Abstract
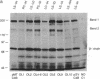
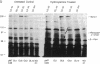
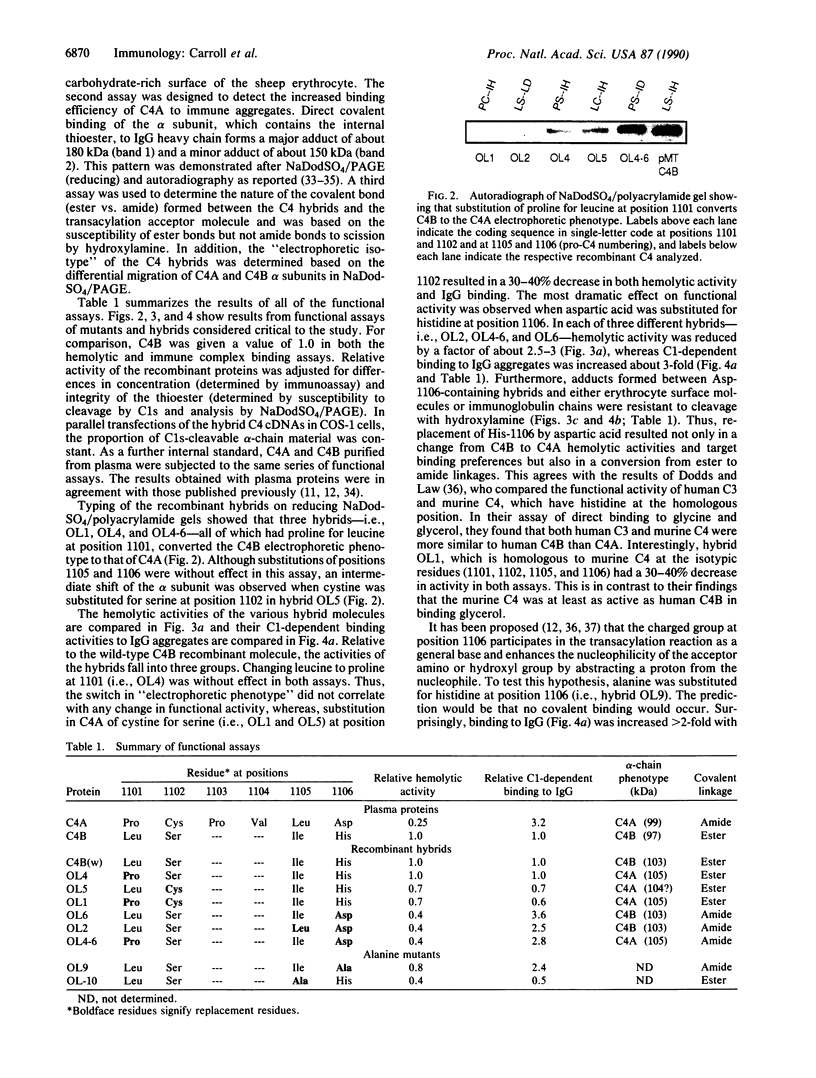
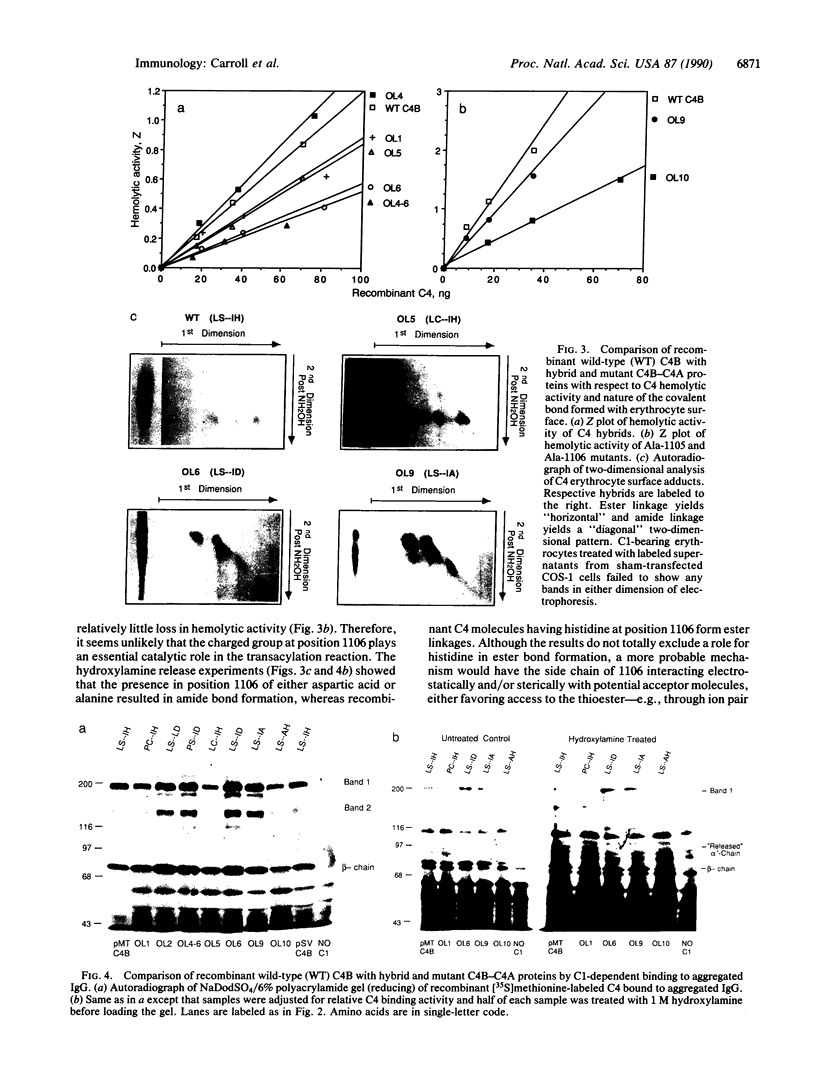
The C4B isotype of the fourth component of human complement (C4) displays 3- to 4-fold greater hemolytic activity than does its other isotype C4A. This correlates with differences in their covalent binding efficiencies to erythrocytes coated with antibody and complement C1. C4A binds to a greater extent when C1 is on IgG immune aggregates. The differences in covalent binding properties correlate only with amino acid changes between residues 1101 and 1106 (pro-C4 numbering)--namely, Pro-1101, Cys-1102, Leu-1105, and Asp-1106 in C4A and Leu-1101, Ser-1102, Ile-1105, and His-1106 in C4B, which are located in the C4d region of the alpha chain. To more precisely identify the residues that are important for the functional differences, C4A-C4B hybrid proteins were constructed by using recombinant DNA techniques. Comparison of these by hemolytic assay and binding to IgG aggregates showed that the single substitution of aspartic acid for histidine at position 1106 largely accounted for the change in functional activity and nature of the chemical bond formed (ester vs. amide). Surprisingly, substitution of a neutral residue, alanine, for histidine at position 1106 resulted in an increase in binding to immune aggregates without subsequent reduction in the hemolytic activity. This result strongly suggests that position 1106 is not "catalytic" as previously proposed but interacts sterically/electrostatically with potential acceptor sites and serves to "select" binding sites on potential acceptor molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcolea J. M., Antón L. C., Marqués G., Sánchez-Corral P., Vivanco F. Formation of covalent complexes between the fourth component of human complement and IgG immune aggregates. Complement. 1987;4(1):21–32. doi: 10.1159/000463004. [DOI] [PubMed] [Google Scholar]
- Awdeh Z. L., Alper C. A. Inherited structural polymorphism of the fourth component of human complement. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3576–3580. doi: 10.1073/pnas.77.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Belt K. T., Yu C. Y., Carroll M. C., Porter R. R. Polymorphism of human complement component C4. Immunogenetics. 1985;21(2):173–180. doi: 10.1007/BF00364869. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Dodds A. W., Porter R. R. The binding of human complement component C4 to antibody-antigen aggregates. Biochem J. 1980 Jul 1;189(1):67–80. doi: 10.1042/bj1890067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Porter R. R. Cloning of a human complement component C4 gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):264–267. doi: 10.1073/pnas.80.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Müller-Eberhard H. J. A comparison of methods for the molecular quantitation of the fourth component of human complement. Immunochemistry. 1968 Mar;5(2):155–169. doi: 10.1016/0019-2791(68)90100-6. [DOI] [PubMed] [Google Scholar]
- Dodds A. W., Law S. K. Structural basis of the binding specificity of the thioester-containing proteins, C4, C3 and alpha-2-macroglobulin. Complement. 1988;5(2):89–97. doi: 10.1159/000463039. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fielder A. H., Walport M. J., Batchelor J. R., Rynes R. I., Black C. M., Dodi I. A., Hughes G. R. Family study of the major histocompatibility complex in patients with systemic lupus erythematosus: importance of null alleles of C4A and C4B in determining disease susceptibility. Br Med J (Clin Res Ed) 1983 Feb 5;286(6363):425–428. doi: 10.1136/bmj.286.6363.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann G., Goetz J., Uring-Lambert B., Grosshans E. Component deficiencies. 2. The fourth component. Prog Allergy. 1986;39:232–249. [PubMed] [Google Scholar]
- Howard P. F., Hochberg M. C., Bias W. B., Arnett F. C., Jr, McLean R. H. Relationship between C4 null genes, HLA-D region antigens, and genetic susceptibility to systemic lupus erythematosus in Caucasian and black Americans. Am J Med. 1986 Aug;81(2):187–193. doi: 10.1016/0002-9343(86)90250-0. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Young J. R. Covalent binding properties of the C4A and C4B isotypes of the fourth component of human complement on several C1-bearing cell surfaces. J Immunol. 1986 Apr 1;136(7):2542–2550. [PubMed] [Google Scholar]
- Isenman D. E., Young J. R. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. J Immunol. 1984 Jun;132(6):3019–3027. [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Davies M. V. Translational efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 1987 Jan;6(1):187–193. doi: 10.1002/j.1460-2075.1987.tb04737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore N., Shah D., Skanes V. M., Levine R. P. The fluid-phase binding of human C4 and its genetic variants, C4A3 and C4B1, to immunoglobulins. Mol Immunol. 1988 Sep;25(9):811–819. doi: 10.1016/0161-5890(88)90117-4. [DOI] [PubMed] [Google Scholar]
- Law S. K., Dodds A. W., Porter R. R. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J. 1984 Aug;3(8):1819–1823. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauff G., Alper C. A., Awdeh Z., Batchelor J. R., Bertrams J., Bruun-Petersen G., Dawkins R. L., Démant P., Edwards J., Grosse-Wilde H. Statement on the nomenclature of human C4 allotypes. Immunobiology. 1983 Mar;164(2):184–191. doi: 10.1016/s0171-2985(83)80009-6. [DOI] [PubMed] [Google Scholar]
- Paul L., Skanes V. M., Mayden J., Levine R. P. C4-mediated inhibition of immune precipitation and differences in inhibitory action of genetic variants, C4A3 and C4B1. Complement. 1988;5(3):110–119. doi: 10.1159/000463045. [DOI] [PubMed] [Google Scholar]
- Porter R. R. Complement polymorphism, the major histocompatibility complex and associated diseases: a speculation. Mol Biol Med. 1983 Jul;1(1):161–168. [PubMed] [Google Scholar]
- Reveille J. D., Arnett F. C., Wilson R. W., Bias W. B., McLean R. H. Null alleles of the fourth component of complement and HLA haplotypes in familial systemic lupus erythematosus. Immunogenetics. 1985;21(4):299–311. doi: 10.1007/BF00430796. [DOI] [PubMed] [Google Scholar]
- Rittner C., Meier E. M., Stradmann B., Giles C. M., Köchling R., Mollenhauer E., Kreth H. W. Partial C4 deficiency in subacute sclerosing panencephalitis. Immunogenetics. 1984;20(4):407–415. doi: 10.1007/BF00345615. [DOI] [PubMed] [Google Scholar]
- Roos M. H., Mollenhauer E., Démant P., Rittner C. A molecular basis for the two locus model of human complement component C4. Nature. 1982 Aug 26;298(5877):854–856. doi: 10.1038/298854a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld S. I., Ruddy S., Austen K. F. Structural polymorphism of the fourth component of human complement. J Clin Invest. 1969 Dec;48(12):2283–2292. doi: 10.1172/JCI106194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli J. A., Steiger G., Hauptmann G., Spaeth P. J., Sjöholm A. G. Formation of soluble immune complexes by complement in sera of patients with various hypocomplementemic states. Difference between inhibition of immune precipitation and solubilization. J Clin Invest. 1985 Dec;76(6):2127–2133. doi: 10.1172/JCI112217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli J. A., Steiger G., Paccaud J. P., Sjöholm A. G., Hauptmann G. Difference in the biological properties of the two forms of the fourth component of human complement (C4). Clin Exp Immunol. 1986 Feb;63(2):473–477. [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Müller-Eberhard H. J. Fourth component of human complement: description of a three polypeptide chain structure. J Exp Med. 1974 Nov 1;140(5):1324–1335. doi: 10.1084/jem.140.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack B. F. The beta-Cys-gamma-Glu thiolester bond in human C3, C4, and alpha 2-macroglobulin. Springer Semin Immunopathol. 1983;6(4):259–282. doi: 10.1007/BF02116276. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y. P., Levine R. P. The esterase-like activity of covalently bound human third complement protein. Mol Immunol. 1988 Sep;25(9):821–828. doi: 10.1016/0161-5890(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Yu C. Y., Belt K. T., Giles C. M., Campbell R. D., Porter R. R. Structural basis of the polymorphism of human complement components C4A and C4B: gene size, reactivity and antigenicity. EMBO J. 1986 Nov;5(11):2873–2881. doi: 10.1002/j.1460-2075.1986.tb04582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]