Abstract
Thought disorder (TD) has long been associated with schizophrenia (SZ) and is now widely recognized as a symptom of mania and other psychotic disorders as well. Previous studies have suggested that the TD found in the clinically unaffected relatives of SZ, schizoaffective and bipolar probands is qualitatively similar to that found in the probands themselves. Here, we examine which quantitative measures of TD optimize the distinction between patients with diagnoses of SZ and bipolar disorder with psychotic features (BP) from nonpsychiatric controls (NC) and from each other. In addition, we investigate whether these same TD measures also distinguish their respective clinically unaffected relatives (RelSZ, RelBP) from controls as well as from each other. We find that deviant verbalizations are significantly associated with SZ and are co-familial in clinically unaffected RelSZ, but are dissociated from, and are not co-familial for, BP disorder. In contrast, combinatory thinking was nonspecifically associated with psychosis, but did not aggregate in either group of relatives. These results provide further support for the usefulness of TD for identifying potential non-penetrant carriers of SZ-risk genes, in turn enhancing the power of genetic analyses. These findings also suggest that further refinement of the TD phenotype may be needed in order to be suitable for use in genetic studies of bipolar disorder.
Keywords: schizophrenia, bipolar disorder, heterogeneity, thought disorder, endophenotype, genetics
Introduction
Cognitive dysfunction is a fundamental component of serious mental illness (SMI),1–10 has a major impact on functional disability and psychosocial outcome8,11–19 and is a risk factor for conversion to psychosis in high-risk samples.20–27 One key feature of cognitive dysfunction is thought disorder (TD). TD has been regarded as a hallmark of schizophrenia (SZ) since Kraepelin28 and Bleuler29,30 first described the disordered thought processes of SZ patients as “derailments” and “loosening of associations,” respectively. Subsequent investigations confirmed the presence of disordered thinking not only in SZ, but also in affective and other psychotic conditions and in some organic brain diseases.31–43
There is now agreement that TD is a transdiagnostic symptom, even though different clinical disorders have distinct TD profiles and some features of TD are nonspecific.38–42,44–46 Andreasen and colleagues, eg, found that poverty of speech and content were more characteristic of SZ than of mania. Pressured speech, clanging, distractible speech, and circumstantiality were more strongly associated with mania. The 2 groups did not differ in the frequency of other indicators of TD (derailment, tangentiality, illogicality, incoherence, and loss of goal).38 Using the Thought Disorder Index (TDI), Holzman and colleagues reported that extravagantly (and often playfully) combined ideas (combinatory thinking) and irrelevant intrusions characterized mania, whereas disorganization and frequent idiosyncratic word usage characterized SZ.41,42,45 The TD of schizoaffective patients tended to resemble primarily that seen in SZ.39,41,45,46 The fact that TD transcends formal diagnostic categories is consistent with the nonspecificity of many symptoms of SMI and with the nonspecificity of many cognitive and biological phenotypes in probands and in their clinically unaffected relatives.6,47–60 This nonspecificity is also consistent with the substantial (but incomplete61,62) overlap in genetic susceptibility loci across psychiatric disorders63–70 and with recent work documenting that the familial aggregation of psychiatric disorders is much less specific than had been thought.71–77
Both Kraepelin and Bleuler observed that the biological family members of SZ patients often displayed what appeared to be attenuated SZ traits (eg, odd speech), and concluded that the psychopathology of SZ was not limited to the psychotic form of the illness. Bleuler referred to such states as latent SZ, which he considered to be a “diluted” form of the illness that included the central feature of “loosening of associations.” He also observed that latent SZ was more prevalent among the biological relatives of SZ patients than was manifest psychosis. In the formulations of the “schizotype” by Rado78 and Meehl,79,80 mild TD, or “cognitive slippage,” was considered a core feature. Meehl considered cognitive slippage to be a reflection of a genetic liability for SZ; in his view, most individuals with the hypothetical schizotaxic predisposition remained nonpsychotic and non-schizoptypic.79
An extensive body of empirical research, using a variety of measures to assess TD, has confirmed the presence of mild TD or odd speech in clinically unaffected relatives of SZ patients42,44,81–98 and in high-risk offspring.20,99–106 Notably, the biological relatives of SZ (RelSZ) adoptees have significantly higher TD scores than adoptive RelSZ.92 Similarly, idiosyncratic verbalizations are significantly more prevalent in adopted biological offspring of SZ mothers than in adopted offspring of control mothers.107 Also, the biological parents of SZ individuals have significantly more deviant associations than do their adoptive parents.108 The results of these adoption studies strongly support the conclusion that genetic factors are involved in the multiple independent replications of the familial aggregation of thought, language and communication disorders in SZ families, although an interaction between genetic vulnerability and psychosocial risk factors, such as parental communication deviance, may also play a role.109,110 Of particular importance, the TD found in clinically unaffected biological RelSZ tends to be qualitatively similar to (but milder than) that found in the probands.
Like TD, communication and language deviance show a significant propensity to aggregate among biological RelSZ.82–84,95,103,111–116 The strong association of thought, language and communication disorders with SZ and their over-representation in clinically unaffected first-degree relatives suggest that one or more aspects of these behaviors may productively inform genetic studies. Idiosyncratic verbalizations, which involve unusual semantic formulations, are a core characteristic of TD, anomalous use of language, and communication disturbances. Semantic anomalies, in particular, seem to be a consistently identified linguistic component of the disease and possibly of genetic liability for it. Indeed, idiosyncratic word usage is a component of most scales used to assess TD,44,117–119 language and thought,120,121 and language and speech122 (reviewed in detail in ref.123). Importantly, the findings in clinically unaffected individuals provide evidence that most TD scales are sensitive even to the mild TD that can be present in the absence of psychosis.
Despite the recognition that TD is a component of affective disorders, mania in particular, it has not been as extensively studied in these patients as in SZ patients and even less so in their biological relatives.98
In the current study we extend previous work based on the TDI in order to characterize the quantitative features of TD in substantially larger samples of patients and relatives. The 2 goals are: (1) to identify the quantitative measures of TD that optimize the distinction between probands with diagnoses of SZ and bipolar disorder with psychotic features (BP) and between each proband group and controls, and (2) to determine whether the same quantitative TD features optimize the distinction between their respective relatives groups and distinguish each group of relatives from controls. The results bear on the usefulness of different TD profiles as endophenotypes for SZ and BP disorder, and on the potential utility of TD as a transdiagnostic endophenotype.
Methods
Participants
The subject groups included patients with a diagnosis of SZ (n = 102), schizoaffective disorder (SA; n = 141), and bipolar I disorder with psychotic features (BP; n = 79; most recent episode manic, n = 50; most recent episode depressed, n = 12; most recent episode mixed, n = 17); nonpsychiatric control (NC) subjects (n = 184); and clinically unaffected first-degree biological relatives of the patient groups: relatives of SZ patients (RelSZ; n = 121), relatives of schizoaffective patients (RelSA; n = 151), and relatives of bipolar disorder patients (RelBP; n = 40). All probands were assessed as outpatients and were recruited approximately 6 months after discharge from McLean Hospital. All relatives were relatives of these probands. Demographic characteristics of the sample are presented in table 1. These subjects were recruited over a 15-year period. Relatives were considered clinically unaffected if they did not meet DSM-IV criteria for any psychotic disorder (lifetime), bipolar disorder without psychotic features, or a SZ-spectrum personality disorder. The NC group was restricted to individuals who met the criteria for being clinically unaffected in relatives of probands but also had no family history of psychosis, suicide, or psychiatric hospitalizations.
Table 1.
Demographic Characteristics (Mean/SD) of the Study Sample
| Group | N | Age | Gender (% Male) | Years of Education | Duration of Illnessa | BPRSb | GASc |
|---|---|---|---|---|---|---|---|
| Schizophrenia (SZ) patients | 242 | 38.7 (9.5) | 55.0%d | 14.0 (2.3)e | 15.6 (9.7)f | 48.0 (14.7)g | 37.4 (9.8)h |
| Bipolar patients | 79 | 35.8 (10.4) | 36.7% | 15.4 (2.4) | 11.2 (9.5) | 34.3 (9.2) | 51.1 (11.7) |
| Normal control subjects | 184 | 39.1 (15.0) | 40.8% | 15.0 (2.4) | — | — | — |
| Relatives of SZ patients | 272 | 51.3 (16.7)i | 34.2% | 15.3 (2.6) | — | — | — |
| Relatives of bipolar patients | 40 | 42.0 (12.1) | 22.5% | 15.6 (2.5) | — | — | — |
Note: RelSZ, relatives of schizophrenia patients; RelBP, relatives of bipolar disorder patients; GAS, Global Assessment Scale; BPRS, Brief Psychiatric Rating Scale.
aDuration of illness is defined as number of years since first hospitalization.
bBPRS was missing for 4 SZ and 1 BP.
cGAS was missing for 1 SZ and 1 BP.
dSZ patients were disproportionately male compared with normal controls (P = .040), RelSZ (P < .001), and RelBP (P = .003); the difference between the proportion of men in the SZ and BP groups did not reach statistical significance (P = .05).
eSZ patients had significantly fewer years of education compared to all other groups (P < .001 for each pairwise comparison).
fSZ patients had significantly longer duration of illness compared to BP (P = .001).
gSZ patients had significantly higher BPRS than BP (P < .001).
hSZ patients had significantly lower GAS than BP (P < .001).
iRelSZ were significantly older than all other groups (P < .001 for each pairwise comparison using the Tukey-Kramer test).
Axis I disorders were assessed in all subject groups using the Structured Clinical Interview for DSM-IV, Patient Edition.124 Schizotypal, schizoid, and paranoid personality disorders were assessed in the NC subjects and the relatives groups using a modified version of the Structured Interview for Schizotypal Symptoms.125 An experienced clinician administered the interviews, and an independent group of senior diagnosticians reviewed the interview material and all available hospital records and assigned consensus Axis I and Axis II diagnoses based on best estimate methods126 using Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) criteria.127 Agreement between pairs of diagnosticians is excellent (0.84–1.00) for individual Axis I and Axis II diagnoses, and for no diagnosis. The interviewers and diagnosticians were both blinded to group membership and the results of the TD procedures. The following exclusion criteria applied to all participants: (1) lack of fluency in English; (2) history of serious head trauma or diagnosed organic brain disease; (3) history of substance abuse or dependence during the past 2 years or previous chronic dependence. All participants provided written informed consent.
Procedure
All subjects were administered a 10-card Rorschach Test128 following the procedures described in Rapaport, Gill, and Shafer.129 All sessions were audiotaped and subsequently transcribed verbatim. The protocols were scored for TD according to the revised TDI scoring manual119 by a consensus team of expert raters who were blinded to group membership. Both the Rorschach administration and TDI scoring were performed independently of the diagnostic interviewers and by different individuals.
The TDI distinguishes 23 categories of TD that are weighted along a continuum of severity (.25, .50, .75, and 1.0), with the .25 level representing very mild forms and the 1.0 level reflecting the most severe forms of TD. The total TDI score has high inter-rater reliability130 and is unrelated to race, gender, socioeconomic status, or IQ.44,131 Detailed descriptions and examples of the individual TD scoring categories are provided elsewhere.44,119,123,132
Quantitative TD Phenotypes.
The total TDI score was calculated as previously described.119 The total TDI score reflects the total amount of TD, but does not identify the nature of the TD that is present. That is, the same total score may be comprised of very different TD dimensions. Two dimensions that were identified in previous studies to distinguish between patient and relatives groups are deviant verbalizations (DVs) and combinatory thinking (CT).41,42,45,98 For each subject we calculated a DV score and a CT score, each of which was based on the sum of instances of TD in each of these categories (eg, peculiar, queer, absurd responses for DVs; incongruous and fabulized combinations, playful and other confabulations for CT).
Examples of SZ-related DVs include: “rectangularly speaking”; “the mineral of its substance”; “an x axis in origin”; “posterior pronunciations”; “the outform of the map”; and “a nonverbal misrepresentation leading to an unformulated thought.” DVs are readily identifiable in settings independent of the formal assessment of TD, as in these chief complaints: “I’d like to discontinue zyprexa and SZ tool bar disorder;” “I am an aspect recruit of biophysics.” Stilted or awkward word usage can occur in nonpsychiatrically ill individuals but usually lacks the malignant quality of the idiosyncratic word usage seen in SZ-related DVs.
CT involves finding relationships between unrelated things, but these vary qualitatively in different contexts. Mild, infrequent instances of CT are not pathological, but more severe forms generally accompany psychotic conditions. In CT, in the context of mania, loosely linked ideas are extravagantly combined and elaborated, as is also seen clinically in the grandiosity and expansiveness of mania. Manic CT is often playful and humorous, even in the context of outlandish embellishments. Some examples of mania-related CT from the TDI include: “parasitic orchids living on puddles of blood;” “babies playing the saxophone and peeing daffodils”; “two women who just had babies are thinking about how much they love their babies, which is why their thoughts are shaped like fetuses and their hearts are popping out of their bodies.” Counterparts exist for psychotic depression and non-affective psychosis, including SZ and delusional disorder, each of which has distinctively recognizable qualities. Thus, more severe manifestations of CT are a component of all SMI, but are manifested differently in different clinical conditions.
Statistical Analyses
The dependent measures were the total number of DV responses, the total number of CT responses, and the total TDI score. The Kruskal-Wallis and Fisher’s Exact tests were used to compare patient groups on the DV and CT scores. For any significant overall group differences, post hoc pairwise comparisons were conducted; in order to account for multiple comparisons, we used corrected Wilcoxon rank sum tests (for continuous outcomes)133–135 and Bonferroni-corrected Fisher’s exact tests (for categorical outcomes). In order to account for the nonindependence of observations in relatives of patients and among NCs, we also used generalized linear mixed models to compare groups. The concordance index, or area under the ROC curve, was used as a measure of discriminative power.
Following previous research,136 we applied a novel hierarchical finite mixture model that accommodates correlated (ie, nonindependent) observations to model the DV and CT scores. Hierarchical modeling is appropriate because subjects were drawn from 314 families (151 NC families, 29 BP families, and 134 SZ families). Mixture modeling approaches are especially useful for analyzing traits that are heterogeneously distributed in a group. Endophenotypes are a good example, in that some relatives perform abnormally on an endophenotype measure and others perform normally, which may be related to the fact that a sample of unaffected relatives is likely a mixture of (non-penetrant) gene carriers and non-gene carriers.
The model used has been discussed in detail elsewhere136; a condensed description is provided here (see also supplementary material for further details). For each of DV and CT, we fit a series of finite mixture models:
Model 1: One hierarchical, zero-inflated Poisson (ZIP)137 was fit to the data with the assumption of no mixture.
Model 2: A 2-component mixture of 1 ZIP and 1 Poisson was fit to the data with the assumption that the same mixing proportion was applicable for all 3 groups.
The Poisson distributed component is assumed to have a larger mean score than the ZIP component and corresponds to the high-risk group. Assuming the mixing proportion (ie, the probability of belonging to the high-risk class) does not differ by group implies that RelSZ and RelBP are at no higher risk for TD as measured by DV or CT than normal controls.
Model 3: A 2-component mixture of 1 ZIP and 1 Poisson was fit to the data. Different mixing proportions were assumed for each of the 3 groups.
As with model 2, the Poisson component corresponds to the high-risk class. Permitting the mixing proportion to vary by subject group allows a test of whether RelSZ or RelBP have significantly greater risk of TD than NC.
All analyses were conducted using SAS version 9.4 (SAS Institute). For all analyses, P < .05 was considered statistically significant.
Results
Neither the DV or CT score nor the Total TDI score differed between SZ and SA patients, or between the respective unaffected relatives of these patient groups (all P values > .05). Thus, in the analyses these groups were combined into a SZ patient group, and an unaffected relatives of SZ patients (RelSZ) group. Table 2 presents the means and standard deviations of the quantitative TD phenotypes in the subject groups.
Table 2.
Thought Disorder Measures (Mean/SD) and Severity (n, %) by Subject Group
| Subject Groups | |||||
|---|---|---|---|---|---|
| Schizophrenia (SZ) Patients (N = 242) | Bipolar Patients (N = 79) | Normal Control Subjects (N = 184) | Relatives of SZ Patients (N = 272) | Relatives of Bipolar Patients (N = 40) | |
| Deviant verbalizations | 5.8 (5.6) | 1.9 (3.3) | 1.9 (3.4) | 3.4 (4.5) | 1.2 (1.7) |
| Combinatory thinking | 3.5 (3.0) | 2.7 (2.3) | 1.6 (2.2) | 1.5 (2.3) | 1.2 (2.1) |
| Total TDI score | 23.2 (23.3) | 10.5 (10.4) | 6.5 (8.4) | 8.7 (10.4) | 4.3 (5.2) |
| TD severity | |||||
| None | 16 (6.6%) | 14 (17.7%) | 56 (30.4%) | 47 (17.3%) | 15 (37.5%) |
| Mild to moderate | 72 (29.8%) | 36 (45.6%) | 87 (47.3%) | 162 (59.6%) | 19 (47.5%) |
| Severe | 154 (63.6%) | 29 (36.7%) | 41 (22.3%) | 63 (23.2%) | 6 (15.0%) |
Note: TD, thought disorder; TDI, Thought Disorder Index.
Total TDI scores differed significantly among the 5 subject groups (P < .001).The SZ group had significantly higher Total TDI scores than NC and BP patients (P < .001, for both), with estimated effect sizes (ES) of 0.90 and 0.61, respectively. Similarly, the RelSZ had significantly higher total TDI scores than the NC (P = .016) and RelBP (P = .027), with estimated ES of 0.23 and 0.44, respectively. Bipolar patients also had significantly higher total TDI scores than NC (P = .003; ES = 0.44), but their relatives did not significantly differ from NC (P > .2). Results for all pairwise comparisons can be found in the supplementary material.
We also examined whether the subject groups differed in relation to the proportion of individuals who showed any TD (ie, a total TDI score >0). A significantly larger proportion of SZ showed TD than BP (P = .033), NC (P < .001), RelBP (P < .001) and RelSZ (P = .003). A significantly larger proportion of RelSZ also showed TD than NC (P = .010) and RelBP (P = .033; table 2).
In addition, we examined the distribution of TD severity in the 5 subject groups (table 2). The vast majority of instances of TD is in the mild to moderate range. Severe instances of TD occur intermittently, usually in the presence of mild-moderate instances of TD, and are typically found in psychotic individuals. Subjects were classified as having severe TD if they had at least one instance of TD at the 0.75 or 1.0 level (regardless of whether they also had instances of mild-moderate severity TD). Subjects were classified as having mild-moderate TD if all instances of TD were at the 0.25 or 0.50 severity levels only. Among subjects with TD, SZ had a higher proportion of severe TD than BP (P = .006), NC (P < .001), RelBP (P < .001) and RelSZ (P < .001). The other groups did not significantly differ from one another in the rate of severe TD.
DV scores significantly differed among the 5 subject groups (P < .001). Histograms of the DV scores by subject group are displayed in figures 1A–E, and show that the distributions of DV scores for SZ and RelSZ are distinct from those of the other groups. SZ patients produced significantly more DVs than BP patients and NC subjects (P < .001, for both). Similarly, the RelSZ had significantly more DVs than NC subjects (P < .001) and RelBP patients (P = .012). The estimated ES for the comparisons of SZ and RelSZ with NC were 0.82 and 0.38, respectively (figure 1F). DVs had a concordance index of 0.76 for distinguishing between SZ and NC and a concordance index of 0.62 for distinguishing between RelSZ and NC, indicating good discrimination.138 Neither BP patients nor RelBP differed from NC in DVs (P > .20, for both), corresponding to estimated ES of 0.01 and −0.22, respectively.
Fig. 1.
Distributions of DVs in (A), RelSZ (B), BP (C), RelBP (D), and NC (E). Average DV for each group with SE bars are shown in 1F. Note: DVs, deviant verbalizations; BP, bipolar disorder with psychotic features; RelSZ, relatives of schizophrenia patients; NC, nonpsychiatric control; RelBP, relatives of bipolar disorder patients.
One hundred fifty-five (57.0%) of the clinically unaffected RelSZ had nonpsychotic Axis I disorders (eg, major depressive, anxiety disorders). DV scores for this subgroup did not significantly differ from the 117 (43.0%) RelSZ who did not meet criteria for any Axis I disorder (P > .1). This result suggests that non-SZ-related Axis I disorders do not account for the increased DV scores in clinically unaffected RelSZ.
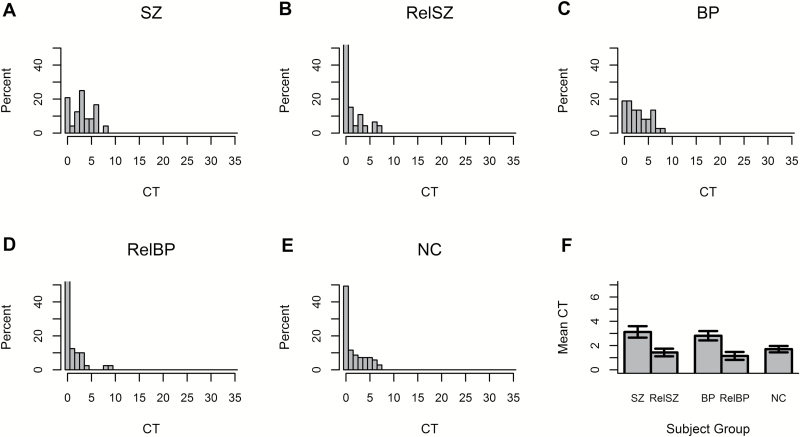
CT scores also significantly differed among the 5 subject groups (P < .001). Both SZ and BP patients produced significantly more instances of CT than NC subjects (P < .001, for both) with ES of 0.74 and 0.50, respectively; SZ and BP subjects did not differ from each other (P > .2). CT had estimated concordance indexes of 0.72 and 0.66 for distinguishing between SZ and NC and between BP and NC, respectively, indicating good discrimination. The 59 BP patients who met criteria for a current BPD with psychotic features and the 20 BP who were in either full or partial remission did not differ in CT score or total TDI score (P > .20, for both), indicating that elevated CT and total TDI scores in BP are not dependent on current psychotic state. Furthermore, neither CT score nor total TDI score significantly differed by subtype of most recent BP episode (P > .2). Neither RelSZ nor RelBP differed from NC in CT (P > .20, for both), corresponding to estimated ES of −0.03 and −0.19, respectively.
In order to get a preliminary idea if further refinement of the CT phenotype would optimize the discrimination of patient and relatives’ groups from controls, we blindly classified instances of CT as “BP” or “non-BP” in a random subset of subjects (24 SZ, 37 BP, 46 RelSZ, 40 RelBP, and 69 NC) and summed the scores for this subtype.
Both BP probands (P < .001) and RelBP (P = .019) showed significantly more “BP” CT than NC, with concordance indexes of 0.64 and 0.58, respectively, consistent with poor discrimination that may be a function of the relatively small sample sizes. Notably, the estimated ES for the comparisons of BP and RelBP with NC were substantially larger with the more narrowly defined CT score: 0.88 and 0.51, respectively. Neither SZ nor RelSZ differed from NC in “BP” CT (P > .2, for both; see supplementary figure 1).
Mixture Models
Random effects were included in each model to accommodate nonindependence of family members. As can be seen in figures 1 and 2, there is a relatively large proportion of zero-valued observations in all subject groups (ie, DV or CT scores of zero) suggesting that zero-inflated distributions are also needed to appropriately model these data. Due to a previous finding that DVs were more common in male subjects,136 sex effects were included in each model. The Akaike information criterion (AIC) was used to compare models139; a lower value of AIC indicates a better fitting model.
Fig. 2.
Distributions of CTs in (A), RelSZ (B), BP (C), RelBP (D), and NC (E). Average DV for each group with SE bars are shown in 2F. Note: CT, combinatory thinking; DV, deviant verbalizations; BP, bipolar disorder with psychotic features; RelSZ, relatives of schizophrenia patients; NC, nonpsychiatric control; RelBP, relatives of bipolar disorder patients.
For DV, model 3 (AIC = 2048.8) provided a better fit to the data than either model 1 (AIC = 2415.3) or model 2 (AIC = 2065.4), indicating that the 2-component mixture with different mixing proportions for each group best described the distribution of DV scores. Results for this model (table 3) show that RelSZ are significantly more likely to be at high risk for DV than both NC (P < .001) and RelBP (P = .034). The mixing proportions for RelBP and NC did not differ (P > .2). For a RelSZ, the probability of belonging to the high-risk class is an estimated 25.5%; for NC and RelBP, the probabilities are 10.0% and 4.3%, respectively. These results indicate that there is significant heterogeneity in DV scores and that the distribution of DV is significantly different in RelSZ compared with NC or RelBP. In this larger sample, male subjects continued to have significantly higher DV scores than female subjects (P = .004).
Table 3.
Mixture Model Results
| DV | High-risk Class | Low-risk Class |
|---|---|---|
| Poisson mean | ||
| Male | 11.0 (9.6, 12.7) | 1.9 (1.5, 2.3) |
| Female | 8.4 (7.1, 10.0) | 1.4 (1.1, 1.8) |
| Rate of zero-inflation | — | 33.1% (25.9%, 41.2%) |
| Probability of belonging to high-risk class | ||
| NC | 10.0% (6.0%, 16.3%) | |
| RelBP | 4.3% (0.7%, 22.8%) | |
| RelSZ | 25.5 (19.5%, 32.7%) | |
| CT | High-risk class | Low-risk class |
| Poisson mean | 4.7 (3.7, 6.0) | 1.2 (0.7, 2.0) |
| Rate of zero-inflation | — | 44.7% (34.7%, 44.9%) |
| Probability of belonging to high-risk class | ||
| NC | 20.3% (11.3%, 33.5%) | |
| RelBP | (same as NC) | |
| RelSZ | (same as NC) | |
Note: DV, deviant verbalizations; CT, combinatory thinking; RelSZ, relatives of schizophrenia patients; NC, nonpsychiatric control; RelBP, relatives of bipolar disorder patients.
For CT, model 2 (AIC = 1640.8) fit the data better than both model 1 (AIC = 1702.3) and model 3 (AIC = 1642.2; table 3). Thus, all subject groups shared the same probability of being at high risk for high CT scores. That is, a 2-component mixture with common mixing proportions provided the best fit, suggesting that there is significant heterogeneity in CT scores, but this heterogeneity is not tied to subject group. An estimated 20.3% of subjects belong to the high-risk class. There was no significant sex effect for CT scores (P > .2).
Discussion
Our data indicate that a quantitative TD phenotype, DVs, is significantly associated with SZ and is co-familial in clinically unaffected RelSZ, but is dissociated from, and is not co-familial for, BP disorder. RelSZ had a significantly higher probability of having a high DV score than RelBP or NC, supporting the usefulness of this measure in identifying a subgroup of RelSZ who do not have SZ-related clinical conditions yet have elevated amounts of SZ-related TD. Importantly, increased DV in RelSZ are independent of the presence of non-SZ-related nonpsychotic Axis I disorders. In contrast, CT was nonspecifically associated with psychosis among probands and did not aggregate in either group of relatives. As expected, the Total TDI score also was nonspecifically associated with psychosis. Although RelSZ had significant increases in this score, the ES were substantially smaller than for DV. These findings, based on by far the largest proband and relatives’ samples studied with the TDI to date, provide independent confirmation of previous empirical findings and phenomenological observations of distinctive qualitative similarities between the TD found in SZ probands and in their nonschizophrenic first-degree biological relatives.41,42,44,98 Thus, the psychotic form of the illness is not a necessary condition for the presence of TD. Rather, TD is both a symptom of SZ and a potential indicator of a genetic predisposition that becomes exacerbated after disease onset.
The selective familial aggregation of DVs in RelSZ suggests that this TD phenotype may be a pleiotropic expression of risk genes. In that case, this “cognitive biomarker” may be useful in identifying non-penetrant gene carriers among clinically unaffected RelSZ. Indeed, this is one of the key justifications for incorporating endophenotypes into genetic studies of psychiatric disorders.140–145 Despite the highly polygenic collective contribution of common variants, the ES for individual variants are quite small.146,147 This fact underscores the value of endophenotypes.148–151 Endophenotypes have a role in gene discovery, either alone or in combination with diagnosis, by identifying subgroups with a particularly large genetic signal. Endophenotypes also reduce genetic heterogeneity and help to clarify the role of specific genes in disease risk; indeed, they are “essential to the interpretation of genetic findings,” in part because smaller sample sizes are likely to be needed to identify loci with larger ES: “… even a small increase in the mean locus-specific effect size has a substantial impact on power…”.150 Using TDI data to illustrate, we have shown elsewhere that, for a specific sample size, a linkage analysis based on an endophenotype has much more power than one based on the disease when the penetrance of the endophenotype is much higher than that of the disease.152
The quantitative TD scores may be especially useful in improving the accuracy of polygenic risk scores (PGRSs) to detect non-penetrant carriers among clinically unaffected relatives. The PGRS aggregates the effects of individual risk alleles into a cumulative genetic risk estimate and can have predictive utility when a large contribution to a trait is polygenic. Although the predictive accuracy of PGRS is currently limited,153–155 it will increase as samples get larger, more risk alleles are identified, and linkage disequilibrium is taken into account.156–159 In addition, multivariate analysis maximizes the predictive accuracy of PGRS, showing a significant advantage to using data from multiple correlated traits.160 Thus, using the DV TD phenotype to define homogeneous subgroups of patients and relatives in the context of PGRS scores may distinguish between non-penetrant carriers and noncarriers of risk genes among relatives or between patients with differing degrees of polygenic risk. Including environmental factors that may interact with TD or with genetic risk may also improve predictive accuracy.110,161–163
The findings reported above are much more encouraging for a SZ-related TD phenotype than for a BP-related TD phenotype. This discrepancy may, in part, reflect the enhanced power of the much larger sample sizes of the SZ and RelSZ groups (the larger sample sizes of these groups reflect the recruitment strategy at the time the data were collected, as the study was designed to primarily assess TD in SZ and RelSZ, with BP and RelBP being included as psychiatric control groups). Even with comparatively smaller samples, however, the narrowly defined “BP CT” score had larger estimated ES in both the BP and RelBP groups than the CT score. Taken together, these results provide preliminary support for the relatively selective association of “BP” CT with BP, the relatively selective familial aggregation of “BP” CT in RelBP, and the dissociation of “BP” CT from SZ. Independent replication of these results and further refinement of this TD phenotype in larger samples of BP and RelBP is clearly warranted. It will be of interest to apply this hierarchical finite mixture model technique to subtypes of CT in whom heterogeneity may be linked to group. Conceivably, it may one day be possible to develop a transdiagnostic TD phenotype164 that can be used to enhance the identification of non-penetrant SMI gene carriers.
We evaluated dimensions of TD that are most salient using the TDI. Other TD dimensions have been described and there are a number of different TD scales.165,166 It would be potentially informative to compare the same individuals on different TD scales and to examine the longitudinal patterns of TD across scales.
Our patient sample consisted entirely of outpatients, indicating that the elevated TD we observed in these groups was not dependent on being in an acute clinical state. Although severity of TD does fluctuate with clinical state, the risk of TD false negatives is relatively low, especially in relation to SZ.46,165,167,168 Notably, over 80% of BP patients continued to show detectable TD as outpatients, whether or not they were in full or partial remission or still met criteria for BP. Furthermore, for BP who showed some TD, subtype of most recent episode (manic, depressed, or mixed) was not significantly associated with the severity of the TD. The presence of detectable TD in substantial proportions of clinically unaffected relatives also supports the relative independence of TD from clinical state per se.
Our proband, relatives, and control groups were orders of magnitude larger than previous samples that have been studied with the TDI (at least 3 to as much as 8 times larger).41,42,44,45,98 The findings are consistent with those previously reported for SZ and RelSZ and represent a significant advance in characterizing the TD associated with BP disorder and with its co-familiality and in clarifying the distinct characteristics of the TD profiles in the 2 proband and relatives groups.
Some NC (n = 43, 23.4%) showed either high DV scores and had at least one instance of severe TD. We reviewed all of the personal and informant material on these individuals in order to try to understand these results. Barring misrepresentation of personal or family history that would have excluded them from participating, we could find no plausible explanation. Our dataset consists entirely of cross-sectional samples of TD; it would be useful to know whether longitudinal data would show a similar pattern of findings in these individuals.
Finally, our results for TD are much more consistent with diagnostic distinctions than findings for various biological measures, whose heterogeneity seems less disease-related,169,170 or with the substantial overlap in genetic susceptibility loci across disorders (see above). It could be especially probative to examine the patterns observed when the combined effects of such biomarkers, TD phenotypes and other cognitive markers are considered.
Supplementary Material
Supplementary data are found at Schizophrenia Bulletin online.
Funding
This work was supported by the National Institutes of Health P01 MH31154, R01 MH049487, R01 MH31340, NIMH R01 071523, the Brain and Behavior Foundation, the Sidney R. Baer, Jr. Foundation, the Ellison Foundation, and Anonymous Foundation. The funding organizations played no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Supplementary Material
Acknowledgments
We thank the subjects who participated in this research. We would also like to thank Anne Gibbs for subject recruitment and Nancy R. Mendell for helpful comments on the paper. None of the authors has any conflicts of interest, including specific financial interests and relationships relevant to the subject of the manuscript. The Principal Investigator and Corresponding Author had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors, not necessarily of the funding organizations.
References
- 1. Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–1112. [DOI] [PubMed] [Google Scholar]
- 2. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 3. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 4. Murphy FC, Sahakian BJ. Neuropsychology of bipolar disorder. Br J Psychiatry. 2001;41:s120–s127. [PubMed] [Google Scholar]
- 5. Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res. 2005;78:27–34. [DOI] [PubMed] [Google Scholar]
- 6. Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK. Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis. 2006;194:255–260. [DOI] [PubMed] [Google Scholar]
- 7. Lewandowski KE, Cohen BM, Keshavan MS, Ongür D. Relationship of neurocognitive deficits to diagnosis and symptoms across affective and non-affective psychoses. Schizophr Res. 2011;133:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewandowski KE, Cohen BM, Keshavan MS, Sperry SH, Ongür D. Neuropsychological functioning predicts community outcomes in affective and non-affective psychoses: a 6-month follow-up. Schizophr Res. 2013;148:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buchanan RW, Carpenter WT. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis. 1994;182:193–204. [PubMed] [Google Scholar]
- 10. Kendler KS. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiatry. 2016;73:1082–1092. [DOI] [PubMed] [Google Scholar]
- 11. Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(suppl 9):3–8. [PubMed] [Google Scholar]
- 13. Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med. 2013;43:1187–1196. [DOI] [PubMed] [Google Scholar]
- 14. Depp CA, Mausbach BT, Harmell AL, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012;14:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martínez-Arán A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–270. [DOI] [PubMed] [Google Scholar]
- 16. Martínez-Arán A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. [DOI] [PubMed] [Google Scholar]
- 17. Martinez-Aran A, Torrent C, Tabares-Seisdedos R, et al. Neurocognitive impairment in bipolar patients with and without history of psychosis. J Clin Psychiatry. 2008;69:233–239. [DOI] [PubMed] [Google Scholar]
- 18. Roche E, Lyne J, O’Donoghue B, et al. The prognostic value of formal thought disorder following first episode psychosis. Schizophr Res. 2016;178:29–34. [DOI] [PubMed] [Google Scholar]
- 19. Yalmçetin B, Ulaş H, Var L, et al. Relation of formal thought disorder to symptomatic remission and social functioning in schizophrenia. Compr Psychiat. 2016;70:98–104. [DOI] [PubMed] [Google Scholar]
- 20. Gooding DC, Coleman MJ, Roberts SA, et al. Thought disorder in offspring of schizophrenic parents: findings from the New York High-Risk Project. Schizophr Bull. 2012;38:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rusaka M, Rancāns E. A prospective follow-up study of first-episode acute transient psychotic disorder in Latvia. Ann Gen Psychiatry. 2014;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perkins DO, Jeffries CD, Cornblatt BA, et al. Severity of thought disorder predicts psychosis in persons at clinical high-risk. Schizophr Res. 2015;169:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Katsura M, Ohmuro N, Obara C, et al. A naturalistic longitudinal study of at-risk mental state with a 2.4 year follow-up at a specialized clinic setting in Japan. Schizophr Res. 2014;158:32–38. [DOI] [PubMed] [Google Scholar]
- 24. Nelson B, Yuen HP, Wood SJ, et al. Long-term follow-up of a group at ultra high risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70:793–802. [DOI] [PubMed] [Google Scholar]
- 25. Salokangas RK, Dingemans P, Heinimaa M, et al. Prediction of psychosis in clinical high-risk patients by the Schizotypal Personality Questionnaire. Results of the EPOS project. Eur Psychiatry. 2013;28:469–475. [DOI] [PubMed] [Google Scholar]
- 26. Thompson A, Nelson B, Yung A. Predictive validity of clinical variables in the “at risk” for psychosis population: international comparison with results from the North American Prodrome Longitudinal Study. Schizophr Res. 2011;126:51–57. [DOI] [PubMed] [Google Scholar]
- 27. Thompson A, Nelson B, Bruxner A, et al. Does specific psychopathology predict development of psychosis in ultra high-risk (UHR) patients?Aust N Z J Psychiatry. 2013;47:380–390. [DOI] [PubMed] [Google Scholar]
- 28. Kraepelin E.Dementia Praecox and Paraphrenia. Chicago, IL: Chicago Medical Book Company; 1896/1919. [Google Scholar]
- 29. Bleuler E.Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1911/1950. [Google Scholar]
- 30. Bleuler E.Textbook of Psychiatry. New York, NY: MacMillan; 1924. [Google Scholar]
- 31. Cameron N. Experimental analysis of schizophrenic thinking. In: Kasanin JS, ed. Language and Thought in Schizophrenia. New York, NY: Norton; 1944:50–64. [Google Scholar]
- 32. Carlson GA, Goodwin FK. The stages of mania. A longitudinal analysis of the manic episode. Arch Gen Psychiatry. 1973;28:221–228. [DOI] [PubMed] [Google Scholar]
- 33. Clayton PJ, Pitts FN, Winokur G. Affective disorder IV. Mania. Compr Psychiat 1965;6:313–322. [DOI] [PubMed] [Google Scholar]
- 34. Edell WS. Role of structure in disordered thinking in borderline and schizophrenic disorders. J Pers Assess. 1987;51:23–41. [DOI] [PubMed] [Google Scholar]
- 35. Goldstein K. Methodical approach to the study of schizophrenic thought disorder. In: Kasanin JS, ed. Language and Thought in Schizophrenia. New York, NY: Norton; 1944:17–40. [Google Scholar]
- 36. Makowski DG, Lajonchere C, Dicker R, et al. Characterization of thought disorder in early onset schizophrenia. Schizophr Res. 1995;15:15. [DOI] [PubMed] [Google Scholar]
- 37. Kestnbaum Daniels E, Shenton ME, Holzman PS, et al. Patterns of thought disorder associated with right cortical damage, schizophrenia, and mania. Am J Psychiatry. 1988;145:944–949. [DOI] [PubMed] [Google Scholar]
- 38. Andreasen N. Thought, language, and communication disorders: II. Diagnostic significance. Arch Gen Psychiatry. 1979b;36:1325–1330. [DOI] [PubMed] [Google Scholar]
- 39. Andreasen N, Grove W. Thought, language and communication in schizophrenia: diagnosis and prognosis. Schizophr Bull. 1986;12:348–359. [DOI] [PubMed] [Google Scholar]
- 40. Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Arch Gen Psychiatry. 1996;53:358–364. [DOI] [PubMed] [Google Scholar]
- 41. Shenton ME, Solovay MR, Holzman P. Comparative studies of thought disorders. II. Schizoaffective disorder. Arch Gen Psychiatry. 1987;44:21–30. [DOI] [PubMed] [Google Scholar]
- 42. Solovay MR, Shenton ME, Holzman PS. Comparative studies of thought disorders. I. Mania and schizophrenia. Arch Gen Psychiatry. 1987;44:13–20. [DOI] [PubMed] [Google Scholar]
- 43. Yalincetin B, Bora E, Binbay T, et al. Formal thought disorder in schizophrenia and bipolar disorder: a systematic review and meta-analysis. Schizophr Res. 2016. [DOI] [PubMed] [Google Scholar]
- 44. Johnston MH, Holzman PS.Assessing Schizophrenic Thinking. San Francisco, CA: Jossey-Bass, Inc; 1979. [Google Scholar]
- 45. Holzman PS, Shenton M, Solovay M. Quality of thought disorder in differential diagnosis. Schizophr Bull. 1986;12:360–372. [DOI] [PubMed] [Google Scholar]
- 46. Spohn HE, Coyne L, Larson J, et al. Episodic and residual thought pathology in chronic schizophrenia. Schizophr Bull. 1986;12:394–407. [DOI] [PubMed] [Google Scholar]
- 47. Glahn D, Almasy L, Blangero J, et al. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet. 2007;144B:242–249. [DOI] [PubMed] [Google Scholar]
- 48. Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. [DOI] [PubMed] [Google Scholar]
- 49. Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. [DOI] [PubMed] [Google Scholar]
- 51. Reichenberg A, Harvey PD, Bowie CR, et al. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr Bull. 2009;35:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ivleva EI, Morris DW, Moates AF, et al. Genetics and intermediate phenotypes of the schizophrenia—bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921. [DOI] [PubMed] [Google Scholar]
- 53. Ivleva EI, Morris DW, Osuji J, et al. Cognitive endophenotypes of psychosis within dimension and diagnosis. Psychiatry Res. 2012;196:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. [DOI] [PubMed] [Google Scholar]
- 55. Hall M-H, Schulze K, Bramon E, et al. Genetic overlap between P300, P50 and duration mismatch negativity. Am J Med Genet B Neuropsychiatr Genet. 2006;141:336–343. [DOI] [PubMed] [Google Scholar]
- 56. Hall MH, Rijsdijk F, Picchioni M, et al. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164:804–812. [DOI] [PubMed] [Google Scholar]
- 57. Hall MH, Rijsdijk F, Kalidindi S, et al. Genetic overlap between bipolar illness and event-related potentials. Psychol Med. 2007;37:667–678. [DOI] [PubMed] [Google Scholar]
- 58. Hamm JP, Ethridge LE, Shapiro JR, et al. Spatiotemporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and bipolar disorder with psychosis. Psychophysiology. 2012;49:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. Am J Psychiatry. 2005;162:43–49. [DOI] [PubMed] [Google Scholar]
- 60. Mathew I, Gardin TM, Tandon N, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–777. [DOI] [PubMed] [Google Scholar]
- 61. Andreassen OA Harbo HF Wang Y,. et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Craddock N, O’Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. [DOI] [PubMed] [Google Scholar]
- 65. Perlis RH, Purcell S, Fagerness J, et al. Family-based association study of lithium-related and other candidate genes in bipolar disorder. Arch Gen Psychiatry. 2008;65:53–61. [DOI] [PubMed] [Google Scholar]
- 66. Fallin MD, Lasseter VK, Avramopoulos D, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DePaulo JR. Genetics of bipolar disorder: where do we stand?Am J Psychiatry. 2004;161:596–597. [DOI] [PubMed] [Google Scholar]
- 68. Berrettini WH. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet. 2003;123C:59–64. [DOI] [PubMed] [Google Scholar]
- 69. Cross-Disorder Group of the Psychiatric Genomics Consortium ; Smoller JW, Craddock N, et al. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going. But still not gone. Br J Psychiatry. 2010;196:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sullivan PF, Magnusson C, Reichenberg A, et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209–1215. [DOI] [PubMed] [Google Scholar]
- 74. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gottesman II, Laursen TM, Bertelsen A, Mortensen PB. Severe mental disorders in offspring with 2 psychiatrically ill parents. Arch Gen Psychiatry. 2010;67:252–257. [DOI] [PubMed] [Google Scholar]
- 76. Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull. 2014;40:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chou IJ, Kuo C-F, Huang Y-S, et al. Familial aggregation and heritability of schizophrenia and co-aggregation of psychiatric illnesses in affected families [published online ahead of print November 21, 2016]. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rado S, Buchenholz B, Dunton H, Karlen S, Senescu R. Schizotypal organization. Preliminary report on a clinical study of schizophrenia. In: Rado S, Daniels G, eds. Changing Concepts of Psychoanalytic Medicine. New York, NY: Grune & Stratton; 1956:225–235. [Google Scholar]
- 79. Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 80. Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. J Pers Disord. 1990;4:1–99. [Google Scholar]
- 81. Perry W, Minassian A, Cadenhead K, Sprock J, Braff D. The use of the Ego Impairment Index across the schizophrenia spectrum. J Pers Assess. 2003;80:50–57. [DOI] [PubMed] [Google Scholar]
- 82. Singer MT, Wynne LC. Differentiating characteristics of parents of childhood schizophrenics, childhood autistics, and young adult schizophrenics. Am J Psychiatry. 1963;120:234–243. [DOI] [PubMed] [Google Scholar]
- 83. Singer MT, Wynne LC. Thought disorder and family relations of schizophrenics. III: Methodology using projective techniques. Arch Gen Psychiatry. 1965a;12:187–212. [DOI] [PubMed] [Google Scholar]
- 84. Singer MT, Wynne LC. Thought disorder and family relations of schizophrenics. IV. Results and implications. Arch Gen Psychiatry. 1965;12:201–212. [DOI] [PubMed] [Google Scholar]
- 85. Hain C, Maier W, Hoechst-Janneck S, Franke P. Subclinical thought disorder in first-degree relatives of schizophrenic patients. Results from a matched-pairs study with the Thought Disorder Index. Acta Psychiatr Scand. 1995;92:305–309. [DOI] [PubMed] [Google Scholar]
- 86. Docherty NM. Linguistic reference performance in parents of schizophrenic patients. Psychiatry. 1995;58:20–27. [DOI] [PubMed] [Google Scholar]
- 87. Vaever MS, Licht DM, Møller L, et al. Thinking within the spectrum: schizophrenic thought disorder in six Danish pedigrees. Schizophr Res. 2005;72:137–149. [DOI] [PubMed] [Google Scholar]
- 88. Baskak B, Ozel ET, Atbasoglu EC, Baskak SC. Peculiar word use as a possible trait marker in schizophrenia. Schizophr Res. 2008;103:311–317. [DOI] [PubMed] [Google Scholar]
- 89. Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296–303. [DOI] [PubMed] [Google Scholar]
- 90. Kendler KS. Diagnostic approaches to schizotypal personality disorder: a historical perspective. Schizophr Bull. 1985;11:538–553. [DOI] [PubMed] [Google Scholar]
- 91. McConaghy N. the use of an object sorting test in elucidating the hereditary factor in schizophrenia. J Neurol Neurosurg Psychiatry. 1959;22:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kinney DK, Holzman PS, Jacobsen B, et al. Thought disorder in schizophrenic and control adoptees and their relatives. Arch Gen Psychiatry. 1997;54:475–479. [DOI] [PubMed] [Google Scholar]
- 93. Romney DM. Formal thought disorder among the first-degree relatives of schizophrenics: a new look at some old data. J Clin Psychol. 1984;40:51–52. [DOI] [PubMed] [Google Scholar]
- 94. Romney DM. Thought disorder among the relatives of schizophrenics. A reaction to Callahan and Saccuzzo. J Nerv Ment Dis. 1988;176:364–367. [DOI] [PubMed] [Google Scholar]
- 95. Docherty NM, Gordinier SW, Hall MJ, Cutting LP. Communication disturbances in relatives beyond the age of risk for schizophrenia and their associations with symptoms in patients. Schizophr Bull. 1999;25:851–862. [DOI] [PubMed] [Google Scholar]
- 96. Docherty NM, Gottesman I. A twin study of communication disturbances in schizophrenia. J Nerv Ment Dis. 2000;188:395–401. [DOI] [PubMed] [Google Scholar]
- 97. Singer M, Wynne L. Communication styles in parents of normals, neurotics and schizophrenics. Psychiatr Res Rep. 1966a;20:25–38. [PubMed] [Google Scholar]
- 98. Shenton ME, Solovay MR, Holzman PS, Coleman M, Gale HJ. Thought disorder in the relatives of psychotic patients. Arch Gen Psychiatry. 1989;46:897–901. [DOI] [PubMed] [Google Scholar]
- 99. Griffith JJ, Mednick SA, Schulsinger F, Diderichsen B. Verbal associative disturbances in children at high risk for schizophrenia. J Abnorm Psychol. 1980;89:125–131. [DOI] [PubMed] [Google Scholar]
- 100. Arboleda C, Holzman PS. Thought disorder in children at risk for psychosis. Arch Gen Psychiatry. 1985;42:1004–1013. [DOI] [PubMed] [Google Scholar]
- 101. Watt NF, Anthony EJ.Children at Risk for Schizophrenia. New York, NY: Cambridge University Press; 1984. [Google Scholar]
- 102. Parnas J, Schulsinger F, Schulsinger H, Mednick S, Teasdale T. Behavioral precursors of the schizophrenia spectrum. Arch Gen Psychiatry. 1982;39:658–664. [DOI] [PubMed] [Google Scholar]
- 103. Harvey PD, Weintraub S, Neale JM. Speech competence of children vulnerable to psychopathology. J Abnorm Child Psychol. 1982;10:373–387. [DOI] [PubMed] [Google Scholar]
- 104. Squires-Wheeler E, Skodol AE, Bassett A, Erlenmeyer-Kimling L. DSM-III-R schizotypal personality traits in offspring of schizophrenic disorder, affective disorder, and normal control parents. J Psychiatr Res. 1989;23:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Metsänen M, Wahlberg KE, Saarento O, et al. Early presence of thought disorder as a prospective sign of mental disorder. Psychiatry Res. 2004;125:193–203. [DOI] [PubMed] [Google Scholar]
- 106. Gooding DC, Ott SL, Roberts SA, Erlenmeyer-Kimling L. Thought disorder in mid-childhood as a predictor of adulthood diagnostic outcome: findings from the New York High-Risk Project. Psychol Med. 2013;43:1003–1012. [DOI] [PubMed] [Google Scholar]
- 107. Wahlberg KE, Wynne LC, Oja H, et al. Thought disorder index of Finnish adoptees and communication deviance of their adoptive parents. Psychol Med. 2000;30:127–136. [DOI] [PubMed] [Google Scholar]
- 108. Zahn TP. Word association in adoptive and biological parents of schizophrenics. Arch Gen Psychiatry. 1968;19:501–503. [DOI] [PubMed] [Google Scholar]
- 109. Wahlberg KE, Wynne LC, Oja H, et al. Gene-environment interaction in vulnerability to schizophrenia: findings from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry. 1997;154:355–362. [DOI] [PubMed] [Google Scholar]
- 110. Roisko R, Wahlberg KE, Hakko H, Tienari P. Association of adoptive child’s thought disorders and schizophrenia spectrum disorders with their genetic liability for schizophrenia spectrum disorders, season of birth and parental Communication Deviance. Psychiatry Res. 2015;226:434–440. [DOI] [PubMed] [Google Scholar]
- 111. Docherty NM. Communication deviance, attention, and schizotypy in parents of schizophrenic patients. J Nerv Ment Dis. 1993;181:750–756. [DOI] [PubMed] [Google Scholar]
- 112. Docherty NM, Miller TN, Lewis MA. Communication disturbances in the natural speech of schizophrenic patients and their nonschizophrenic parents. Acta Psychiatr Scand. 1997;95:500–507. [DOI] [PubMed] [Google Scholar]
- 113. Docherty NM, Rhinewine JP, Labhart RP, Gordinier SW. Communication disturbances and family psychiatric history in parents of schizophrenic patients. J Nerv Ment Dis. 1998;186:761–768. [DOI] [PubMed] [Google Scholar]
- 114. Wynne LC, Singer MT. Thought disorder and family relations of schizophrenics. Arch Gen Psychiatry. 1965;12:187–200. [DOI] [PubMed] [Google Scholar]
- 115. Hirsch SR, Leff JP.Abnormalities in Parents of Schizophrenics. London, UK: Oxford University Press; 1975. [Google Scholar]
- 116. Wynne LC, Singer MT, Bartko J, Toohey ML. Schizophrenics and their families: Recent research on parental communication. In: Tanner JM, ed. Developments in Psychiatric Research. Seven Oaks, Kent, England: Hodder & Stroughton; 1977:254–286. [Google Scholar]
- 117. Harrow M, Prosen M. Schizophrenic thought disorders: bizarre associations and intermingling. Am J Psychiatry. 1979;136:293–296. [DOI] [PubMed] [Google Scholar]
- 118. Harrow M, Quinlan D.Disordered Thinking and Schizophrenic Psychopathology. New York, NY: Gardner press; 1985. [Google Scholar]
- 119. Solovay M, Shenton M, Gasperetti C, et al. Scoring manual for the thought disorder index. Schizophr Bull. 1986;12:483–496. [DOI] [PubMed] [Google Scholar]
- 120. Andreasen NC. Thought, language, and communication disorders. I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36:1315–1321. [DOI] [PubMed] [Google Scholar]
- 121. Liddle PF, Ngan ET, Caissie SL, et al. Thought and Language Index: an instrument for assessing thought and language in schizophrenia. Br J Psychiatry. 2002;181:326–330. [DOI] [PubMed] [Google Scholar]
- 122. Chen EYH, Lam LCW, Kan CS, et al. Language disorganization in schizophrenia: validation and assessment with a new clinical rating instrument. Hong Kong J Psychiatry. 1996;6:4–13. [Google Scholar]
- 123. Levy DL, Coleman MJ, Sung H, et al. The genetic basis of thought disorder and language and communication disturbances in schizophrenia. J Neurolinguistics. 2010;23:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Spitzer R, Williams J, Gibbon M, First M.Structured Clinical Interview for DSM-IV. Patient Edition ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 125. Kendler KS.Structured Interview for Schizotypal Symptoms (SISS, version 1.5). Ruchmond, VA: Department of Psychiatry, Medical College of Virginia; 1989. [Google Scholar]
- 126. Leckman J, Sholomskas D, Thompson W, Belanger A, Weissman M. Best estimate of lifetime psychiatric diagnosis. Arch Gen Psychiatry. 1982;39:879–883. [DOI] [PubMed] [Google Scholar]
- 127. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 128. Rorschach H.Psychodiagnostics. New York, NY: Grune & Stratton; 1949. [Google Scholar]
- 129. Rapaport D, Gill MM, Schafer R.Diagnostic Psychological Testing. Rev. ed. ed. New York, NY: International Universities Press; 1968. [Google Scholar]
- 130. Coleman MJ, Carpenter JT, Waternaux C, et al. The Thought Disorder Index: a reliability study. Psychol Assess. 1993;3:336–342. [Google Scholar]
- 131. Haimo SF, Holzman PS. Thought disorder in schizophrenics and normal controls: social class and race differences. J Consult Clin Psychol. 1979;47:963–967. [DOI] [PubMed] [Google Scholar]
- 132. Holzman PS, Levy DL, Johnston MH. The use of the Rorschach technique for assessing formal thought disorder. In: Bornstein RF, Masling JM, eds. Scoring the Rorschach: Eight Validated Systems. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 133. Dwass M. Some k-sample rank-order tests. In: Olkin I, Ghurye SG, Hoeffding W, Madow WG, Mann HB, eds. Contributions to Probability and Statistics. Stanford, CA: Stanford University Press; 1960:198–202. [Google Scholar]
- 134. Steel RGD. A rank sum test for comparing all pairs of treatments. Technometrics. 1960;2:197–207. [Google Scholar]
- 135. Critchlow DE, Fligner MA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory. 1991;20:127–139. [Google Scholar]
- 136. Morgan CJ, Lenzenweger MF, Rubin DB, Levy DL. A hierarchical finite mixture model that accommodates zero-inflated counts, non-independence, and heterogeneity. Stat Med. 2014;33:2238–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lambert D. Zero-inflated Poisson regression with an application to defects in manufacturing. Technometrics. 1992;34:1–14. [Google Scholar]
- 138. Homer DW, Lemeshow S.Applied Logistic Regression. 2nd ed. New York, NY: Wiley; 2000. [Google Scholar]
- 139. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. [Google Scholar]
- 140. Matthysse S, Parnas J. Extending the phenotype of schizophrenia: implications for linkage analysis. J Psychiatr Res. 1992;26:329–344. [DOI] [PubMed] [Google Scholar]
- 141. Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for Mendelian disease, future approaches for complex disease. Nat Genet. 2003;33(suppl):228–237. [DOI] [PubMed] [Google Scholar]
- 142. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 143. Holzman PS, Matthysse S. The genetics of schizophrenia: a review. Psychol Sci. 1990;1:279–286. [Google Scholar]
- 144. Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull. 2007;33:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Freedman R, Adler LE, Leonard S. Alternative phenotypes for the complex genetics of schizophrenia. Biol Psychiatry. 1999;45:551–558. [DOI] [PubMed] [Google Scholar]
- 146. Ripke S, O’Dushlaine C, Chambert K, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Glahn DC, Knowles EE, McKay R, et al. Arguments for the sake of endophenotypes. Am J Med Genet. 2014;165B:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gottesman II, McGue M. Endophenotypes. In: Cautin RL, Lilienfeld SO, eds. The Encyclopedia of Clinical Psychology. New York, NY: John Wiley & Sons, Inc; 2015. [Google Scholar]
- 150. Flint J, Timpson N, Munafò M. Assessing the utility of intermediate phenotypes for genetic mapping of psychiatric disease. Trends Neurosci. 2014;37:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989. [DOI] [PubMed] [Google Scholar]
- 152. Sunga H, Ji F, Levy DL, Matthysse S, Mendell NR. The power of linkage analysis of a disease-related endophenotype using asymmetrically ascertained sib pairs. Comput Stat Data Anal. 2009;53:1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Need AC, Goldstein DB. Schizophrenia genetics comes of age. Neuron. 2014;83:760–763. [DOI] [PubMed] [Google Scholar]
- 154. Roussos P, Mitchell AC, Voloudakis G, et al. A role for noncoding variation in schizophrenia. Cell Rep. 2014;9:1417–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kavanagh DH, Tansey KE, O’Donovan MC, Owen MJ. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry. 2015;20:72–76. [DOI] [PubMed] [Google Scholar]
- 156. Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chatterjee N, Wheeler B, Sampson J, et al. Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat Genet. 2013;45:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wray NR, Yang J, Hayes BJ, et al. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vilhjalmsson BJ, Yang J, Finucane HK, et al. Modeling linkage disequilibrium increases accuracy of polygenic risk scores. Am J Hum Genet. 2015;97:576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Maier R, Moser G, Chen GB, et al. Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder, and major depressive disorder. Am J Hum Genet. 2015;96:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Roisko R, Wahlberg KE, Miettunen J, Tienari P. Association of parental communication deviance with offspring’s psychiatric and thought disorders. A systematic review and meta-analysis. Eur Psychiatry. 2014;29:20–31. [DOI] [PubMed] [Google Scholar]
- 162. Benros ME, Trabjerg BB, Meier S, et al. Influence of polygenic risk scores on the association between infections and schizophrenia. Biol Psychiatry. 2016;80:609–616. [DOI] [PubMed] [Google Scholar]
- 163. Agerbo E, Sullivan PF, Vilhjálmsson BJ, et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a danish population-based study and meta-analysis. JAMA Psychiatry. 2015;72:635–641. [DOI] [PubMed] [Google Scholar]
- 164. Reininghaus U, Böhnke JR, Hosang G, et al. Evaluation of the validity and utility of a transdiagnostic psychosis dimension encompassing schizophrenia and bipolar disorder. Br J Psychiatry. 2016;209:107–113. [DOI] [PubMed] [Google Scholar]
- 165. Roche E, Creed L, MacMahon D, Brennan D, Clarke M. The epidemiology and associated phenomenology of formal thought disorder: a systematic review. Schizophr Bull. 2015;41:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Peralta V, Cuesta MJ, de Leon J. Formal thought disorder in schizophrenia: a factor analytic study. Compr Psychiatry. 1992;33:105–110. [DOI] [PubMed] [Google Scholar]
- 167. Marengo JT, Harrow M. Schizophrenic thought disorder at follow-up. A persistent or episodic course?Arch Gen Psychiatry. 1987;44:651–659. [DOI] [PubMed] [Google Scholar]
- 168. Marengo JT, Harrow M. Longitudinal courses of thought disorder in schizophrenia and schizoaffective sisorder. Schizophr Bull. 1997;23:273–285. [DOI] [PubMed] [Google Scholar]
- 169. Hall MH, Smoller JW, Cook NR, et al. Patterns of deficits in brain function in bipolar disorder and schizophrenia: a cluster analytic study. Psychiatry Res. 2012;200:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.