ABSTRACT
L-type voltage gated Ca2+ channels are considered to be the primary source of calcium influx during the myogenic response. However, many vascular beds also express T-type voltage gated Ca2+ channels. Recent studies suggest that these channels may also play a role in autoregulation. At low pressures (40–80 mmHg) T-type channels affect myogenic responses in cerebral and mesenteric vascular beds. T-type channels also seem to be involved in skeletal muscle autoregulation. This review discusses the expression and role of T-type voltage gated Ca2+ channels in the autoregulation of several different vascular beds. Lack of specific pharmacological inhibitors has been a huge challenge in the field. Now the research has been strengthened by genetically modified models such as mice lacking expression of T-type voltage gated Ca2+ channels (CaV3.1 and CaV3.2). Hopefully, these new tools will help further elucidate the role of voltage gated T-type Ca2+ channels in autoregulation and vascular function.
KEYWORDS: autoregulation, blood flow, calcium, T-type channel
Introduction
In many organs such as kidneys, brain, heart and skeletal muscles changes in perfusion pressure only elicits a minimal change in steady-state blood flow. This phenomenon, autoregulation, depends on the active response of resistance vessels independently of neurohumoral and endothelial stimuli. Increases in perfusion pressure lead to vasoconstriction whereas decreases in perfusion pressure leads to vasodilation. Autoregulation operates only within specific pressure ranges. In the kidney this range extends between 80 mmHg and 180 mmHg1 and in the brain between 60 and 130 mmHg.2 Changes in perfusion pressure beyond these limits will induce changes in blood flow. During pathophysiological conditions such as hypertension these limits will adapt to the new blood pressure.3
The aim of overall autoregulation is to maintain constant organ perfusion and stabilization of capillary pressure when acute every-day changes in blood pressure occur. The main mechanism in autoregulation is the myogenic response (Bayliss response) intrinsic to vascular smooth muscle cells (VSMC).4 In the kidney a second local mechanism, the tubuloglomerular feedback mechanism (TGF), also participates in renal autoregulation.5
Autoregulation maintains a near-constant blood flow but changes in metabolism still affect organ blood flow.6 Increased oxygen consumption increases flow, but flow is still autoregulated if acute changes in perfusion pressure occur. This metabolic hyperemia is also a local event eliciting vasodilation of small arterioles. To be effective the vasodilatory response needs to spread through the vascular tree. A vascular conducted response is believed to travel through gap junctions coupling either via the endothelial cell or vascular smooth muscle cell layers.7,8 In addition, a local vasodilation may cause increased flow and endothelial shear stress in upstream feed arteries thus contributing to the increased organ blood flow.9,10 Thus, myogenic tone provides the set point from which local metabolic signals, conducted vasomotor signals and shear stress-mediated signaling can regulate vessel diameter.
Role of membrane potential and ion channels in regulation of vascular tone
The tone of resistance vessels, and thus vascular resistance, is dependent on the intracellular Ca2+ concentration ([Ca2+]i) in VSMC. Ca2+ is recruited from intracellular stores and/or via entry from the extracellular space.11-13 The increase in [Ca2+]i varies temporally and spatially between different vascular beds and different stimuli. Thus, it can be transient or sustained, cover the whole cell or only restricted compartments.14 In VSMC global increases in [Ca2+]i is supposed to play a prominent role in the myogenic response.15 The Ca2+ entry occurs largely via voltage-gated Ca2+-channels (VGCC).11,13 However, nonselective non voltage dependent cation channels such as transient receptor potential (TRP) may also play a role in several vascular beds, also in the initiation of the myogenic response.16,17 As the VGCC are the dominant Ca2+-entry pathway in most vascular beds, VSMC membrane potential greatly affects the control of vascular tone. The major VGCC in most resistance vessels is the dihydropyridine-sensitive L-type channel (CaV 1.2).18,19 In addition, T-type channels have been found in several vascular beds including renal pre- and postglomerular20 but their role in regulating intracellular Ca2+ is controversial and remains to be clarified.
The cell membrane potential (Vm) in VSMC is determined by the chemical gradients and the relative ion conductances over the VSMC membrane. Endothelial cells may affect Vm of VSMC via gap junctions but it is mainly ion gradients and permeabilities over the VSMC membrane that governs Vm15. In practice, it is the intra- and extracellular concentrations of K+, Na+ and Cl− ions and their membrane conductances that control Vm (see eq. 1; 21).
| (eq. 1) |
Ex is the equilibrium potential and gx the conductance for the particular ion x
K+ channels are the dominant cation channels in VSMC.22,23 Therefore Vm is primarily determined by EK as the conductance for K+ normally exceeds that of the other ions. EK is ∼ − 90 mV but due to the conductance for other ions, Vm in VSMC is more positive in the range of −55 to −40 mV.15,24 These values are close to the activation potential of L-type channels but more positive than the activation potential for T-type channels. However, they are in the range for T-type window currents and thus a role for these channels in the maintenance of Vm is possible (for review, see25). There are 4 major classes of vascular K+ channels: ATP sensitive (KATP), calcium activated (KCa), voltage activated (KV) and inward rectifier (KIR). Vasoconstriction elicited by the myogenic response may theoretically be exerted by inactivation of K+ channels with concomitant depolarization and activation of VOCC.23,26 It is suggested that the general closure of K+ channels may be orchestrated by activation of protein kinase C (PKC).27 Conversely, vasodilation could be initiated by activation of VSMC K+ channels.27 Some VSMC, e.g. from renal cortical efferent arterioles, lack L-type VOCC but activation of K+ channels will still cause hyperpolarization.
The permeability of Cl− channels also participates in the regulation of VSMC Vm. In accord with this, Cl− channels have been suggested to participate in the myogenic response.28,29 Influx of Na+ into the VSMC also leads to depolarization. This influx may occur via members of the TRP channel family.16,17 These channels also carry an influx of Ca2+ which directly increase [Ca2+]i. Different members of the TRP channel family are activated by stimulation of cell surface receptors, emptying of intracellular Ca2+ stores and/or mechanical stretch.17 It has been suggested that activation of TRPM4 and/or TRPC6 channels contribute to the depolarization leading to the myogenic response30-33
Voltage gated calcium channels
In mammalian cells, VGCCs are expressed by at least 10 different genes that encode Ca2+ selective channels with somewhat different biophysical and regulatory properties, expression patterns, and thus physiologic roles. The CaV1 and CaV2 families of channels belong to the so-called high-voltage activated (HVA) type as they require relatively large depolarization to be activated. Conversely, the CaV3 channel family belongs to the low-voltage activated (LVA) channels as they are activated by small depolarization at relatively hyperpolarized resting membrane potentials.34,35 The CaV1 family (CaV1.1–1.4 channels) carries the Ca2+ currents known as L-type (Large, Long-Lasting). This current is characterized by the relatively large unitary currents and long opening times due to slow inactivation kinetics (reviewed by36). The CaV1 channels are primarily expressed in muscle (smooth, skeletal, cardiac), neurons and endocrine cells where they serve important roles in excitation-contraction (EC) -coupling and regulation of gene transcription, hormone- and neurotransmitter release (see34). In resistance-sized arteries, CaV1.2 channels are primarily responsible for EC-coupling and control of blood pressure.15,37,38 The L-type channels are all blocked by dihydropyridines, phenylalkylamines and benzothiazepines with similar potencies; however, some peptide toxins (calciseptine, FS-2) may specifically inhibit CaV1.2 channels.20,39,40 The CaV1.2 α1-subunit isoforms cloned from human intestinal smooth muscle and the heart were both shown to be inherently mechano-sensitive when expressed in HEK-293 cells.41
The CaV2 family (HVA) encoding the P/Q-type (CaV2.1a/b: splice variants of the Cacna1a gene), N-type (CaV2.2) and R-type (CaV2.3) currents are primarily expressed and functional in neurons where they participate in neurotransmitter release, action potentials, and local Ca2+ transients. Several peptide toxins such as ω-agatoxins (P/Q-type), some ω-conotoxins (N-type) and SNX-482 (R-type) inhibit the CaV2 channels specifically and are therefore valuable pharmacological tools (see34). The P/Q-type channels seem to be expressed in renal preglomerular arterioles,42 but none of the P/Q-, N- or R-type channels are expressed and functional in mesenteric-terminal arterioles.40
The CaV3 family (LVA) T-type (Tiny, Transient) channels consisting of small and fast-inactivating CaV3.1–3 currents are primarily expressed in the peripheral and central nervous system where they are important for repetitive firing of action potentials (for review, see Perez-Reyes, 200335). However, CaV3.1 and CaV3.2 channels are also expressed in the heart where they are involved in pacemaking in the sinoatrial node (CaV3.1; 43) as well as in ventricular hypertrophy where CaV3.1 and CaV3.2 channels seem to play opposite roles.44,45 Moreover, in resistance vessels CaV3.1 and CaV3.2 T-type channel protein expression has been demonstrated by immunolocalization to VSMCs46-51 as well as ECs.48,49,51,52 Interestingly, in human cerebral arteries the T-type isoforms expressed are the CaV3.2 and CaV3.3 channels, with no expression of CaV3.1 channels.53 Application of 15 mmHg extracellular pressure to SW620 human cancer cells causes mechanical activation of Ca2+ influx through CaV3.3 T-type channels (but not through CaV3.1 or CaV3.2 channels), which activates PKC-β and stimulates proliferation of cancer cells.54 There are no reports of direct mechano-sensitivity of CaV3.1 channels. Although not directly mechano-sensitive, the CaV3.2 T-type channels are important for normal excitability of skin D-hair receptors in mice. The high sensitivity of D-hair receptors to light touch is facilitated by CaV3.2 channel expression due to a lowering of the threshold for mechanical activation of action potentials.55-57 No specific T-type channel blockers are available, so knockout mice are currently the golden standard for deciphering the function of these channels in the resistance vasculature. Mibefradil and its derivative NNC 55-0396 are used to investigate the physiologic role of T-type channels, but they are shown to block HVA channels in a use-dependent manner,46,58,59 and to lower blood pressure through inhibition of L-type channels.60 Thus, the exact function of CaV3.1 and CaV3.2 channels in the resistance vasculature is currently under investigation using knockout mice.46,50,61-64
Myogenic response
The myogenic response is considered a local response where changes in transmural pressure affect the circumferential stress in the vascular smooth muscle cell.65 How the change in stress is translated into changes in vascular diameter is still not fully understood. Several suggestions including changes in extracellular matrix connections, cytoskeleton structure, mechano-sensitive TRP channels and micro-domains in the membrane are currently under investigation (reviewed in66).
The resting membrane potential (RMP) of VSMC in most vascular beds during physiologic pressures ranges between −60 mV to −35 mV.67-70 This range of RMP is a prerequisite for setting the basal tone upon which vasodilators can act. Increases in perfusion pressure further depolarize VSMC.71 As mentioned above this RMP is within the activation window for voltage-gated (L-type) Ca2+ channels but also affects KV channels and BKCa channels to modify myogenic constriction.71,72 Activation of BKCa depends on subunit composition, membrane potential and Ca2+ levels73 and therefore one could speculate that tissue specific activation exists.
Inhibition of L-type Ca2+ channels significantly reduces the myogenic response in most vascular beds indicting a need for Ca2+ entry.74-77 Recently T-type channels have also been implicated in the myogenic response of several vascular beds although some studies show that the influence of T-type channels on the myogenic response is more pronounced in the lower pressure range where the VSMC are less depolarized.46,78,79
In the renal vascular bed inhibition of T-type channels reduce autoregulation,80,81 but whether this effect is caused by an attenuation of the myogenic response alone or also affects the TGF is unknown. Both mechanisms affect resistance in the afferent arteriole. The myogenic response is induced by increased pressure but TGF is a conducted vasoconstriction elicited most likely by ATP or adenosine. Thus, the pathway inducing vasoconstriction is not similar and may affect T-type channels differently. However, in cremaster,82 cerebral,83 mesenteric46 and retinal79 arterioles T-type channels have been suggested to play a role in the myogenic response. The following paragraphs will review the literature on T-type channels and autoregulation in several vascular beds.
Renal autoregulation
In the renal microcirculation expression of T-type channels (CaV3.1 and CaV3.2) has been shown in both pre- and postglomerular vessels.20 Using patch-clamp studies in VSMC isolated from rat afferent arterioles Smirnov et al. failed to demonstrate T-type currents while they were able to detect these currents in VSMC from the rat tail artery.84 Nevertheless, T-type currents have been found in larger preglomerular vessels such as interlobar and arcuate arteries.85 In contrast, L-type channels are only expressed in preglomerular vessels.86,87 Preglomerular vessels are the main effectors of the renal autoregulation as both the myogenic response and the TGF exert their action in these vessels.88,89 Inhibition of L-type channels abolishes renal autoregulation.90,91 Some studies have also shown that inhibition of renal T-type channels significantly affects autoregulation.80,81 However, due to the lack of specific inhibitors of T-type channels results have been difficult to interpret.
In vivo experiments using inhibitors of T-type channels have revealed no effect on baseline renal blood flow indicating that the channels are not significantly activated at normal physiologic perfusion pressures.61,80,92,93 However, recent findings from T-type knockout mice showed that lack of CaV3.1 led to increased renal blood flow (RBF) whereas lack of CaV3.2 led to increased glomerular filtration rate (GFR).94 This suggests that CaV3.1 maintains afferent tone whereas CaV3.2 supports efferent dilatation.
The expression pattern of voltage gated Ca2+ channels in the renal vasculature suggests a dual role in the renal autoregulation. Renal autoregulation affects both RBF and GFR. The renal circulation is a portal system where the glomerular capillaries are positioned between the afferent and efferent arterioles. Increased afferent resistance reduces both RBF and GFR whereas increases in efferent resistance decreases RBF and increases GFR within certain limits. Inhibition of L-type channels abolishes autoregulation of both RBF and GFR whereas the role of T-type channels are less elucidated. Inhibitors of L-type channels are often used in treatment of hypertension and it has been suggested that this leads to increased glomerular capillary pressure due to loss of renal autoregulation leading to increased proteinuria and glomerulosclerosis.95,96 Therefore focus has increased on the role of T-type channels in renal protection97,98 as they are hypothesized to reduce glomerular pressure.
Examinations of the role of T-type channels in renal autoregulation are limited. In isolated perfused rat kidneys pimozide significantly attenuated the afferent vasoconstriction in response to increases in renal perfusion pressure.81 In rats treated with mibefradil in the drinking water for 2–4 d renal autoregulation was also significantly attenuated. However, the effects of these T-type inhibitors are very concentration-dependent and the above-mentioned results are likely to reflect an L-type channel effect as well.99 In isolated kidneys from mice lacking the expression of CaV3.1 we showed that T-type channels play no apparent role in the afferent arteriolar autoregulation at renal perfusion pressures between 75 and 155 mmHg.61 Also, in normo - and hypertensive rats the changes in RBF after acute increases in renal perfusion pressure was identical in saline-treated and mibefradil-treated rats.61 The concentration of mibefradil used in this study is suggested to be specific for T-type channels but nevertheless these results have to be interpreted with caution.100 Taken together it appears as there is no major role for T-type channels in the renal autoregulation at pressures within the physiologic range. Whether they play a role at lower pressures as in the mesenteric circulation46 is unknown.
Coronary circulation
Perfusion of cardiac tissue is tightly coupled to metabolic demands and thus hyperemic mechanisms are the prime determinants of flow. As hyperemia induces vasodilation, it only works in the presence of basal tone in part determined by the myogenic response. Isolated coronary vessels have significant tone and demonstrate myogenic vasoconstriction in the physiologic pressure range.101 The myogenic response relies on calcium influx via L-type channels as demonstrated by its sensitivity to L-type channel blockers.102
Few studies have addressed the role of T-type channels in the coronary circulation, but knockout of CaV3.2 blunts the vasodilation to ACh and NO103 in coronary arteries. A possible mechanism may be that localized calcium influx through CaV3.2 activates BKCa channels, leading to hyperpolarization and a reduction of global calcium influx. Since direct vasodilation by NO was also affected, it is unlikely that CaV3.2 acts by modifying NO production in the endothelial cells. The study by Chen et al only implicates CaV3.2 in vasodilation and the authors found no effect on vessel diameter (at 40 mmHg) or on agonist induced constriction.103 Although this argues against a role for CaV3.2 in coronary vasoconstriction, the study did not specifically address the myogenic mechanism and the application of 40 mmHg is probably too low to expect a detectable myogenic response.
Knockout of CaV3.1 affected the myogenic response of mesenteric arteries, but only in the low pressure range.46 The relevance of this phenomenon has not been investigated in coronary vessels, but no overt coronary phenotype has been reported for the CaV3.1 KO mice.
Skeletal muscle circulation
Both CaV3.1 and CaV3.2 are present in arterioles from rat cremaster muscle64 and pharmacological evidence suggests that T-type currents contribute significantly to myogenic tone in this vascular bed82 at physiologic pressures (75 mmHg). Furthermore, T-type channels contributed to basal tone in cremaster muscle vessels in vivo and their contribution was increased at reduced NO levels, most likely via an increase in reactive oxygen species.64 Such regulation may explain differences between studies and should be considered when comparing experiments from different species, vascular beds and experimental settings. However, a role for T-type channels in the autoregulation of blood flow in skeletal muscles seems possible at pressures within the physiologic range.
Cerebral autoregulation
In normotensive humans, autoregulation of cerebral blood flow is kept constant at approx. 50 mL/min/100 g brain tissue at mean arterial pressures between 60–130 mmHg and is believed to be entirely dependent on a myogenic response of cerebral arteries and arterioles.2 Increases in transmural pressure from 10 to 100 mmHg caused a graded depolarization of rat small cerebral artery VSMCs from about −63 mV to about −36 mV. Addition of the VGCC blockers diltiazem (30 µM) or nisoldipine (10 nM) inhibited intracellular Ca2+ increases and a myogenic response in this pressure range.104 This and later studies have unequivocally demonstrated a pivotal role of L-type channel mediated Ca2+ entry in the myogenic response and thus cerebral autoregulation. Activation of whole-cell L-type currents in VSMCs occurs at a Vm between −55 mV and 10 mV, with window currents through overlapping activation and inactivation curves in the range from approx. −30 mV to 0 mV.36 Therefore, it may be speculated that T-type channels play a role in myogenic tone at lower pressures at which VSMC membrane potential is outside the activation range of L-type channels. This hypothesis was independently confirmed by 2 studies showing that several T-type channel blockers inhibited myogenic tone at lower pressures in rat middle cerebral artery78 and that genetic deletion of Cav3.1 T-type channels abolished myogenic tone in the pressure range from 40–80 mmHg in mouse small mesenteric arteries.46 However, also at higher pressure (100 mmHg) did low concentration of mibefradil (60 nM) significantly reduce cerebral myogenic responses.83
Conversely, myogenic tone in rat middle cerebral arteries was increased by the CaV3.2 T-type channel blocker Ni2+ (50 µM)105 which is in line with the results found in the renal vasculature in CaV3.2 T-type KO mice94 where efferent arteriolar resistance increased. This increase in myogenic tone following addition of Ni2+ was not seen in mesenteric arteries from CaV3.2−/− mice.50,63,105 The increased tone was mimicked by addition of the specific BKCa channel blocker paxilline (1 µM) in rat cerebral arteries,105 and this effect of paxilline was not seen in small mesenteric arteries from young CaV3.2-deficient mice.50 These results are generally in agreement with the hypothesis that pressure-dependent activation of CaV3.2 channels in the VSMC plasma membrane causes activation of Ca2+ sparks via Ryanodine receptors (RyRs) closely apposed to Sarcoplasmatic Reticulum (SR). This leads to activation of spontaneous transient outward currents (STOC) via BKCa channels, and negative feedback on the cerebral myogenic tone,63,105 as previously suggested for the involvement of CaV3.2 channels in relaxation of coronary arteries.103 These effects of CaV3.2 channel deletion on myogenic tone were only observed in young mice (2–4 months) but not in mature adult mice (7–12 months) leading to the conclusion that CaV3.2 channel expression may protect against excessive tone and high blood pressure in young individuals.50 Human cerebral arteries express the CaV3.2 and CaV3.3 isoforms, and interestingly Ni2+ (50 µM) also increased myogenic tone in human arteries.53 Moreover, the T-type blocker NNC 55-0396 (1 µM) inhibited the myogenic tone at lower pressures in human cerebral arteries in the presence of the L-type blocker nifedipine (200 nM), an effect that is reminiscent of the CaV3.1-mediated effects on myogenic tone in rodent arteries46,78 but might be mediated via CaV3.3 channels in human cerebral arteries53
Mesenteric circulation
The superior mesenteric artery supplying the small intestine carries >10% of cardiac output at rest. Sympathetic nerves densely innervate mesenteric arteries and arterioles, and α1-adrenoceptor mediated sympathetic vasoconstriction is of paramount importance during maximal physical activity, fight-or-flight syndrome, and hypotensive crises. Adjustment of O2 delivery occurs partly at the level of O2 uptake, which is maintained constant over a wide range of perfusion pressures. Autoregulation does occur but seems to be mainly coupled to release of local metabolic factors, such as adenosine, interstitial K+ or altered osmolality. Rat mesenteric arteries (2nd-3rd order) do not possess much myogenic tone in vitro unless it is triggered by low agonist-induced tone.106,107 However, 5th order rat mesenteric arteries, as well as 2nd-3rd order mouse mesenteric arteries do exhibit spontaneous myogenic tone in vitro, and the diameter-pressure curve usually has a negative slope in the pressure range from 60–120 mmHg108-110 suggesting that the mesenteric arteries have the inherent capacity to autoregulate blood flow via a myogenic mechanism. As a large body of mechanistic evidence has been gathered in studies using isolated mesenteric arteries we will present data on VGCCs and the myogenic response from this vascular bed.
While the essential role of L-type channels in myogenic tone in rat and mouse mesenteric arteries is well established,111,112 the role of other HVA channels remains unexplored. However, specific peptide toxins targeting P/Q-type, N-type and R-type VGCCs did not inhibit high-KCl induced Ca2+ entry in rat mesenteric arterioles,40 so most likely these HVA channels do not play a role in myogenic tone.
Previous studies have shown abundant expression of CaV3.1 and CaV3.2 T-type LVA channels at the mRNA and protein level in rat and mouse mesenteric arteries and arterioles.40,46,48,50,113 In small mesenteric arteries from wild-type mice nifedipine (1 µM) abolished myogenic tone at higher pressures, whereas there was no effect on tone at 40 mmHg. In age-matched mice deficient in the CaV3.1 T-type channels, myogenic tone was abolished at lower pressures (40–80 mmHg), but at pressures above 80 mmHg the spontaneous myogenic tone was abolished by 1 µM nifedipine.46 These data are consistent with the hypothesis that at lower pressures, at which the VSMC membrane potentials are relatively hyperpolarized, the L-type channels are inactive whereas the CaV3.1 channels are active and sustain myogenic tone possibly via a window-type current. At higher pressures, where the myogenic depolarization activates the L-type channels, the CaV3.1 channels will inactivate accounting for the complete inhibition of myogenic tone by nifedipine at pressures above 80 mmHg.46
Conversely, the expression of CaV3.2 T-type channels in mesenteric artery VSMCs seems to participate in a negative feedback mechanism on the myogenic tone. In young mice, deletion of CaV3.2 channels causes a significant enhancement of myogenic tone at pressures above 40–60 mmHg.50,63 This was shown to be dependent on generation of Ca2+ sparks via superficial SR ryanodine receptors causing activation of spontaneous transient outward currents via BKCa channels and hyperpolarization to give negative feedback modulation on myogenic tone.63 A potential role of CaV3.2 channels in protection of the myocardium against excessive arterial tone and age-dependent hypertension was indicated by the finding that the role of CaV3.2 channels in opposing myogenic tone found in young mice was completely absent in mature adult mice.50 This protection could also be relevant in the kidney to protect glomerular capillaries as CaV3.2 deletion in mice resulted in an increased GFR.94 This effect of aging was not caused by a reduced mRNA expression of CaV3.2 channels or BKCa α1 and β1 subunits, but it might be coupled to a dramatic reduction of CaV3.1 channel expression in VSMCs, which was seen at the mRNA and protein level in mature adult mice.50 How this putative coupling between CaV3.1 and CaV3.2 channels can exert an effect on myogenic tone remains to be established.
Both CaV1.2 and CaV3.3 channels were previously shown to be directly activated by pressure or stretch.41,54 However, a direct pressure-dependent activation of Ca2+ influx via VGCCs cannot play a major role in the myogenic response, since myogenic depolarization is dependent on opening of non-selective cation channels,30,33 and is not affected by application of the L-type antagonist nifedipine.114,115 The role of CaV3.1 channels (and perhaps Cav3.3 channels in human MCAs) in myogenic tone at lower pressures can be explained by a corresponding low myogenic depolarization in the range from approximately −65 to −50 mV, which activates a non-inactivation window-type Ca2+ current through these channels. CaV3.1/CaV3.3 might be situated in plasma membrane micro-domains in close apposition with Ca2+-dependent signaling molecules (such as Cl− channels, PLA2, PLC, PKC, RhoA/Rho-kinase, etc.), and this could potentially amplify the development of myogenic tone due to opening of T-type channels via further depolarization and/or Ca2+ sensitization. This micro-domain model of CaV3.1/CaV3.3 may exist in parallel with the already demonstrated role of the Cav3.2 T-type channels, which upon activation to an increase in pressure causes stimulation of SR Ca2+ spark activity, spontaneous outward currents, VSMC hyperpolarization and negative feedback on the myogenic tone development. Fig. 1 shows a model in which the suggested signaling pathways upon pressure-dependent activation of T-type channels in VSMCs are shown.
Figure 1.
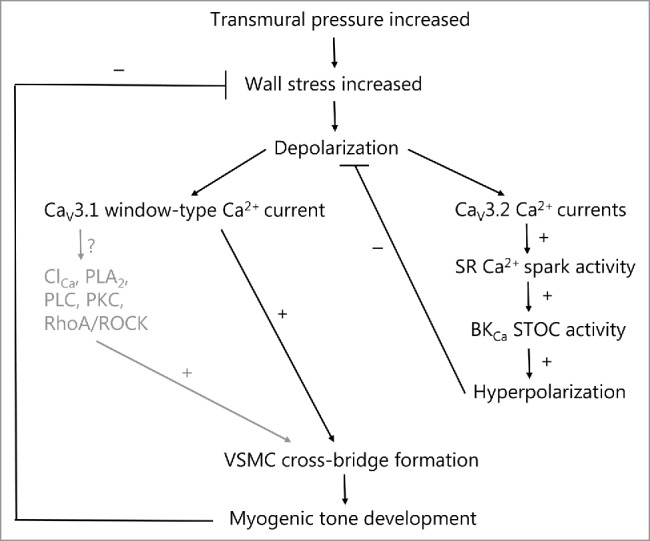
T-type calcium channels in spontaneous myogenic tone development in the rat middle cerebral artery and mouse small mesenteric artery, as explained in the text. The well-established role of L-type calcium channels is omitted here. At VSMC membrane potentials more hyperpolarized than the activation threshold for L-type channels, increases in pressure in the low range from 40–80 mmHg leads to low voltage-activation of CaV3.1 and/or CaV3.2 T-type calcium channels. The activation of CaV3.1 in this pressure range leads to myogenic tone development either via low sustained window-type Ca2+ currents, or as proposed here, via subsequent activation of Ca2+-dependent signaling molecules in membrane micro-domains (caveolae). The activation of CaV3.2 channels leads to Ca2+-dependent activation of RyRs in closely apposed SR causing increased Ca2+ spark activity. This in turn will activate nearby BKCa channels to increase STOC activity leading to hyperpolarization of VSMCs, which causes a negative feedback on the pressure-dependent depolarization and on spontaneous myogenic tone development. Thus activation of T-type channels at lower pressures may cause both an activation as well as an inhibition of myogenic tone, and the balance between the 2 opposing roles presumably relies on the vascular bed and vessel diameter. Text shown in gray are proposed signaling mechanisms that are so far unexplored. Stimulation and inhibition of an activity is shown as + or −, respectively. SR (sarcoplasmic reticulum); BKCa (large-conductance calcium-activated potassium channel); STOC (spontaneous transient outward current), ClCa (calcium-activated chloride channel, such as TMEM16A); PLA2 (Ca2+-dependent cytosolic phospholipase A2); PLC (calcium-dependent phospholipase C-δ); PKC (protein kinase C-α/β); RhoA/ROCK (small G-protein RhoA/Rho-kinase pathway).
Concluding remarks
The role of voltage gated T-type Ca2+ channels in autoregulation is still unsettled. Depending on the vascular bed investigated the results vary. In the renal and coronary vascular beds there seems to be no role for T-type channels whereas a role has been shown in the skeletal, cerebral and mesenteric autoregulation. Furthermore, the actual pressures experienced by the vasculature in the organs have been shown to greatly affect the results. At low pressures (40–80 mmHg) T-type channels have been show to affect myogenic responses in cerebral and mesenteric vascular beds. However, also at higher pressures do T-type channels seem to play a role in the cerebral autoregulation. The lack of specific pharmacological inhibitors has been a huge challenge in the field but now the research has been strengthened by genetically modified models such as mice lacking expression of CaV3.1 and CaV3.2 channels. Hopefully, these new tools will help further elucidate the role of voltage gated T-type Ca2+ channels in autoregulation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Reference
- [1].Shipley RE, Study RS. Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. Am J Physiol 1951; 167:676-88. [DOI] [PubMed] [Google Scholar]
- [2].Lassen NA, Christensen MS. Physiology of cerebral blood flow. Br J Anaesth 1976; 48:719-34; PMID:7284 [DOI] [PubMed] [Google Scholar]
- [3].Sorensen CM, Leyssac PP, Skott O, Holstein-Rathlou NH. NO mediates downregulation of RBF after a prolonged reduction of renal perfusion pressure in SHR. Am J Physiol Regul Integr Comp Physiol 2003; 285:R329-R38; PMID:12714352; https://doi.org/ 10.1152/ajpregu.00063.2003 [DOI] [PubMed] [Google Scholar]
- [4].Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 1902; 28:220-31; PMID:16992618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol 1978; 234:F357-F70; PMID:347950 [DOI] [PubMed] [Google Scholar]
- [6].Laird JD, Breuls PN, van der Meer P, Spaan JA. Can a single vasodilator be responsible for both coronary autoregulation and metabolic vasodilation? Basic Res Cardiol 1981; 76:354-8; PMID:7283936 [DOI] [PubMed] [Google Scholar]
- [7].Gustafsson F, Holstein-Rathlou NH. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand 1999; 167:11-21; PMID:10519972; https://doi.org/ 10.1046/j.1365-201x.1999.00603.x [DOI] [PubMed] [Google Scholar]
- [8].Segal SS, Duling BR. Propagation of vasodilation in resistance vessels of the hamster: development and review of a working hypothesis. Circ Res 1987; 61:II20-II5; PMID:3664984 [PubMed] [Google Scholar]
- [9].Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res 1991; 69:697-705; PMID:1873864 [DOI] [PubMed] [Google Scholar]
- [10].Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol 1990; 258:H916-H20; PMID:2316704 [DOI] [PubMed] [Google Scholar]
- [11].Salomonsson M, Arendshorst WJ. Calcium recruitment in renal vasculature: NE effects on blood flow and cytosolic calcium concentration. Am J Physiol 1999; 276:F700-F10; PMID:10330052 [DOI] [PubMed] [Google Scholar]
- [12].Somlyo AP, Somlyo AV. Ultrastructural aspects of activation and contraction of vascular smooth muscle. Fed Proc 1976; 35:1288-93; PMID:770202 [PubMed] [Google Scholar]
- [13].van BC, Farinas BR, Gerba P, McNaughton ED. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res 1972; 30:44-54; PMID:5007527 [DOI] [PubMed] [Google Scholar]
- [14].Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 2011; 3:a004549; PMID:21709182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 1990; 259:C3-18; PMID:2164782 [DOI] [PubMed] [Google Scholar]
- [16].Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010; 119:19-36; PMID:20370719; https://doi.org/ 10.1042/CS20090641 [DOI] [PubMed] [Google Scholar]
- [17].Dietrich A, Kalwa H, Gudermann T. TRPC channels in vascular cell function. Thromb Haemost 2010; 103:262-70; PMID:20126834; https://doi.org/ 10.1160/TH09-08-0517 [DOI] [PubMed] [Google Scholar]
- [18].Navar LG, Champion WJ, Thomas CE. Effects of calcium channel blockade on renal vascular resistance responses to changes in perfusion pressure and angiotensin-converting enzyme inhibition in dogs. Circ Res 1986; 58:874-81; PMID:3013463 [DOI] [PubMed] [Google Scholar]
- [19].McCall D. Excitation-contraction coupling in cardiac and vascular smooth muscle: modification by calcium-entry blockade. Circulation 1987; 75:V3-14; PMID:2436829 [PubMed] [Google Scholar]
- [20].Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 2001; 89:630-8; PMID:11577029 [DOI] [PubMed] [Google Scholar]
- [21].Sten-Knudsen O. Stoftransport, membranpotentialer og elektriske impulser over biologiske membraner [Substance transport, membrane potentials and electrical impulses across biological membranes]. Copenhagen: Akademisk Forlag; 1995. [Google Scholar]
- [22].Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995; 268:C799-C822; PMID:7733230 [DOI] [PubMed] [Google Scholar]
- [23].Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 2005; 12:113-27; PMID:15804979; https://doi.org/ 10.1080/10739680590896072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Buhrle CP, Nobiling R, Mannek E, Schneider D, Hackenthal E, Taugner R. The afferent glomerular arteriole: immunocytochemical and electrophysiological investigations. J Cardiovasc Pharmacol 1984; 6 Suppl 2:S383-S93; PMID:6206347 [PubMed] [Google Scholar]
- [25].Jensen LJ, Holstein-Rathlou NH. Is there a role for T-type Ca2+ channels in regulation of vasomotor tone in mesenteric arterioles? Can J Physiol Pharmacol 2009; 87:8-20; PMID:19142211; https://doi.org/ 10.1139/Y08-101 [DOI] [PubMed] [Google Scholar]
- [26].Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Role of vascular potassium channels in the regulation of renal hemodynamics. Am J Physiol Renal Physiol 2012; 302:F505-F18; PMID:22169005 [DOI] [PubMed] [Google Scholar]
- [27].Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 2008; 44:65-81; PMID:18552454 [DOI] [PubMed] [Google Scholar]
- [28].Matchkov VV, Secher Dam V, Bodtkjer DM, Aalkjaer C. Transport and function of chloride in vascular smooth muscles. J Vasc Res 2013; 50:69-87; PMID:23172353; https://doi.org/ 10.1159/000345242 [DOI] [PubMed] [Google Scholar]
- [29].Nelson MT, Conway MA, Knot HJ, Brayden JE. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J Physiol 1997; 502(Pt 2):259-64; PMID:9263908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 2004; 95:922-9; PMID:15472118; https://doi.org/ 10.1161/01.RES.0000147311.54833.03 [DOI] [PubMed] [Google Scholar]
- [31].Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 2007; 292:H2613-H22; PMID:17293488; https://doi.org/ 10.1152/ajpheart.01286.2006 [DOI] [PubMed] [Google Scholar]
- [32].Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 2010; 299:C1195-C202; PMID:20826763; https://doi.org/ 10.1152/ajpcell.00269.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 2002; 90:248-50; PMID:11861411 [DOI] [PubMed] [Google Scholar]
- [34].Catterall WA, Striessnig J, Snutch TP, Perez-Reyes E. International Union of Pharmacology. XL. Compendium of voltage-gated ion channels: calcium channels. Pharmacol Rev 2003; 55:579-81; PMID:14657414; https://doi.org/ 10.1124/pr.55.4.8 [DOI] [PubMed] [Google Scholar]
- [35].Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 2003; 83:117-61; PMID:12506128; https://doi.org/ 10.1152/physrev.00018.2002 [DOI] [PubMed] [Google Scholar]
- [36].McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 1994; 74:365-507; PMID:8171118 [DOI] [PubMed] [Google Scholar]
- [37].Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 2003; 22:6027-34; PMID:14609949; https://doi.org/ 10.1093/emboj/cdg583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Smirnov SV, Aaronson PI. Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol 1992; 457:455-75; PMID:1338463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Weille JR, Schweitz H, Maes P, Tartar A, Lazdunski M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc Natl Acad Sci U S A 1991; 88:2437-40; PMID:1848702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jensen LJ, Salomonsson M, Jensen BL, Holstein-Rathlou NH. Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels. Br J Pharmacol 2004; 142:709-18; PMID:15172957; https://doi.org/ 10.1038/sj.bjp.0705841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 2002; 283:C1001-8; PMID:12176756; https://doi.org/ 10.1152/ajpcell.00140.2002 [DOI] [PubMed] [Google Scholar]
- [42].Hansen PB, Jensen BL, Andreasen D, Friis UG, Skott O. Vascular smooth muscle cells express the alpha(1A) subunit of a P-/Q-type voltage-dependent Ca(2+)Channel, and It is functionally important in renal afferent arterioles. Circ Res 2000; 87:896-902; PMID:11073885 [DOI] [PubMed] [Google Scholar]
- [43].Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, et al.. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 2006; 98:1422-30; PMID:16690884; https://doi.org/ 10.1161/01.RES.0000225862.14314.49 [DOI] [PubMed] [Google Scholar]
- [44].Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, et al.. The Ca(v)3.2 T-type Ca(2+) channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res 2009; 104:522-30; PMID:19122177; https://doi.org/ 10.1161/CIRCRESAHA.108.184051 [DOI] [PubMed] [Google Scholar]
- [45].Nakayama H, Bodi I, Correll RN, Chen X, Lorenz J, Houser SR, Robbins J, Schwartz A, Molkentin JD. alpha1G-dependent T-type Ca2+ current antagonizes cardiac hypertrophy through a NOS3-dependent mechanism in mice. J Clin Invest 2009; 119:3787-96; PMID:19920353; https://doi.org/ 10.1172/JCI39724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bjorling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein-Rathlou NH, Jensen LJ. Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated CaV 3.1 T-type Ca(2+) channel. Acta Physiol (Oxf) 2013; 207:709-20; PMID:23356724; https://doi.org/ 10.1111/apha.12066 [DOI] [PubMed] [Google Scholar]
- [47].Blanks AM, Zhao ZH, Shmygol A, Bru-Mercier G, Astle S, Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol 2007; 581:915-26; PMID:17446221; https://doi.org/ 10.1113/jphysiol.2007.132126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res 2009; 46:138-51; PMID:18765948; https://doi.org/ 10.1159/000151767 [DOI] [PubMed] [Google Scholar]
- [49].Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab 2010; 30:1226-39; PMID:20125181; https://doi.org/ 10.1038/jcbfm.2010.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mikkelsen MF, Bjorling K, Jensen LJ. Age-dependent impact of CaV 3.2 T-type calcium channel deletion on myogenic tone and flow-mediated vasodilatation in small arteries. J Physiol 2016; 594(20):5881-5898; https://doi.org/ 10.1113/JP271470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Poulsen CB, Al-Mashhadi RH, Cribbs LL, Skott O, Hansen PB. T-type voltage-gated calcium channels regulate the tone of mouse efferent arterioles. Kidney Int 2011; 79:443-51; PMID:21068717; https://doi.org/ 10.1038/ki.2010.429 [DOI] [PubMed] [Google Scholar]
- [52].Svenningsen P, Andersen K, Thuesen AD, Shin HS, Vanhoutte PM, Skott O, Jensen BL, Hill C, Hansen PB. T-type Ca(2+) channels facilitate NO-formation, vasodilatation and NO-mediated modulation of blood pressure. Pflugers Arch 2014; 466:2205-14; PMID:24627154; https://doi.org/ 10.1007/s00424-014-1492-4 [DOI] [PubMed] [Google Scholar]
- [53].Harraz OF, Visser F, Brett SE, Goldman D, Zechariah A, Hashad AM, Menon BK, Watson T, Starreveld Y, Welsh DG. CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries. J Gen Physiol 2015; 145:405-18; PMID:25918359; https://doi.org/ 10.1085/jgp.201511361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Basson MD, Zeng B, Downey C, Sirivelu MP, Tepe JJ. Increased extracellular pressure stimulates tumor proliferation by a mechanosensitive calcium channel and PKC-beta. Mol Oncol 2015; 9:513-26; PMID:25454347; https://doi.org/ 10.1016/j.molonc.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci 2003; 6:724-30; PMID:12808460; https://doi.org/ 10.1038/nn1076 [DOI] [PubMed] [Google Scholar]
- [56].Dubreuil AS, Boukhaddaoui H, Desmadryl G, Martinez-Salgado C, Moshourab R, Lewin GR, Carroll P, Valmier J, Scamps F. Role of T-type calcium current in identified D-hair mechanoreceptor neurons studied in vitro. J Neurosci 2004; 24:8480-4; PMID:15456821; https://doi.org/ 10.1523/JNEUROSCI.1598-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang R, Lewin GR. The Cav3.2 T-type calcium channel regulates temporal coding in mouse mechanoreceptors. J Physiol 2011; 589:2229-43; PMID:21486775; https://doi.org/ 10.1113/jphysiol.2010.203463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967). Mol Pharmacol 1995; 48:540-9; PMID:7565636 [PubMed] [Google Scholar]
- [59].Jimenez C, Bourinet E, Leuranguer V, Richard S, Snutch TP, Nargeot J. Determinants of voltage-dependent inactivation affect Mibefradil block of calcium channels. Neuropharmacology 2000; 39:1-10; PMID:10665814 [DOI] [PubMed] [Google Scholar]
- [60].Moosmang S, Haider N, Bruderl B, Welling A, Hofmann F. Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res 2006; 98:105-10; PMID:16306443; https://doi.org/ 10.1161/01.RES.0000197851.11031.9c [DOI] [PubMed] [Google Scholar]
- [61].Frandsen RH, Salomonsson M, Hansen PB, Jensen LJ, Braunstein TH, Holstein-Rathlou NH, Sorensen CM. No apparent role for T-type Ca channels in renal autoregulation. Pflugers Arch 2015; 468(4):541-50; PMID:26658945; https://doi.org/ 10.1007/s00424-015-1770-9 [DOI] [PubMed] [Google Scholar]
- [62].Hansen PB. Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: news from the world of knockout mice. Am J Physiol Regul Integr Comp Physiol 2015; 308:R227-R37; PMID:25519728; https://doi.org/ 10.1152/ajpregu.00276.2014 [DOI] [PubMed] [Google Scholar]
- [63].Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol 2015; 35:1843-51; PMID:26069238; https://doi.org/ 10.1161/ATVBAHA.115.305736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res 2013; 98:449-57; PMID:23436820; https://doi.org/ 10.1093/cvr/cvt043 [DOI] [PubMed] [Google Scholar]
- [65].Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 1999; 79:387-423; PMID:10221985 [DOI] [PubMed] [Google Scholar]
- [66].Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc 2006; 34:67-79; PMID:16543619 [PubMed] [Google Scholar]
- [67].Videbaek LM, Aalkjaer C, Mulvany MJ. Pinacidil opens K+-selective channels causing hyperpolarization and relaxation of noradrenaline contractions in rat mesenteric resistance vessels. Br J Pharmacol 1988; 95:103-8; PMID:3219470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bratz IN, Falcon R, Partridge LD, Kanagy NL. Vascular smooth muscle cell membrane depolarization after NOS inhibition hypertension. Am J Physiol Heart Circ Physiol 2002; 282:H1648-H55; PMID:11959627; https://doi.org/ 10.1152/ajpheart.00824.2001 [DOI] [PubMed] [Google Scholar]
- [69].Monos E, Raffai G, Contney SJ, Stekiel WJ, Cowley AW Jr.. Axial stretching of extremity artery induces reversible hyperpolarization of smooth muscle cell membrane in vivo. Acta Physiol Hung 2001; 88:197-206; PMID:12162578; https://doi.org/ 10.1556/APhysiol.88.2001.3-4.2 [DOI] [PubMed] [Google Scholar]
- [70].Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation 2004; 11:279-93; PMID:15280082; https://doi.org/ 10.1080/10739680490425985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wesselman JP, Schubert R, VanBavel ED, Nilsson H, Mulvany MJ. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am J Physiol 1997; 272:H2241-H9; PMID:9176292 [DOI] [PubMed] [Google Scholar]
- [72].Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 1998; 508(Pt 1):211-21; PMID:9490841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 2010; 584:2033-42; PMID:20178789; https://doi.org/ 10.1016/j.febslet.2010.02.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mace PJ, Stallard TJ, Littler WA. The effect of felodipine on forearm haemodynamics and the myogenic response of the forearm resistance vessels in normal man. Br J Clin Pharmacol 1985; 20:383-6; PMID:4074606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].VanBavel E, Wesselman JP, Spaan JA. Myogenic activation and calcium sensitivity of cannulated rat mesenteric small arteries. Circ Res 1998; 82:210-20; PMID:9468192 [DOI] [PubMed] [Google Scholar]
- [76].Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Signalling mechanisms underlying the myogenic response in human subcutaneous resistance arteries. Cardiovasc Res 2001; 49:828-37; PMID:11230983 [DOI] [PubMed] [Google Scholar]
- [77].Jeppesen P, Aalkjaer C, Bek T. Myogenic response in isolated porcine retinal arterioles. Curr Eye Res 2003; 27:217-22; PMID:14562172 [DOI] [PubMed] [Google Scholar]
- [78].Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development. Am J Physiol Heart Circ Physiol 2013; 304:H58-H71; PMID:23103495; https://doi.org/ 10.1152/ajpheart.00476.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fernandez JA, McGahon MK, McGeown JG, Curtis TM. CaV3.1 T-Type Ca2+ channels contribute to myogenic signaling in rat retinal arterioles. Invest Ophthalmol Vis Sci 2015; 56:5125-32; PMID:26241400; https://doi.org/ 10.1167/iovs.15-17299 [DOI] [PubMed] [Google Scholar]
- [80].Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 2004; 286:F1136-F43; PMID:14996672; https://doi.org/ 10.1152/ajprenal.00401.2003 [DOI] [PubMed] [Google Scholar]
- [81].Feng MG, Li M, Navar LG. T-type calcium channels in the regulation of afferent and efferent arterioles in rats. Am J Physiol Renal Physiol 2004; 286:F331-F7; PMID:14583435; https://doi.org/ 10.1152/ajprenal.00251.2003 [DOI] [PubMed] [Google Scholar]
- [82].VanBavel E, Sorop O, Andreasen D, Pfaffendorf M, Jensen BL. Role of T-type calcium channels in myogenic tone of skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 2002; 283:H2239-H43; PMID:12388244; https://doi.org/ 10.1152/ajpheart.00531.2002 [DOI] [PubMed] [Google Scholar]
- [83].Lam E, Skarsgard P, Laher I. Inhibition of myogenic tone by mibefradil in rat cerebral arteries. Eur J Pharmacol 1998; 358:165-8; PMID:9808266 [DOI] [PubMed] [Google Scholar]
- [84].Smirnov SV, Loutzenhiser K, Loutzenhiser R. Voltage-activated Ca(2+) channels in rat renal afferent and efferent myocytes: no evidence for the T-type Ca(2+) current. Cardiovasc Res 2013; 97:293-301; PMID:23042470; https://doi.org/ 10.1093/cvr/cvs310 [DOI] [PubMed] [Google Scholar]
- [85].Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol 1994; 266:F325-F41; PMID:8141333 [DOI] [PubMed] [Google Scholar]
- [86].Reslerova M, Loutzenhiser R. Divergent mechanisms of ATP-sensitive K+ channel-induced vasodilation in renal afferent and efferent arterioles. Evidence of L-type Ca2+ channel-dependent and -independent actions of pinacidil. Circ Res 1995; 77:1114-20; PMID:7586223 [DOI] [PubMed] [Google Scholar]
- [87].Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca(2+) influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca(2+) entry. Circ Res 2000; 87:551-7; PMID:11009559 [DOI] [PubMed] [Google Scholar]
- [88].Briggs JP, Wright FS. Feedback control of glomerular filtration rate: site of the effector mechanism. Am J Physiol 1979; 236:F40-F7; PMID:434154 [DOI] [PubMed] [Google Scholar]
- [89].Steinhausen M, Blum M, Fleming JT, Holz FG, Parekh N, Wiegman DL. Visualization of renal autoregulation in the split hydronephrotic kidney of rats. Kidney Int 1989; 35:1151-60; PMID:2770100 [DOI] [PubMed] [Google Scholar]
- [90].Wang X, Aukland K, Iversen BM. Autoregulation of total and zonal glomerular filtration rate in spontaneously hypertensive rats during antihypertensive therapy. J Cardiovasc Pharmacol 1996; 28:833-41; PMID:8961082 [DOI] [PubMed] [Google Scholar]
- [91].Just A, Wittmann U, Ehmke H, Kirchheim HR. Autoregulation of renal blood flow in the conscious dog and the contribution of the tubuloglomerular feedback. J Physiol (Lond) 1998; 506:275-90; PMID:9481688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Griffin KA, Picken M, Bakris GL, Bidani AK. Comparative effects of selective T- and L-type calcium channel blockers in the remnant kidney model. Hypertension 2001; 37:1268-72; PMID:11358939 [DOI] [PubMed] [Google Scholar]
- [93].Sorensen CM, Giese I, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Closure of multiple types of K+ channels is necessary to induce changes in renal vascular resistance in vivo in rats. Pflugers Arch 2011; 462:655-67; PMID:21874333; https://doi.org/ 10.1007/s00424-011-1018-2 [DOI] [PubMed] [Google Scholar]
- [94].Thuesen AD, Andersen H, Cardel M, Toft A, Walter S, Marcussen N, Jensen BL, Bie P, Hansen PB. Differential effect of T-type voltage-gated Ca2+ channel disruption on renal plasma flow and glomerular filtration rate in vivo. Am J Physiol Renal Physiol 2014; 307:F445-F52; PMID:24966091; https://doi.org/ 10.1152/ajprenal.00016.2014 [DOI] [PubMed] [Google Scholar]
- [95].Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive treatment of patients with proteinuric renal diseases: risks or benefits of calcium channel blockers? Kidney Int 1998; 53:1559-73; PMID:9607186; https://doi.org/ 10.1046/j.1523-1755.1998.00912.x [DOI] [PubMed] [Google Scholar]
- [96].Epstein M. Calcium antagonists and renal hemodynamics: implications for renal protection. Clin Invest Med 1991; 14:590-5; PMID:1794210 [PubMed] [Google Scholar]
- [97].Ohishi M, Takagi T, Ito N, Terai M, Tatara Y, Hayashi N, Shiota A, Katsuya T, Rakugi H, Ogihara T. Renal-protective effect of T-and L-type calcium channel blockers in hypertensive patients: an Amlodipine-to-Benidipine Changeover (ABC) study. Hypertens Res 2007; 30:797-806; PMID:18037772; https://doi.org/ 10.1291/hypres.30.797 [DOI] [PubMed] [Google Scholar]
- [98].Yamamoto E, Kataoka K, Dong YF, Nakamura T, Fukuda M, Nako H, Ogawa H, Kim-Mitsuyama S. Benidipine, a dihydropyridine L-type/T-type calcium channel blocker, affords additive benefits for prevention of cardiorenal injury in hypertensive rats. J Hypertens 2010; 28:1321-9; PMID:20224431; https://doi.org/ 10.1097/HJH.0b013e3283388045 [DOI] [PubMed] [Google Scholar]
- [99].Enyeart JJ, Biagi BA, Day RN, Sheu SS, Maurer RA. Blockade of low and high threshold Ca2+ channels by diphenylbutylpiperidine antipsychotics linked to inhibition of prolactin gene expression. J Biol Chem 1990; 265:16373-9; PMID:1697857 [PubMed] [Google Scholar]
- [100].Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res 1994; 75:144-8; PMID:8013072 [DOI] [PubMed] [Google Scholar]
- [101].Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol 1988; 255:H1558-H62; PMID:2462367 [DOI] [PubMed] [Google Scholar]
- [102].Nyborg NC, Mikkelsen EO. Comparison of the inhibitory effects of nifedipine and nimodipine on mechanical responses of isolated rat coronary small arteries. J Cardiovasc Pharmacol 1987; 9:519-24; PMID:2439831 [PubMed] [Google Scholar]
- [103].Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, et al.. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 2003; 302:1416-8; PMID:14631046; https://doi.org/ 10.1126/science.1089268 [DOI] [PubMed] [Google Scholar]
- [104].Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 1998; 508(Pt 1):199-209; PMID:9490839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, et al.. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res 2014; 115:650-61; PMID:25085940; https://doi.org/ 10.1161/CIRCRESAHA.114.304056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chlopicki S, Nilsson H, Mulvany MJ. Initial and sustained phases of myogenic response of rat mesenteric small arteries. Am J Physiol Heart Circ Physiol 2001; 281:H2176-H83; PMID:11668080 [DOI] [PubMed] [Google Scholar]
- [107].Watanabe J, Keitoku M, Hangai K, Karibe A, Takishima T. alpha-Adrenergic augmentation of myogenic response in rat arterioles: role of protein kinase C. Am J Physiol 1993; 264:H547-H52; PMID:8383459 [DOI] [PubMed] [Google Scholar]
- [108].Bund SJ. Spontaneously hypertensive rat resistance artery structure related to myogenic and mechanical properties. Clin Sci (Lond) 2001; 101:385-93; PMID:11566076 [PubMed] [Google Scholar]
- [109].Gros R, Van WR, You X, Thorin E, Husain M. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol 2002; 282:H380-H8; PMID:11748085 [DOI] [PubMed] [Google Scholar]
- [110].Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension 2001; 38:833-9; PMID:11641295 [DOI] [PubMed] [Google Scholar]
- [111].Wesselman JP, VanBavel E, Pfaffendorf M, Spaan JA. Voltage-operated calcium channels are essential for the myogenic responsiveness of cannulated rat mesenteric small arteries. J Vasc Res 1996; 33:32-41; PMID:8603124 [DOI] [PubMed] [Google Scholar]
- [112].Zhang J, Berra-Romani R, Sinnegger-Brauns MJ, Striessnig J, Blaustein MP, Matteson DR. Role of Cav1.2 L-type Ca2+ channels in vascular tone: effects of nifedipine and Mg2+. Am J Physiol Heart Circ Physiol 2007; 292:H415-H25; PMID:16980345; https://doi.org/ 10.1152/ajpheart.01214.2005 [DOI] [PubMed] [Google Scholar]
- [113].Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou N. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca(2+) channels. Am J Physiol Heart Circ Physiol 2001; 280:H582-H90; PMID:11158955 [DOI] [PubMed] [Google Scholar]
- [114].Potocnik SJ, Murphy TV, Kotecha N, Hill MA. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca(2+). Br J Pharmacol 2000; 131:1065-72; PMID:11082112; https://doi.org/ 10.1038/sj.bjp.0703650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol 2005; 289:H1326-34; PMID:15863456; https://doi.org/ 10.1152/ajpheart.00323.2005 [DOI] [PubMed] [Google Scholar]


