 1513±171 |
| Clinical factor scores (mean ± SD) | |||
| Global psychopathologya,b,c | 0.53±0.44 | −0.36±0.43 | 0.08±0.44 |
| Anxiety-misery | −0.05±0.38 | −0.05±0.33 | −0.03±0.34 |
| Behaviora,c | 0.19±1.27 | −0.47±0.93 | 0.20±1.28 |
| Feara,c | 0.09±0.70 | −0.13±0.52 | 0.06±0.62 |
| Unusual thoughts/perceptionsa,b,c | 1.18±0.84 | −0.59±0.68 | −0.24±0.73 |
| Special abilities/persecutiona,b,c | 0.95±1.04 | −0.44±0.73 | −0.19±0.80 |
| Negative/disorganizeda,b,c | 0.97±0.99 | −0.61±0.71 | −0.16±0.78 |
Note: Significant group differences.
aOther psychopathology vs healthy controls.
bPsychosis spectrum vs other psychopathology.
cPsychosis spectrum vs healthy controls.
Table 2.
Frequency Distributions of Subjects Meeting Clinical Inclusion Criteria
| Psychosis Spectrum (n = 386) | Healthy Controls (n = 359) | Other Psychopathology (n = 521) | |
|---|---|---|---|
| Psychosis spectrum criteria | a | ||
| PRIME score > 2 SD for age | 117 | 0 | 0 |
| Scale of Prodromal Symptoms neg/disorg Score > 2 SD for age | 87 | 0 | 0 |
| Kiddie Schedule for Affective Disorders and Schizophrenia: Hallucinations or delusions | 176 | 0 | 0 |
| Individual PRIME item ratings (3 items rated 5 or 1 item rated 6) | 230 | 0 | 0 |
| Other psychopathology criteria | b | c | |
| Mood disorders | 100 | 0 | 87 |
| Anxiety disorders | 266 | 0 | 387 |
| Eating disorders | 11 | 0 | 10 |
| Behavioral disorders (attention deficit/hyperactivity, oppositional-defiant, conduct disorder) | 246 | 0 | 284 |
Note: aIncludes 147 PS subjects who met more than one “psychosis spectrum” inclusion criterion.
bIncludes 215 PS who met more than one “Other Psychopathology” inclusion criterion.
cIncludes 208 OP subjects who met more than one “Other Psychopathology” inclusion criterion.
Clinical Psychopathology Factor Analysis
To parse this heterogeneous and overlapping psychopathology into orthogonal dimensions to relate to imaging measures, bifactor modeling was applied to the item-level clinical psychopathology measures, across the entire PNC cohort (n = 9498)30 (supplementary methods). The bifactor modeling approach32 effectively models highly correlated data sets that have a strong underlying factor plus various individual factors. As detailed in Calkins et al,30 this yielded an overall psychopathology factor and 4 orthogonal symptom clusters: “anxious-misery” (mood and anxiety disorders), “behavior” (attention deficit/hyperactivity disorder, conduct disorder, and oppositional-defiant disorder), “fear” (phobias), and psychosis spectrum. The psychosis factor was further parsed into 3 specific subfactors: “special abilities/persecution,” “unusual thoughts/perceptions,” and “negative/disorganized” symptoms. Thus, 8 psychopathology scores were generated for each subject.
Cognitive Assessment
A computerized neurocognitive battery was administered to all participants.33 This included 14 tests covering a broad range of cognitive domains including executive control, episodic memory, complex cognition, social cognition, and sensorimotor and motor speed. A global cognition measure was calculated by averaging age-normed accuracy z-scores (based on the full sample of 9498 subjects) across all domains.
Neuroimaging Acquisition and Image Processing
MRI scans were acquired on one 3T Siemens Tim Trio whole-body scanner and 32-channel head coil, in a 1-hour session that included both structural and functional scans. Cortical reconstruction and measurement of T1-weighted structural images was performed using FreeSurfer 5.3 (http://surfer.nmr.mgh.harvard.edu/). Using the FreeSurfer image analysis suite, regions of interest were automatically segmented from each subject’s MPRAGE.34 Intracranial volume (ICV) was obtained using FreeSurfer’s process of estimation based on linear transformation to MNI305 space.35 Automated quality assurance procedures and visual inspection of the resulting gray–white matter segmentation were performed on all images.36 As noted, 145 subjects were excluded due to poor image quality. (See supplementary methods for acquisition parameters and quality assurance procedures.)
Measures of cortical gray matter volume were extracted for 7 regions in both left and right temporal lobes. EC (which, as delineated in Freesurfer, also incorporates the perirhinal cortex) and adjacent TP were the 2 regions previously contrasted in our schizophrenia study.11 To further test the specificity of a hypothesized EC volume decrement, we also examined hippocampus, amygdala (AM), inferior temporal gyrus, superior temporal gyrus (STG), and parahippocampal gyrus. These are all regions reported as compromised in schizophrenia imaging studies, but their status in high-risk samples is unclear.37–40
Statistical Analyses
Demographic, clinical, and cognitive differences were examined with analyses of variance or chi-square tests, as indicated. The initial volumetric analysis applied a general linear model implemented in Statistica 12 (Dell Inc., 2015), with 7 regions of interest (ROIs) and hemisphere (left and right) as within-subject factors, group (PS, OP, HC) and sex as between-subject categorical factors, and age and ICV as continuous covariates. To meaningfully compare regions with intrinsically different volumes, all brain measures were converted to standardized z-scores, with HC subjects having mean = 0 and SD = 1. Significant (p < .05) main effects or interactions observed in this omnibus model were followed by appropriate within-region contrasts.
Relationships between brain abnormalities and clinical phenomenology were examined using a single general linear model applied to all subjects, with age, sex, ICV, and all abnormal brain regions included as independent predictors, and clinical factor scores and cognitive index as multivariate dependent measures. Univariate effects with p < .05 were considered significant.
The predictive utility of these MRI measures as “real-world” biomarkers for the psychosis spectrum was explored by fitting a multinomial logistic regression model, with group as the categorical dependent measure and all abnormal MRI measures as predictors. The logistic regression model computes a probability between 0 and 1, representing the best fit estimate of inclusion for each specific category. Each case was assigned to the category with highest probability. A comparison of actual and assigned categories allowed the calculation of MRI sensitivity and specificity for predicting PS status.
Results
The 3 groups were comparable in education, but differed in age [F(2,1263) = 8.56, p = .0002], sex [χ2(2) = 8.49, p = .014], and racial composition [χ2(4) = 48.66, p < .0001] (table 1). The PS sample was ~1 year older with proportionally more African American subjects, while the HC sample included more Caucasians. The OP group contained relatively more females. The groups also differed in maternal education [F(2,1246) = 12.13, p < .0001], global cognition [F(2,1261) = 7.76, p = .0004], and ICV [F(2,1263) = 13.25, p < .0001]. ICV and cognitive differences persisted after controlling for age and sex and, in paired contrasts, distinguished the PS group from both HC and OP [ICV: PS vs HC F(1,1259) = 29.10, p < .0001; PS vs OP F(1,1259) = 9.86, p = .002] [Cognition: PS vs HC F(1,1257) = 35.91, p < .0001; PS vs OP F(1,1257) = 27.75, p < .0001]. OP subjects also had lower ICV than HC [F(1,1259) = 7.09, p = .008], but did not differ in cognitive ability [F(1,1257) = 1.44, p = .23].
As expected, there were robust group differences on each of the clinical factor scores (all p < .0001) with the exception of anxious-misery (p = .51). PS subjects had greater levels of overall psychopathology than OP subjects and, consistent with the method by which they were classified, higher scores on each of the psychosis spectrum subfactors. However, there were no differences between these 2 clinical groups on any of the specific psychopathology subfactors (ie, anxious-misery, behavior, and fear; all p > .40). The OP group, therefore, represented a clinical control sample that was otherwise quite similar to PS except for the added presence of psychosis spectrum symptoms in the PS sample.
ROI Volumetric Analysis
There were significant main effects, across all regions, of group [F(2,1258) = 3.60, p = .028], sex [F(1,1258) = 52.69, p < .0001], age [F(1,1258) = 35.11, p < .0001], and total ICV [F(1,1258) = 998.64, p < .0001]. There were, in addition, significant 2-way and 3-way interactions of Sex × Region [F(6,7548) = 5.71, p < .0001] and Sex × Region × Hemisphere [F(6,7548) = 5.12, p = .0001]. Most importantly, given our overarching hypothesis, there was a significant 3-way interaction of Group × Region × Hemisphere [F(12,7548) = 1.92, p = .028]. However, there were no significant interactions of group with sex [Group × Sex: F(2,1258) = 0.65, p = .52; Group × Sex × Region: F(12,7548) = 0.50, p = .92; Group × Sex × Region × Hemisphere: F(12,7548) = 0.71, p = .74]. Excluding PS and OP subjects taking psychoactive medications did not alter these results—the Group × Region × Hemisphere interaction was unchanged [F(12, 6768) = 1.90, p = .029].
Given the differences in racial stratification across the groups, we repeated the analysis with race included as a between-subjects factor. Although there was a main effect of race [F(2,1255) = 40.53, p < .0001] and interactions of race with region [F(12,7530) = 6.67, p < .0001] and Region × Hemisphere [F(12,7530) = 4.37, p < .0001], there were no interactions between race and clinical group. Moreover, the inclusion of race in the model enhanced, rather than attenuated, the significance of the clinical Group × Region × Hemisphere interaction [F(12,7530) = 2.47, p = .003]. Univariate contrasts delineating the effects of sex and race within each region of interest are presented in supplementary tables S2 and S3.
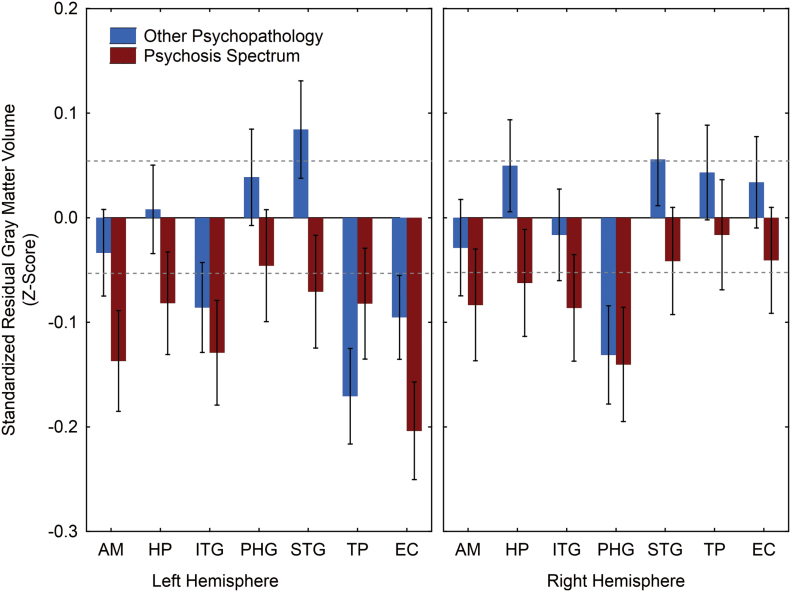
Table 3 presents the univariate analyses to parse the Group × Region × Hemisphere interaction. In contrast to sex effects, which were evident for every ROI in at least 1 hemisphere, there was only a single region that exhibited a significant difference across the 3 clinical groups. This was the left EC [F(2,1258) = 4.73, p = .009]. However, in separate paired contrasts of each clinical group with HC, PS differed significantly from HC in both the left EC [F(1,1258) = 9.40, p = .002] and, marginally, in the left AM [F(1,1258) = 4.12, p = .04]. The OP sample was indistinguishable from HC in both regions, but instead differed in the left TP [F(1,1258) = 5.44, p = .02]. But, in direct PS–OP comparisons, the only region that emerged as significantly different was the left STG [F(1,1258) = 4.48, p = .035], a region where neither group differed from controls. Figure 1 presents the regional gray matter volumes, separately for PS and OP, as standardized residual z-scores relative to HC.
Table 3.
Region-Of-Interest Analyses: Diagnostic Group Effects
| Least Squares Meana (mm3 ± SE) | F Statistic | ||||
|---|---|---|---|---|---|
| Region | PS (n = 386) | OP (n = 521) | HC (n = 359) | (df 2,1258) | P Value |
| Amygdala | |||||
| Left | 1421±8.76 | 1441±7.57 | 1447±9.04 | 2.32 | .10 |
| Right | 1440±8.75 | 1449±7.56 | 1454±9.03 | 0.64 | .53 |
| Hippocampus | |||||
| Left | 4082±19.63 | 4118±16.97 | 4116±20.26 | 1.11 | .33 |
| Right | 4230±19.81 | 4276±17.12 | 4256±20.45 | 1.50 | .22 |
| Inferior temporal gyrus | |||||
| Left | 11751±78.26 | 11821±67.64 | 11957±80.77 | 1.70 | .18 |
| Right | 11438±75.19 | 11544±64.98 | 11569±77.60 | 0.85 | .43 |
| Parahippocampal gyrus | |||||
| Left | 2382±18.34 | 2412±15.86 | 2399±18.93 | 0.73 | .48 |
| Right | 2207±16.21 | 2213±14.01 | 2251±16.73 | 2.09 | .12 |
| Superior temporal gyrus | |||||
| Left | 13530±66.90 | 13718±57.82 | 13621±69.05 | 2.26 | .10 |
| Right | 13116±65.54 | 13236±56.65 | 13169±67.65 | 0.99 | .37 |
| Temporal pole | |||||
| Left | 2635±21.79 | 2601±18.84 | 2669±22.49 | 2.75 | .06 |
| Right | 2439±18.34 | 2462±15.85 | 2446±18.93 | 0.45 | .64 |
| Entorhinal cortex | |||||
| Left | 1761 ± 17.69 | 1804 ± 15.29 | 1839 ± 18.26 | 4.73 | .009 |
| Right | 1642±17.15 | 1669±14.82 | 1656±17.70 | 0.69 | .50 |
Note: HC, healthy controls; OP, other psychopathology; PS, psychosis spectrum.
aControlling for effects of age, sex, and total intracranial volume.
Fig. 1.
Gray matter volume measurements for PS and OP youths. Mean residual z-scores (±SE) controlling for age, sex, and total intracranial volume are plotted relative to HC who have mean z = 0±1 SD.
AM, amygdala; EC, entorhinal cortex; HC, healthy controls; HP, hippocampus; ITG, inferior temporal gyrus; OP, other psychopathology; PHG, parahippocampal gyrus; PS, psychosis spectrum; STG, superior temporal gyrus; TP, temporal pole.
Relationship to Clinical Symptomatology and Cognition
We applied a general linear model to the entire sample, with age, sex, ICV, and the 4 MRI regions that distinguished any of the groups from each other (left AM, left STG, left TP, and left EC) as predictors and the clinical factor scores and cognition index as dependent measures. As indicated (table 4), age and/or sex were significant predictors of every clinical measure. ICV predicted overall psychopathology and cognitive ability, as well as the positive psychosis spectrum factor “special abilities/persecution,”’ but was not a significant predictor of negative/disorganized symptoms. None of the 4 ROIs predicted any of the primary psychopathology factors. However, reduced left AM volume was selectively associated with the positive psychosis subfactor “special abilities/persecution” and lower EC volume was a significant predictor of both negative/disorganized symptoms and cognitive performance. Restricting the sample to PS subjects, we continued to observe significant differential effects of the AM and EC regions despite the reduced sample size and the more restricted ranges of clinical measures. Left AM remained uniquely associated with the “special abilities/persecution” factor [F(1,360) = 7.18, p = .008], while left EC was a unique predictor of cognitive impairment [F(1,360) = 7.20, p = .007]. However, the association with negative/disorganized symptoms, which had an especially narrow within-group distribution, was no longer significant. Although not statistically significant, it is worth noting that subjects who met the negative symptom criteria for inclusion as PS (n = 87) tended to have smaller left EC volumes than the PS subjects (n = 299) who did not meet these criteria (z = −0.30±0.09 SE vs z=−0.18±0.05 SE).
Table 4.
Predictors of Clinical Psychopathology F Statistic (P Value)
| Clinical Index | Age | Sex | Total ICV | Left Amygdala | Left Superior Temporal Gyrus | Left Temporal Pole | Left Entorhinal Cortex |
|---|---|---|---|---|---|---|---|
| Global psychopathology | 6.01 (.014) | 0.42 (.52) | 3.94 (.047) | 3.31 (.07) | 0.95 (.33) | 1.42 (.23) | 0.42 (.52) |
| Anxious-misery | 8.59 (.003) | 20.15 (<.0001) | 1.16 (.28) | 2.41 (.12) | 0.47 (.49) | 2.04 (.15) | 0.04 (.85) |
| Behavioral disturbance | 9.03 (.003) | 14.20 (.0002) | 0.19 (.67) | 2.98 (.08) | 0.02 (.89) | 0.009 (.93) | 0.01 (.91) |
| Fear | 40.53 (<.0001) | 8.35 (.004) | 2.22 (.14) | 0.42 (.52) | 0.09 (.76) | 2.10 (.15) | 2.47 (.12) |
| Psychosis | 34.57 (<.0001) | 11.57 (.0006) | 1.19 (.28) | 2.90 (.09) | 0.001 (.97) | 0.19 (.67) | 1.10 (.29) |
| Psychosis subfactors | |||||||
| Unusual thoughts/perceptions | 6.80 (.009) | 8.17 (.004) | 2.46 (.12) | 3.26 (.07) | 0.06 (.81) | 0.64 (.42) | 2.08 (.15) |
| Special abilities/persecution | 16.83 (<.0001) | 14.85 (.0001) | 8.85 (.003) | 4.97 (.026) | 0.25 (.62) | 0.17 (.68) | 1.58 (.21) |
| Negative/disorganized Symptoms | 0.001 (.97) | 18.28 (<.0001) | 1.56 (.21) | 3.52 (.06) | 0.20 (.65) | 0.05 (.83) | 5.01 (.025) |
| Cognition | 106.56 (< .0001) | 13.07 (.0003) | 20.79 (<.0001) | 1.24 (.26) | 2.87 (.09) | 3.77 (.06) | 14.57 (.0001) |
Classification Sensitivity and Specificity
To assess the ability of these temporal lobe measures to correctly classify individuals as PS, we conducted multinomial logit regression analyses, with group as the categorical dependent measure and the 4 ROI measures as predictors. Correct classification of PS subjects (sensitivity) was 0.18, while correct exclusion as not PS (specificity) was 0.91 for HC and 0.93 for OP. Conducting the analysis separately in males and females, which eliminated the confounding effects of nonspecific sex-related variability, improved PS sensitivity to 0.31 without reducing specificity. The classification sensitivity of ICV was only 0.02, and the addition of this measure did not further increase either sensitivity or specificity. Subjects correctly classified as PS were older [t(384) = 3.32, p = .001] than other PS subjects, but did not differ in cognitive status or clinical symptom severity.
Discussion
This study was motivated by evidence that olfactory deficits are core to schizophrenia and an observed dissociation, in schizophrenia patients, of 2 adjacent temporal lobe regions, the TP and the perirhinal/entorhinal cortices. Reduced perirhinal/entorhinal cortical volumes hinted at a selective abnormality that might be a developmentally mediated risk marker for psychosis.13 The current study expanded upon this observation by (1) prospectively examining a large sample of psychosis spectrum adolescents from the community; (2) including both a healthy comparison sample and a sample with clinical psychopathology, but without psychosis spectrum symptoms; and (3) examining the remaining cortical and subcortical temporal lobe regions. We found evidence for a diffuse structural abnormality, as indicated by reduced ICV, in both PS and OP subjects, which was more severe in the PS cohort. Left EC emerged as the only region exhibiting a significant difference across the 3 groups, independent of ICV. Like ICV, it was largest in HC and smallest in PS. Additionally, PS had reductions in left AM compared with HC and left superior temporal gyrus compared with OP, though neither clinical group differed from HC in the latter. Finally OP, but not PS, had reduced left TP volumes compared with HC.
Only ICV, EC, and AM—the 3 measures that distinguished PS from HC—were related to clinical features. Across the entire sample, reduced ICV was associated with greater overall psychopathology, reduced cognitive ability, and greater psychotic-like ideation related to special abilities (grandiosity, future prediction, and mind reading), thought control, audible thoughts, superstitiousness, and persecution. Reduced left EC volume was also associated with impaired cognition, but it was also selectively associated with increased negative/disorganized symptoms. Reduced left AM was associated only with increased psychotic-like ideation. There was thus a clinical dissociation of the 2 regions that distinguished PS subjects from HC, one tied to cognition and negative/disorganized symptoms, and the other to positive symptoms. Within the PS sample, with its associated restricted range of clinical measures, the differential association of the AM with delusional symptoms and the EC with cognitive impairment remained quite strong.
There are only a few studies of the EC in patients with psychotic illnesses. While virtually all of these have had positive findings, they have been inconsistent in their regional and clinical specificity. We previously observed selective volume decrements in chronic schizophrenia patients, but these were bilateral and did not correlate with negative symptoms.13 Others have reported deficits that were either left lateralized,14 right lateralized,41 or bilateral but indistinguishable from other medial temporal lobe regions.15 However, a recent large study from the B-SNIP consortium42 reported volume reductions in the left (but not in the right) EC in schizophrenia and schizoaffective, but not in bipolar, patients. This clinical specificity distinguished it from hippocampal volume decrements, which were evident in both clinical groups. Associations of this measure with symptomatology were not reported.
In contrast, there have been many studies of the AM in schizophrenia. The recent ENIGMA consortium meta-analysis confirmed robust bilateral AM reductions in schizophrenia, but with no associations to specific clinical symptomatology.43 Studies in patients are difficult to interpret, given the confounding effects of medications and illness sequelae. While there is a growing literature reporting MRI anomalies during the psychosis prodrome (eg, Cannon et al44 and Satterthwaite et al45), the data on specific abnormalities in these medial temporal lobe regions are still limited. There are no specific findings for the EC, and findings for the AM, based on relatively small samples, have been inconsistent.46–48
The present study addresses this shortcoming by assessing multiple medial temporal lobe regional volumes in a large at-risk sample. Since abnormalities in nearly all of these regions exist in diagnosed psychosis patients, the presence of selective decrements in this preclinical sample implies distinct time courses of disease-related volumetric changes. In particular, we observed no differences between PS and HC in either the hippocampus or the superior temporal gyrus, 2 regions that are perhaps most closely linked to schizophrenia pathology. There is evidence to suggest that cortical thinning in the superior temporal gyrus (as well as parahippocampal gyrus) progresses rapidly during the transition from the at-risk state to overt psychosis.44 There is also evidence that hippocampal shape and volume are abnormal in those at ultra-high risk for psychosis, with hippocampal shape disturbance predicting symptom progression over the next 12 months.49 In this context, our failure to observe robust deficits in these regions could, at least in part, reflect the uniqueness of the PNC sample. As a community ascertained rather than help-seeking cohort, it is on average younger and psychiatrically healthier than typical ultra-high risk samples. We might expect that greater deficits will emerge in at least a subset of subjects, as they approach the point of transition to overt psychosis. It should be noted, in this regard, that PS subjects did exhibit nonsignificant volume reductions across all temporal lobe regions, even after correcting for ICV. However, we also cannot rule out a methodological contribution to the failure to observe significant regional differences. Automated MRI segmentation algorithms like Freesurfer invariably produce results that are discrepant from those obtained with either manual tracings or alternate algorithms.50,51 As a surface-based approach, Freesurfer is especially sensitive to measurement variations in subcortical regions.52 In particular, it’s been shown to overestimate hippocampal volume,53 and this may have reduced its sensitivity to more subtle preclinical differences.
Nevertheless, reduced EC and AM volume in the PS sample indicates that these occur early in the course of illness, perhaps in many cases prior to the social and psychological deterioration that elicits help seeking for PS symptoms. We have suggested that the selectivity of the EC volume reduction may be a marker of aberrant fetal development during an early embryonic risk period that coincides with development of the olfactory system.13 Notably the AM—which is the earliest developing component of the limbic system—also emerges during this same embryonic risk period,54 is spatially contiguous with the EC, and receives synaptic inputs from both the olfactory bulb and cortex.55 It is notable, too, that the deficits we observed were all lateralized to the left hemisphere. This is consistent with the long-standing notion that schizophrenia is characterized by a disruption of the normal pattern of emerging cerebral asymmetry, which primarily affects left hemisphere development56 and leads to more prominent left hemisphere dysfunction.57 Without longitudinal data, it is impossible to determine whether these volume reductions represent developmental anomalies or are the earliest signs of progressive gray matter loss. However, the idea that they are, at least in part, developmental aberrations is supported by a recent examination of the heritability of neurocognitive and neuroanatomic endophenotypes for schizophrenia.58 This study, of a large randomly ascertained pedigree, found reduced EC to be the single strongest measure associated with increased familial liability for schizophrenia.
The 2 clinical features linked to the EC in the PNC sample, cognitive impairment and negative symptoms, are among the most intractable features of schizophrenia. Their presence early in the course of illness is a predictor of poor functional outcome.59,60 However, classification schemes to identify adolescents at elevated psychosis risk have typically focused on subthreshold positive symptoms—for example, attenuated positive symptoms or brief limited intermittent psychotic symptoms.61 It is now clear that not only are cognitive deficits and negative symptoms both present in some individuals in the prepsychotic period, but they are similarly associated with greater levels of functional impairment.62–64 Thus, left EC volume reduction may be a biomarker denoting an especially worrisome preclinical state.
The real-world utility of such MRI measures as predictive biomarkers remains unclear. The multinomial logit regression analysis demonstrated excellent specificity (92%) in classifying PS subjects, but relatively modest sensitivity (31%). On its face, this is an unacceptably low hit rate, which rules out these measures as primary screening metrics. However, this result must be considered in the context of the PNC sample and the clinical classification of PS subjects within that sample. The relatively early psychosis spectrum sample in this study was defined differently than typical prodromal samples. Subjects were not classified as PS based on a clinical assessment of help seekers, but rather on responses to a structured screening interview in a broad community-based sample. Such instruments can be overinclusive in labeling individuals who are not “true positives” (ie, low specificity).65 In the PNC sample, approximately 20% of all subjects were classified as PS,28 which exceeds the number in whom psychosis spectrum symptoms persist or worsen as we conduct comprehensive follow-up evaluations (~50% of PS subjects) and certainly exceeds the number who will eventually manifest psychotic symptoms. Given such overinclusiveness, the high specificity of these MRI measures, which would help to exclude false-positive cases, is probably more important than their low sensitivity. While they are unlikely to be useful during the initial phase of an evaluation, they may serve as a potential second tier (ie, confirmatory) classification tool to be applied following initial clinical screening.66 The delineation of a PS subset of 31% who fit this at-risk MRI profile would reduce the sample of interest from 20% to ~6% of the general population, a number that is much closer to the expected epidemiological incidence of psychosis.
Of course, the extent to which such a prospectively identified subgroup overlaps with the subgroup that ultimately transitions to psychosis still needs to be determined through longitudinal follow-up. However we are hopeful that, by identifying objective neurobiological markers that are present prior to the emergence of diagnosable clinical symptoms, we can enhance our predictive power and, thereby, facilitate earlier identification and intervention.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health (R01MH099156 to B.I.T., K01MH102609 to D.R.R., K23MH098130 to T.D.S., RC2MH089983 to R.E.G, and T32MH019112to R.E.G.)
Supplementary Material
References
- 1. McGorry PD, Yung AR, Phillips LJ. The close-in‚ or ultra high-risk model. Schizophr Bull. 2003;29:771–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. [DOI] [PubMed] [Google Scholar]
- 4. Becker E, Hummel T, Piel E, Pauli E, Kobal G, Hautzinger M. Olfactory event-related potentials in psychosis-prone subjects. Int J Psychophysiol. 1993;15:51–58. [DOI] [PubMed] [Google Scholar]
- 5. Brewer WJ, Wood SJ, McGorry PD, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. [DOI] [PubMed] [Google Scholar]
- 6. Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res. 2010;123:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamath V, Moberg PJ, Calkins ME, et al. An odor-specific threshold deficit implicates abnormal cAMP signaling in youths at clinical risk for psychosis. Schizophr Res. 2012;138:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamath V, Turetsky BI, Calkins ME, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J Biol Psychiatry. 2014;15:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin A, Brewer WJ, Yung AR, Nelson B, Pantelis C, Wood SJ. Olfactory identification deficits at identification as ultra-high risk for psychosis are associated with poor functional outcome. Schizophr Res. 2015;161:156–162. [DOI] [PubMed] [Google Scholar]
- 10. Moberg PJ, Roalf DR, Gur RE, Turetsky BI. Smaller nasal volumes as stigmata of aberrant neurodevelopment in schizophrenia. Am J Psychiatry. 2004;161:2314–2316. [DOI] [PubMed] [Google Scholar]
- 11. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen AD, Pelavin PE, Shenton ME, et al. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: an MRI study. Brain Imaging Behav. 2011;5:252–261. [DOI] [PubMed] [Google Scholar]
- 13. Turetsky BI, Moberg PJ, Roalf DR, Arnold SE, Gur RE. Decrements in volume of anterior ventromedial temporal lobe and olfactory dysfunction in schizophrenia. Arch Gen Psychiatry. 2003;60:1193–1200. [DOI] [PubMed] [Google Scholar]
- 14. Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. [DOI] [PubMed] [Google Scholar]
- 15. Sim K, DeWitt I, Ditman T, et al. Hippocampal and parahippocampal volumes in schizophrenia: a structural MRI study. Schizophr Bull. 2006;32:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bitter T, Brüderle J, Gudziol H, Burmeister HP, Gaser C, Guntinas-Lichius O. Gray and white matter reduction in hyposmic subjects–a voxel-based morphometry study. Brain Res. 2010;1347:42–47. [DOI] [PubMed] [Google Scholar]
- 17. Bakhshi K, Chance SA. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience. 2015;303:82–102. [DOI] [PubMed] [Google Scholar]
- 18. Crespo-Facorro B, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. 2004;132:107–115. [DOI] [PubMed] [Google Scholar]
- 19. Roiz-Santiáñez R, Pérez-Iglesias R, Quintero C, et al. Temporal pole morphology in first-episode schizophrenia patients: clinical correlations. Psychiatry Res. 2010;184:189–191. [DOI] [PubMed] [Google Scholar]
- 20. Liao J, Yan H, Liu Q, et al. Reduced paralimbic system gray matter volume in schizophrenia: correlations with clinical variables, symptomatology and cognitive function. J Psychiatr Res. 2015;65:80–86. [DOI] [PubMed] [Google Scholar]
- 21. Posimo JM, Titler AM, Choi HJ, Unnithan AS, Leak RK. Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo. PLoS One. 2013;8:e58596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farbman AI. Developmental biology of olfactory sensory neurons. Semin Cell Biol. 1994;5:3–10. [DOI] [PubMed] [Google Scholar]
- 23. Kostovic P, Zdravko P, Milos J. Early areal differentiation of the human cerebral cortex: entorhinal area. Hippocampus. 1993;4:447–458. [DOI] [PubMed] [Google Scholar]
- 24. van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. [DOI] [PubMed] [Google Scholar]
- 25. Blaizot X, Mansilla F, Insausti AM, et al. The human parahippocampal region: I. Temporal pole cytoarchitectonic and MRI correlation. Cereb Cortex. 2010;20:2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fogliarini C, Chaumoitre K, Chapon F, et al. Assessment of cortical maturation with prenatal MRI. Part I: Normal cortical maturation. Eur Radiol. 2005;15:1671–1685. [DOI] [PubMed] [Google Scholar]
- 27. Gill KE, Evans E, Kayser J, et al. Smell identification in individuals at clinical high risk for schizophrenia. Psychiatry Res. 2014;220:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Good KP, Tibbo P, Milliken H, et al. An investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosis. Schizophr Res. 2010;124:60–65. [DOI] [PubMed] [Google Scholar]
- 29. Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calkins ME, Merikangas KR, Moore TM, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56:1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Satterthwaite TD, Elliott MA, Ruparel K, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reise SP, Moore TM, Haviland MG. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J Pers Assess. 2010;92:544–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 35. Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. [DOI] [PubMed] [Google Scholar]
- 36. Vandekar SN, Shinohara RT, Raznahan A, et al. Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 2015;35:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cullen AE, De Brito SA, Gregory SL, et al. Temporal lobe volume abnormalities precede the prodrome: a study of children presenting antecedents of schizophrenia. Schizophr Bull. 2013;39:1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roman-Urrestarazu A, Murray GK, Barnes A, et al. Brain structure in different psychosis risk groups in the Northern Finland 1986 birth cohort. Schizophr Res. 2014;153:143–149. [DOI] [PubMed] [Google Scholar]
- 39. Chung Y, Jacobson A, He G, et al. Prodromal symptom severity predicts accelerated gray matter reduction and third ventricle expansion among clinically high risk youth developing psychotic disorders. Mol Neuropsychiatry. 2015;1:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nenadic I, Dietzek M, Schönfeld N, et al. Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophr Res. 2015;161:169–176. [DOI] [PubMed] [Google Scholar]
- 41. Baiano M, Perlini C, Rambaldelli G, et al. Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res. 2008;102:171–180. [DOI] [PubMed] [Google Scholar]
- 42. Mathew I, Gardin TM, Tandon N, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. [DOI] [PubMed] [Google Scholar]
- 43. Van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Satterthwaite TD, Wolf DH, Calkins ME, et al. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry. 2016;73:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. [DOI] [PubMed] [Google Scholar]
- 47. Witthaus H, Mendes U, Brüne M, et al. Hippocampal subdivision and amygdalar volumes in patients in an at-risk mental state for schizophrenia. J Psychiatry Neurosci. 2010;35:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bechdolf A, Wood SJ, Nelson B, et al. Amygdala and insula volumes prior to illness onset in bipolar disorder: a magnetic resonance imaging study. Psychiatry Res. 2012;201:34–39. [DOI] [PubMed] [Google Scholar]
- 49. Dean DJ, Orr JM, Bernard JA, et al. Hippocampal shape abnormalities predict symptom progression in neuroleptic-free youth at ultrahigh risk for psychosis. Schizophr Bull. 2016;42:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grimm O, Pohlack S, Cacciaglia R, et al. Amygdalar and hippocampal volume: a comparison between manual segmentation, Freesurfer and VBM. J Neurosci Methods. 2015;253:254–261. [DOI] [PubMed] [Google Scholar]
- 51. Morey RA, Petty CM, Xu Y, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Powell S, Magnotta VA, Johnson H, Jammalamadaka VK, Pierson R, Andreasen NC. Registration and machine learning-based automated segmentation of subcortical and cerebellar brain structures. Neuroimage. 2008;39:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wenger E, Mårtensson J, Noack H, et al. Comparing manual and automatic segmentation of hippocampal volumes: reliability and validity issues in younger and older brains. Hum Brain Mapp. 2014;35:4236–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132:135–165. [DOI] [PubMed] [Google Scholar]
- 55. Keshavarzi S, Power JM, Albers EHH, et al. Dendritic organization of olfactory inputs to medial amygdala neurons. J Neurosci. 2015;35:13020–13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Crow TJ, Ball J, Bloom SR, et al. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46:1145–1150. [DOI] [PubMed] [Google Scholar]
- 57. Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978;87:226–238. [DOI] [PubMed] [Google Scholar]
- 58. Glahn DC, Williams JT, McKay DR, et al. Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biol Psychiatry. 2015;77:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Green MF, Hellemann G, Horan WP, et al. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang WC, Hui CL, Chan SK, et al. Impact of avolition and cognitive impairment on functional outcome in first-episode schizophrenia-spectrum disorder: a prospective one-year follow-up study. Schizophr Res. 2016;170:318–321. [DOI] [PubMed] [Google Scholar]
- 61. Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 62. Gur RE, March M, Calkins ME, et al. Negative symptoms in youths with psychosis spectrum features: complementary scales in relation to neurocognitive performance and function. Schizophr Res. 2015;166:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niendam TA, Bearden CE, Johnson JK, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100–111. [DOI] [PubMed] [Google Scholar]
- 64. Piskulic D, Addington J, Cadenhead KS, et al. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ising HK, Veling W, Loewy RL, et al. The validity of the 16-item version of the Prodromal Questionnaire (PQ-16) to screen for ultra high risk of developing psychosis in the general help-seeking population. Schizophr Bull. 2012;38:1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah J, Eack SM, Montrose DM, et al. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophr Res. 2012;141:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.