Abstract
The relationship between exercise training and nitric oxide-related parameters was examined in a cross-sectional study and an intervention study. A cross-sectional study using 184 employees was conducted to observe the association of exercise habits with serum arginase (ELISA and activity), l-arginine, l-citrulline, l-ornithine, NOx, exhaled nitric oxide, blood pressure, FEV1%, hs-CRP, HDL-cholesterol, IgE, and life style factors. An intervention study was also conducted to evaluate the changes of serum arginase I, nitric oxide-related parameters, and mRNA levels of anti-oxidant enzymes in blood monocytes before and after 1 h of aerobic exercise training per day for a month. Exercise habits were associated with increased arginase activity and a moderate alcohol drinking habit, after adjustment with several covariates. Aerobic exercise training induced a decrease in l-arginine and diastolic blood pressure and induced an increase in NO2− and urea. Moreover, mRNA expression of anti-oxidant enzymes, such as catalase and GPX1, and a life elongation enzyme, SIRT3, were significantly increased after aerobic exercise. The results that aerobic exercise training increased NO generation, reduced blood pressure, and induced anti-oxidant enzymes via SIRT3 suggest that exercise training may be an important factor for the prevention of disease by inducing intrinsic NO and anti-oxidant enzymes.
Keywords: exercise, blood pressure, l-arginine, nitric oxide, intervention research
Introduction
In recent years, the number of patients with hypertension, dyslipidemia, and lifestyle-related diseases, such as diabetes, are on the increase, and the onset of these diseases is associated with the inheritance of genetic factors, pathogens, toxic substances, external environmental factors such as stressors, and lifestyle factors such as diet, exercise, and drinking. Although lifestyle choices, such as a lack of exercise, westernization of eating habits, drinking, and smoking, may be major factors in cardiovascular disease, there is considerable evidence to show that exercise training reduces many of the related risk factors, including high blood pressure, high cholesterol, obesity, and insulin resistance.(1) A meta-analysis showed that regular exercise reduces mean systolic and diastolic blood pressure.(2) Several mechanisms for the reduction of blood pressure by exercise training have been considered.(3–5) Exercise also stimulates nitric oxide (NO) release from endothelial cells.(6) Despite the obvious benefits of physical training, the main exercise-mediated mechanisms involved in the improvement of vascular function in coronary artery disease have not been clearly identified.(7) However, the mechanical relationship of vascular physiology with NO has been connected to the reduction of blood pressure after exercise training. Physical exercise reduces blood pressure and is now broadly recommended by current American and European hypertension guidelines.(8,9) However, there is also considerable evidence to show the contribution of NO to the regulation of blood pressure after exercise training.(7,10,11) since NO has been discovered to be an endothelial-derived relaxing factor and may play an important role in not only endothelial relaxation but also the prevention of endothelial cell dysfunction.(12,13)
On the other hand, there is an intriguing increase in NO production in skeletal muscle in response to physical exercise. NO, produced by nitric oxide synthase 3 (eNOS) in the vascular endothelium and produced by NOS1 (neuronal NOS), NOS2 (inducible NOS), and NOS3 in skeletal muscle, is synthesized from l-arginine.(14) In skeletal muscle, NOS1 and NOS3 are expressed.(15,16) Chronic exercise, 90 min/day for 8 weeks, increases the expression of NOS1 and NOS3 in skeletal muscle.(17) In skeletal muscle, NO interacts with the metabolic enzyme, AMPK.(18) NO and AMPK cooperatively regulate PGC1α.(19) 70–90% of NO is stored in S-nitrosothiols, the main source of NO in tissues.(20) Moderate exercise increases NO content through the activation of nuclear factor κB.(21)
Arginase, a key enzyme in the urea cycle, is involved in indirect regulation of NO by the consumption of l-arginine, which is a common substrate for NOS. Studies on the induction or activation of arginase have focused on pre-atherosclerotic vascular changes and asthmatic airway inflammation as pathophysiological evidence that the consumption of l-arginine by arginase may lead to the reduction of NO, resulting in the reduced enlargement of endothelial or bronchial smooth muscle-associated vascular damage.(22)
Serum arginase levels were evaluated in various diseases such as asthma,(23–25) type-2 diabetes mellitus,(26) cancer,(27) and atherosclerosis(28) by activity assay and the ELISA method. In a healthy population, arginase I was associated with oxidative stress, exhaled nitric oxide, and l-arginine.(29–31)
Although there is considerable evidence showing the association of exercise training and NO, there are few reports demonstrating the association of exercise training with arginase and NO-related parameters in cross-sectional and intervention studies. Therefore, in this study, we evaluated the interaction of arginase with exercise training in association with NO-related parameters in healthy individuals who were occupational workers and exercise training instructors.
Materials and Methods
Study design
A cross-sectional study on the relationship between arginase or NO-related clinical parameters and exercise training was designed for an industrial company (n = 408) in Hiroshima Prefecture in September 2011, and all participants provided written informed consent. The data of 184 of those who had no previous history of asthma, diabetes mellitus, or other serious disease were used for the analysis, and 224 individuals were excluded due to irregular employment and shift work. The ethics committee of Okayama University approved the study (Number 561).
An intervention study for exercise training was performed in 41 individuals who were employees of a sports club or students of International Pacific University in Japan. The 41 individuals provided written informed consent. The survey period was from May to July 2013. It was divided into two groups. The first intervention, with 19 individuals, was performed from May 8 to June 3, 2013, and the second intervention, with 22 individuals, was performed from June 16 from July 15, 2013. Continuous exercise training was performed five times per week for 4 weeks. Blood for analysis was withdrawn before and after intervention. The ethics committee of Okayama University approved the study, and all subjects gave written informed consent. This intervention study was registered with UMIN (UMINO000024457).
Exercise training
Bicycle exercises were performed using a stationary bicycle with an attached ergometer for one hour a day, five times per week, for 4 weeks. In addition, the exercise intensity was limited to “moderate”, such that the subject’s muscles did not consciously feel “tight”. The exercises were managed by setting a target heart rate relative to the subject’s maximum heart rate, calculated by the following Karvonen formula: “target hear trate = [(220 − age) − resting heart rate] × 0.6 + resting heart rate”. For safety, the exercise intensity was set to 60%. The exercise was carried out under the supervision and guidance of exercise guidance staff.
Measurement of clinical parameters for whole blood and serum
Ten ml of blood samples were collected one day before of beginning of exercise and one day after the day of final exercise. Venous blood samples were collected after 4 h of fasting. Serum for intervention was collected within 10 min and serum for cross-sectional analysis was collected after standing for 1 h to coagulate the blood. Sera were preserved at −80°C until analysis. Serum LDL-cholesterol was measured using automated XE-2100 (Sysmex, Kobe, Japan) and H7700 (Hitachi High-Technologies, Tokyo, Japan), and high-sensitivity CRP (hs-CRP) was measured by a highly sensitive CRP assay (Behring Latex-Enhanced using the Behring Nephelometer BN-100; Behring Diagnostics, Westwood, MA). Arginase activity and IgE were determined using ELISA kits (Montgomery, TX), and arginase I was determined with ELISA kits previously established.(31) Information on lifestyle factors, including cigarette smoking, exercise habits, and alcohol drinking habits, was obtained using self-reported questionnaires or clinical records.
Fractional exhaled NO (FeNO) and FEV1%
Fractional exhaled NO (FeNO) was measured using a portable electrochemical analyzer (Niox Mino, Aerocrine AB, Sweden). This device measures FeNO during a 10-s exhalation with a constant flow of 50 ml/s, according to the international recommendations. All measurements were performed in duplicate, all within 10% deviation, and the mean concentration in parts per billion (ppb) was registered.
FEV1% was measured as a pulmonary function test using a spirometer (CHESTGRAPH Jr.; Chest, Tokyo, Japan) according to international recommendations, measuring FEV1 and FVC, and expressed as a percentage of FEV1/FVC.
Measurement of serum NO2− and NOx
Serum NO2− is gradually oxidized by hemoglobin to NO3− within 10 min. Serum NO3− is derived from NO2−, food intake, and intestinal flora. Therefore, serum NO2− before oxidation is important for NO supply in the blood. NO2− was determined with a NO analyzer (model-280i NOA with the Purge Vessel; Sievers, Boulder, CO).
NOx (NO2− + NO3−) levels in the serum were determined by reduction of NO3− to NO2−. After reduction of NO3− to NO2− with nitrate reductase (Sigma-Aldrich, St. Louis, MO) for 30 min at room temperature, NOx was determined with an NO analyzer (model-280i NOA with the Purge Vessel; Sievers). In the NO analyzer, NO2− was further reduced to NO in the Purge Vessel containing the reducing agent potassium iodide in acetic acid, and generated NO was transported to the NO analyzer using the ozone-chemiluminescence method.
Measurement of serum l-arginine, l-citrulline and l-ornithine
Serum l-arginine was measured using an HPLC system (HITACHI, Tokyo, Japan). l-Arginine was eluted from serum and supplemented with 5 µM monomethylarginine (MMA) as an internal standard, using Oasis MCX solid phase-extraction cartridges (Waters, Milford, MA) conditioned with 2 ml of methanol/water/ammonia solution (50:45:5, v/v/v) and phosphate-buffered saline (PBS). Serum samples containing 5 µM MMA were dissolved in PBS and loaded on an equilibrated SPE column. The column was constitutively washed with 0.1 N HCl (2 ml) and methanol (2 ml). The fraction containing l-arginine was eluted with 1 ml of methanol/water/ammonia solution (50:45:5, v/v/v) and dried in a vacuum centrifuge. After the drying process, the residue was reconstituted with water. Serum samples for l-citrulline and l-ornithine were measured using an HPLC system (HITACHI). Serum samples for l-citrulline and l-ornithine containing 10 µM MMA were precipitated with 1.5 M HClO4. The samples reconstituted with water for l-arginine or the supernatants for l-citrulline and l-ornithine were mixed with an equal amount of derivatizing agent (5 mg/ml ortho-phthaldialdehyde, 10% methanol, 0.5% 3-mercaptopropionic acid in 200 mM borate buffer, pH 9.5), and the reaction was allowed to occur for 30 min at room temperature. The samples for l-arginine were introduced into the fluorescence HPLC system using a TSKgel ODS-100V column (4.6 × 250 mm, 5 µm, Tosoh, Yamaguchi, Japan) with the mobile phase consisting of 9% acetonitrile in acetate buffer (pH 6.3) at a flow rate of 1.5 ml/min. The samples for l-citrulline and l-ornithine were examined with the fluorescence HPLC system using a Supelco C18 column (4.6 × 15 cm, 3 mm, Bellefonte, PA) at a flow rate of 1.1 ml/min of a gradient mixture as follows: mobile phase A, consisting of 0.1 M sodium acetate (pH 7.2) containing of 9% methanol and 0.5% tetrahydrofuran, to mobile phase B consisting of 100% methanol. The detection of each amino acid was performed using excitation and emission wavelengths of 340 and 455 nm, respectively.
Expression of mRNA of anti-oxidative enzymes in peripheral blood monocytes in the intervention study
After intervention, blood with anti-coagulant was put on Polymorphprep (Axis-Shield PoC AS, Oslo, Norway), and a cell layer of monocytes was removed and washed with Hank’s balanced solution. Total RNA was purified from monocytes by ISOGEN (NIPPON GENE, Tokyo, Japan) in combination with High-Salt Precipitation Solution (NIPPON GENE). RNA concentrations were measured on a Nano Drop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The integrity of RNA was verified by denaturing agarose gel electrophoresis. Complementary DNA (cDNA) was synthesized from 1 µg of total RNA in a 20-µl reaction volume using the PrimeScript 1st strand cDNA Synthesis Kit (Takara6110) with the Oligo dT primer, according to the manufacturer’s instructions. An aliquot of cDNA was used as a template for quantitative PCR using SYBR Premix Ex Taq (Tli RNaseH Plus) (Takara RR420) with the ROX dye passive reference on the StepOne Plus Real-time PCR System (Applied Biosystems; the Central Research Laboratory, Okayama University Medical School) operated in the relative gene expression mode using the ROX dye as a passive reference. The primers used are listed in Table 6. Primers were chosen from the PrimerBank(32) or qPrimerDepot(33), such that each amplicon spanned at least one intron. Relative expression levels were calculated by the ΔΔCt method using the GAPDH gene as an endogenous control for normalization, with the following modification: the mean PCR amplification efficiency for each primer set was calculated by performing PCR in duplicate using undiluted and 4-fold diluted cDNAs as templates. Expression levels in each sample represented the geometric means of duplicate measurements with the dilution rate and amplification efficiency being taken into account. In a few samples in which multiple peaks were observed in the melting temperature curve of one of the duplicate measurements, the expression level of only one of the duplicates with a single peak was represented. The mean of the relative expression level of post-intervention to pre-intervention was presented.
Table 6.
Primer sets for real time PCR
| Target gene | Forward primer (5' to 3') | Reverese primer (5' to 3') | Source |
|---|---|---|---|
| SOD1 | CTAGCGAGTTATGGCGACGA | TAATGCTTCCCCACACCTTC | qPrimerDepot |
| SOD2 | GGAGAAGTACCAGGAGGCGT | TAGGGCTGAGGTTTGTCCAG | qPrimerDepot |
| Catalase | TGGGATCTCGTTGGAAATAACAC | TCAGGACGTAGGCTCCAGAAG | PrimerBank |
| GPX1 | CAACCAGTTTGGGCATCAG | AAGAGCATGAAGTTGGGCTC | qPrimerDepot |
| SIRT3 | ACCCAGTGGCATTCCAGAC | GGCTTGGGGTTGTGAAAGAAG | PrimerBank |
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0c for Mac (GraphPad Software, Inc., San Diego, CA) and PASW Statistics 18 for Mac. The differences in means ± SEM of several clinical parameters were analyzed between sex, age (<44 and ≥45) with the Mann-Whitney U test or unpaired t test, and the changes of nitric oxide and l-arginine-related parameters according to exercise degree were analyzed by age and sex-adjusted ANCOVA. We performed logistic regression analysis to investigate the association of exercise with arginase and NO-related clinical parameters using log-transformed values. Covariate for adjustment were log-transformed values of age, MBI, arginase I, arginase activity, l-arginine, l-citrulline, l-ornithine, NOx, FeNO, FEV1%, systolic and diastolic blood pressure, and hs-CRP, HDL-cholesterol, IgE and dichotomized scales variable of smoking habits, exercise, and alcohol consumption. All the probability values for the <0.05 were regarded as statistically significant.
Results
Characteristics of subjects of cross-sectional study
The characteristics of 184 healthy individuals are shown in Table 1. Serum levels of arginase I, arginase activity, l-arginine, and l-ornithine, diastolic blood pressure, and the likelihood of exercising, smoking, and alcohol drinking were significantly higher in males compared with females. l-Ornithine, NOx, and blood pressure were higher in the over-47 age group compared with the under-46 age group. When the degree of exercise was divided into three groups (no exercise training, some exercise training each week, and daily exercise training each week), arginase activity and FENO showed significant differences among the three groups after being adjusted for age and sex by ANOVA (Table 2).
Table 1.
Characterisitcs of arginase, nitric oxide-related clinical parameters
| Variables | Total | Male | Female | p | Age <46 | Age ≥47 | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 184) | (n = 120) | (n = 64) | (n = 92) | (n = 92) | ||||||
| Age | 46.3 ± 1.0 | 45.3 ± 1.3 | 48.0 ± 1.6 | 0.225 | 34.5 ± 0.9 | 58 ± 0.6 | <0.0001 | |||
| Sex (M/F) | 120/64 | 63/29 | 57/35 | 0.353 | ||||||
| BMI | 23.6 ± 0.3 | 23.9 ± 0.3 | 22.9 ± 0.4 | 0.034 | 23.4 ± 0.4 | 23.7 ± 0.4 | 0.576 | |||
| Arginase I | 5.2 ± 0.3 | 5.8 ± 0.3 | 4.0 ± 0.4 | <0.0001 | 5.4 ± 0.4 | 5.0 ± 0.4 | 0.292 | |||
| Arginase activity | 4.3 ± 0.2 | 4.9 ± 0.2 | 3.2 ± 0.3 | <0.0001 | 4.5 ± 0.3 | 4.2 ± 0.3 | 0.323 | |||
| l-Arginine | 197.7 ± 3.5 | 206.7 ± 4.3 | 181.1 ± 5.4 | 0.0005 | 195.3 ± 4.7 | 200.2 ± 5.2 | 0.604 | |||
| l-Citrulline | 315.6 ± 9.0 | 322.7 ± 10.6 | 301.8 ± 16.6 | 0.372 | 317.5 ± 11.6 | 313.7 ± 13.9 | 0.956 | |||
| l-Ornithine | 345.0 ± 10.1 | 359.8 ± 13.4 | 316 ± 13.2 | 0.047 | 324.7 ± 11.2 | 366.4 ± 16.6 | 0.024 | |||
| l-Citru + l-Orni | 614.4 ± 16.2 | 638.3 ± 20.3 | 578 ± 25.0 | 0.037 | 607.4 ± 20.5 | 628.2 ± 24.5 | 0.631 | |||
| l-Arg/l-Citru + l-Orni | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.31 ± 0.1 | 0.677 | 0.30 ± 0.1 | 0.30 ± 0.1 | 0.203 | |||
| l-Arg/l-Citru | 0.70 ± 0.1 | 0.68 ± 0.1 | 0.70 ± 0.1 | 0.280 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.570 | |||
| l-Citru/l-Orni | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.085 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.073 | |||
| l-Arg/l-Orni | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.637 | 0.60 ± 0.1 | 0.60 ± 0.1 | 0.027 | |||
| NOx | 33.3 ± 1.6 | 34.9 ± 2.2 | 30.4 ± 2.0 | 0.113 | 29.6 ± 1.4 | 37.10 ± 2.8 | 0.024 | |||
| FeNO | 20.7 ± 1.4 | 21.70 ± 1.9 | 18.7 ± 2.0 | 0.059 | 22.5 ± 2.5 | 18.90 ± 1.3 | 0.934 | |||
| FVE1% | 88.6 ± 0.6 | 88.8 ± 0.8 | 88.4 ± 1.1 | 0.906 | 91.8 ± 0.8 | 85.50 ± 0.9 | <0.0001 | |||
| Systolic blood pressure | 125.0 ± 1.1 | 126.2 ± 1.3 | 122.6 ± 2.2 | 0.136 | 120.3 ± 1.4 | 129.7 ± 1.7 | <0.0001 | |||
| Diastolic blood pressure | 78.2 ± 0.8 | 79.9 ± 1.0 | 74.9 ± 1.4 | 0.0027 | 75.5 ± 1.1 | 80.9 ± 1.1 | 0.0002 | |||
| hs-CRP | 0.9 ± 0.1 | 0.80 ± 0.1 | 1 ± 0.3 | 0.102 | 0.9 ± 0.2 | 0.80 ± 0.1 | 0.015 | |||
| HDL-c | 57.2 ± 1.2 | 53.9 ± 1.6 | 63.4 ± 1.6 | <0.0001 | 56.5 ± 2.0 | 57.90 ± 1.5 | 0.240 | |||
| IgE | 128.6 ± 12.6 | 138.5 ± 17.1 | 110.2 ± 16.9 | 0.109 | 151.2 ± 20.8 | 106.0 ± 14.0 | 0.065 | |||
| Exercise habit (+/–) | 62/122 | 50/70 | 12/52 | 0.024 | 32/60 | 30/62 | 0.756 | |||
| Smoking habit (+/–) | 72/112 | 60/60 | 12/52 | <0.0001 | 36/57 | 38/55 | 0.765 | |||
| Alcohol drinking (+/–) | 108/76 | 82/38 | 26/38 | 0.0003 | 52/40 | 56/36 | 0.549 |
Values represent mean ± SEM, and bold indicates statistical significance.
Table 2.
Age and sex adjusted mean values of nitric oxide and l-arginine-related clinical variables
| Degree of exercise |
p | |||
|---|---|---|---|---|
| – (n = 119) | + (n = 53) | ++ (n = 11) | ||
| BMI | 23.6 ± 0.4 | 24.3 ± 0.5 | 22.6 ± 1.1 | 0.235 |
| Arginase I | 4.9 ± 0.3 | 5.6 ± 0.5 | 5.7 ± 1.0 | 0.432 |
| Arginase activity | 3.9 ± 0.2 | 4.8 ± 0.3 | 6.3 ± 0.7* | 0.001 |
| l-Arginine | 190.7 ± 4.6 | 182.9 ± 7.3 | 161.7 ± 15.1 | 0.155 |
| l-Citrulline | 304.6 ± 10.7 | 294.9 ± 17.0 | 266.2 ± 35.0 | 0.548 |
| l-Ornithine | 333.6 ± 12.0 | 315.9 ± 18.5 | 285.5 ± 37.9 | 0.395 |
| l-Citru + l-Orni | 615.6 ± 22.8 | 592.9 ± 33.1 | 554 ± 68.5 | 0.625 |
| l-Arg/l-Citru + l-Orni | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.719 |
| l-Arg/l-Citru | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.752 |
| l-Citru/l-Orni | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.593 |
| l-Arg/l-Orni | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.499 |
| NOx | 32.3 ± 2.0 | 34.7 ± 3.1 | 32.2 ± 6.4 | 0.904 |
| FeNO | 18.6 ± 1.7 | 20.3 ± 2.7 | 38.5 ± 5.6*,# | 0.004 |
| FVE1% | 88.9 ± 0.7 | 89.1 ± 1.1 | 86.6 ± 2.3 | 0.611 |
| Systolic blood pressure | 123.9 ± 1.3 | 127.1 ± 2.1 | 125.5 ± 4.3 | 0.418 |
| Diastolic blood pressure | 78.4 ± 0.9 | 77.8 ± 1.5 | 78.8 ± 3.1 | 0.920 |
| hs-CRP | 0.9 ± 0.1 | 0.8 ± 0.2 | 1.7 ± 0.4 | 0.148 |
| HDL-c | 56.4 ± 1.5 | 58.3 ± 2.3 | 51.60 ± 4.8 | 0.439 |
| IgE | 108.9 ± 15.7 | 172.30 ± 24.8 | 108.90 ± 51.4 | 0.096 |
Significant difference (p<0.05) by age and sex-adjusted ANCOVA among the values of clinical parameters by degree of exercise. *p<0.01 vs exercise (–) group and #p<0.05 vs exercise degree (+) group.
Logistic regression analysis for exercise training habits
Significantly high odds of exercise training are shown in Table 3. A positive association between the degree of exercise and arginase activity, with significant p trend, is shown.
Table 3.
Odds of degree of exercise according to arginase activity
| Explanatory variable | Arginase activity |
p trend | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Model 1 | 1 | 1.99 (0.77–5.17) | 2.41 (0.94–6.19) | 4.11 (1.62–10.42) | 0.003 |
| Model 2 | 1 | 1.49 (0.54–4.12) | 1.84 (0.68–4.97) | 2.91 (1.04–8.14) | 0.035 |
| Model 3 | 1 | 1.38 (0.47–4.04) | 1.78 (0.61–5.16) | 3.77 (1.24–11.46) | 0.016 |
Model 1: Odds of degree of exercise according to arginase activity with no adjustment. Model 2: Odds of degree of exercise according to arginase activity, adjusted with age and sex. Model 3: Odds of degree of exercise according to arginase activity, adjusted with age, sex, BMI, l-arginine, l-citrulline, l-ornithine, hs-CRP, FENO, NOx, FEV1%, IgE, HDL-c, cigarette smoking, and alcohol intake.
Characteristics of and changes in clinical and NO-related parameters in the intervention study
The characteristics of the 41 intervention individuals are shown in Table 4. l-Arginine was significantly reduced after exercise intervention compared with pre-intervention. These significant reductions were observed independent of sex and age. A significant increase in NO2− observed after exercise intervention, compared with pre-intervention, was found in males. Diastolic blood pressure decreased significantly after intervention in the older age group. Urea synthesis by arginase was increased in post-intervention compared with pre-intervention. This increase was found in females and younger aged individuals.
Table 4.
Changes in nitric oxide-related parameters before and after chronic exercise training for one month
| Variables | Total | p | Male | p | Female | p | Age ≤34 | p | Age ≥35 | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 41) |
(n = 19) |
(n = 22) |
(n = 22) |
(n = 19) |
|||||||||||
| Pre-exercise (n = 19) | Post-exercise (n = 21) | Pre-exercise | Post-exercise | Pre-exercise | Post-exercise | Pre-exercise | Post-exercise | Pre-exercise | Post-exercise | ||||||
| Age | 34.24 ± 12.0 | 34.68 ± 12.34 | 33.86 ± 11.97 | 24.27 ± 4.01 | 44.55 ± 8.22 | ||||||||||
| Sex (M/F) | 19/22 | 10/12 | 9/10 | ||||||||||||
| Weight | 62.1 ± 1.78 | 61.8 ± 1.8 | 0.842 | 69.6 ± 2.4 | 69.2 ± 2.4 | 0.793 | 55.5 ± 1.6 | 55.4 ± 1.6 | 0.940 | 62.5 ± 2.2 | 62.1 ± 2.2 | 0.769 | 61.6 ± 2.8 | 61.4 ± 2.9 | 0.964 |
| Arginase I | 4.8 ± 2.6 | 4.7 ± 2.5 | 0.766 | 4.8 ± 1.9 | 4.2 ± 1.4 | 0.290 | 4.9 ± 3.1 | 5.1 ± 3.2 | 0.835 | 4.7 ± 2.4 | 4.1 ± 1.7 | 0.252 | 4.9 ± 2.9 | 5.4 ± 3.1 | 0.609 |
| l-Arginine | 156.1 ± 35.4 | 138.3 ± 31.3 | 0.001 | 165.2 ± 35.3 | 146.7 ± 5.5 | 0.040 | 148.3 ± 34.4 | 131.0 ± 35.3 | 0.012 | 148.7 ± 27.8 | 134.6 ± 30.4 | 0.024 | 164.8 ± 41.8 | 142.4 ± 32.5 | 0.019 |
| l-Citrulline | 32.8 ± 8.0 | 34.6 ± 6.8 | 0.168 | 33.7 ± 7.6 | 35.5 ± 7.1 | 0.361 | 32.1 ± 8.3 | 33.9 ± 6.6 | 0.318 | 32.6 ± 8.4 | 35.0 ± 8.3 | 0.266 | 33.1 ± 7.6 | 34.2 ± 4.7 | 0.424 |
| l-Ornithine | 72.8 ± 32.7 | 84.30 ± 33.7 | 0.101 | 68.30 ± 30.2 | 87.5 ± 33.1 | 0.103 | 76.7 ± 34.9 | 81.6 ± 34.6 | 0.566 | 79.1 ± 4.0 | 89.2 ± 3.5 | 0.367 | 65.6 ± 19.4 | 78.7 ± 32.2 | 0.112 |
| NO2 | 141.9 ± 45.5 | 160.4 ± 53.4 | 0.039 | 121.9 ± 24.8 | 145.2 ± 44.1 | 0.043 | 159.21 ± 52.4 | 174 ± 58.2 | 0.298 | 150.3 ± 54.9 | 169.0 ± 60.2 | 0.180 | 132 ± 30.1 | 150.5 ± 43.9 | 0.107 |
| NOx (NO2− + NO3−) | 34.3 ± 20.2 | 33.7 ± 21.0 | 0.883 | 34.80 ± 20.4 | 29.4 ± 13.8 | 0.323 | 33.8 ± 20.7 | 37.3 ± 25.4 | 0.611 | 31.2 ± 16.4 | 30.5 ± 13.7 | 0.863 | 37.9 ± 24.0 | 37.3 ± 27.1 | 0.948 |
| SBP | 130.4 ± 15.2 | 128.2 ± 15.3 | 0.115 | 137.1 ± 15.5 | 136.9 ± 12.4 | 0.944 | 124.7 ± 12.6 | 120.6 ± 13.6 | 0.030 | 126.5 ± 10.4 | 127.1 ± 11.5 | 0.700 | 135.0 ± 18.6 | 129.4 ± 19.1 | 0.026 |
| DBP | 79.2 ± 12.0 | 76.70 ± 11.7 | 0.032 | 83.6 ± 12.8 | 80.7 ± 11.2 | 0.125 | 75.4 ± 9.9 | 73.2 ± 11.3 | 0.150 | 73.3 ± 8.4 | 74.60 ± 9.4 | 0.631 | 83.7 ± 13.9 | 79.1 ± 13.9 | 0.019 |
| Urea | 30.20 ± 9.3 | 39.40 ± 14.3 | <0.001 | 31.1 ± 9.9 | 33.8 ± 9.2 | 0.435 | 29.4 ± 8.9 | 44.3 ± 16.3 | <0.001 | 32.1 ± 9.1 | 45.40 ± 15.7 | 0.002 | 28.1 ± 9.3 | 32.6 ± 8.8 | 0.101 |
The values represent the mean ± SEM, and bold represents statistical significance.
Changes in serum l-arginine, l-citrulline, and l-ornithine in the intervention study
To observe the metabolism of amino acids relating to NO synthesis by NOS and urea synthesis by arginase, the ratios of l-arginine, l-citrulline, and l-ornithine were calculated and compared between pre-intervention and post-intervention (Table 5). The ratio of l-arginine/l-citrulline + l-ornithine was significantly decreased after intervention. l-Arginine/l-citrulline and l-arginine/l-ornithine were also significantly decreased after intervention.
Table 5.
Changes in amino acid ratios between before and after chronic exercise
| Variables | Mean ± SEM (n = 41) |
p | |
|---|---|---|---|
| Pre-intervention | Post-intervention | ||
| Arg/(Cit + Orn) | 1.59 ± 0.56 | 1.22 ± 0.33 | <0.0001 |
| Arg/Cit | 4.88 ± 1.06 | 4.07 ± 0.95 | <0.0001 |
| Arg/Orn | 2.61 ± 1.39 | 1.85 ± 0.75 | <0.001 |
| Cit/Orn | 0.55 ± 0.30 | 0.47 ± 0.20 | 0.111 |
Bold indicates statistical significance.
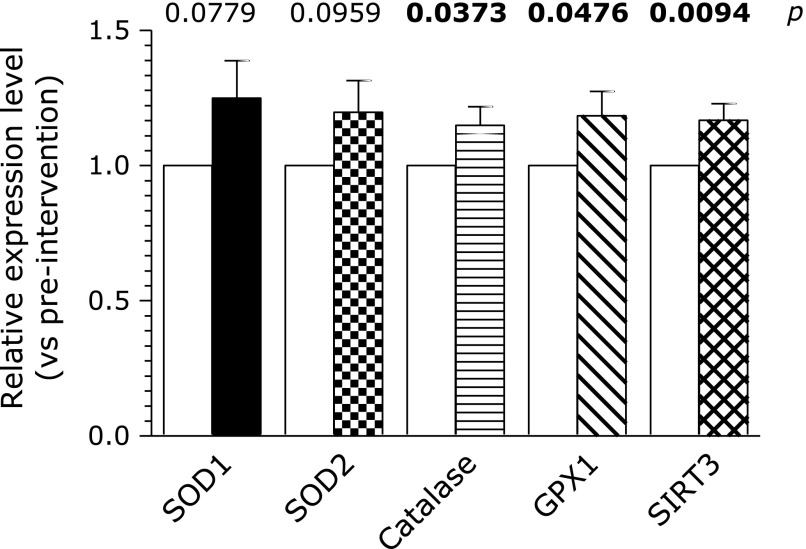
Real-time PCR of mRNA of SIRT3 and anti-oxidative enzymes of superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), catalase, and glutathione peroxidase 1 (GPX1) in the intervention study
The regulation of the mRNA of SIRT3, SOD1, SOD2, catalase, and GPX1 was measured in the intervention study of exercise training by real-time PCR. The messenger RNAs of catalase, GPX1, and SIRT3 were significantly up-regulated in post-exercise compared with pre-exercise (Fig. 1). SOD1 showed a tendency of up-regulation by the intervention of exercise training.
Fig. 1.
Expression of mRNA of SOD1, SOD2, catalase, GPx1 and SIRT3 in the intervention study of exercise training. Relative mean values of mRNA in post-intervention vs pre-intervention are presented. Bold p value represents statistical significance.
Discussion
The cross-sectional study with 184 healthy individuals showed the relationship of the degree of exercise with arginase activity and FeNO. In the intervention study, after exercise training, l-arginine was reduced and NO2− was increased. Moreover, the ratios of l-arginine/l-citrulline + l-ornithine, l-arginine/l-citrulline, and l-arginine/l-ornithine were significantly reduced. These results suggested that NO production may increase with exercise training. Since the arginase I content in serum was not changed between pre-intervention and post-intervention, and although arginase activity was not measured in the intervention study, it was believed that arginase was modified into a nitrosylated molecule, and activity was increased by NO.(34) The NO supply during exercise contributed to the up-regulation of NOS1 and NOS3 in skeletal muscle.(16–18) Moreover, 70–90% of NO is stored in S-nitrosothiols, the main source of NO in tissues.(21) However, it is not known whether the NO generated in NOS1 and NOS3 in skeletal muscle affects serum NO2− levels. NOS3 in vascular endothelial cells has not been ruled out, although there are several controversial studies on exercise-induced changes in NOS3. A 24-week course of swimming did not change the expression of the NOS3 protein in healthy mice.(35) However, exercise is associated with increases in NOS3 expression at the mRNA and protein levels.(36) Moreover, there is considerable evidence to support the idea that the NO supply is regulated by nitrite (NO2−), and NO2− is a physiologically relevant storage reservoir of NO(37) in blood and tissue that can readily be reduced to NO under pathological conditions, such as ischemia or hypoxia.(38) Although NO production is oxygen-dependent in normoxic tissues and is limited in hypoxia, the nitrate-nitrite-NO pathway is significantly facilitated and can complement NOS-based NO production under conditions of exercise or ischemia.(39)
Diastolic blood pressure was reduced by intervention in older aged individuals. A reduction of sympathetic tone is reported consistently in different studies and regarded as a mechanism of crucial relevance.(3,5) In the presence of physical exercise, physical and chemical stimuli control NO production.(11) In the endothelial cells, exercise stimulates NO synthesis through chemical mechanisms. These chemical mechanisms involve the interaction of endogenous/exogenous agonists (acetylcholine, bradykinin, and ATP) with the specific receptors on the endothelial cells. In exercise, the efferent nerve neuromuscular junctions are the physiological source of acetylcholine.(40) Interstitial bradykinin content is increased in the contraction during strenuous exercise.(41) Physical impact on the vascular wall stimulates NO release in the blood vessel.(42) Although the mechanisms of shear stress-induced NO synthesis are not completely understood, increased exercise-induced shear stress stimulates the release of a vasorelaxation factor (NO) and augments NOS3 and NOS1 expressions.(43) Moreover, physical activity increases testosterone level in the blood.(44) Testosterone induced NO generation(45) and vascular relaxation via NO generation.(46) Therefore, in this intervention study, elevation of NO2− may be involved in the up-regulation of NOS3 activity inducing higher levels of testosterone in vascular endothelial cells.
Serum l-arginine levels, the ratio of l-arginine/l-citrulline + l-ornithine, the ratio of l-arginine/l-citrulline, and the ratio of l-arginine/l-ornithine were reduced by the intervention of exercise training. Arginase activity in the cross-sectional study increased in parallel with the increase in degree of exercise.
S-nitrosylation of cysteine residues in the arginase protein molecule by up-regulated NO augment arginase activity.(33) Increased arginase activity may consume l-arginine and up-regulated NOS may consume l-arginine. Therefore, the reduction of l-arginine levels after exercise intervention is understandable.
Physical exercise induced a transient increase in reactive oxygen species (ROS) from mitochondria, and the increased ROS induce the nuclear transcription coactivator, PGC1α/β, and activate several nuclear transcription factors, including PPARγ, and their targets, superoxide dismutase (SOD)1, SOD2, glutathione peroxidase (GPx)1, and catalase (CAT), resulting in the induction of endogenous ROS defense enzymes in skeletal muscle.(47) Exercise training for 6.5 weeks increased SIRT3 and SOD2 via AMPK activation in skeletal muscle.(48) Moderate exercise induced SOD activity.(49) Moreover, induction up-regulated PGC1α/β, which activated the SIRT3 gene and resulted in an increase in GPx1, SOD, and catalase via FOXO3α.(50) Therefore, the up-regulation of GPx1, catalase, and SIRT3 mRNA in peripheral blood monocytes after exercise training intervention supported the involvement of ROS, PGC1α/β, and AMPK and supported an increase in anti-oxidative capacity. It also suggests that mRNA in peripheral blood monocytes is useful in observing the effect of exercise training, even though most previous studies used the mRNA in skeletal muscle.
Although the findings here are significant, several limitations in the study should be noted. First, the sample size was small. Second, casual relationships could not be determined because one of the studies was a cross-sectional study. Third, this intervention study did not have a control study. Forth, some reporting bias may have been introduced because the information on exercise training was obtained via self-reported questionnaires. Fifth, for an analysis of exercise training, information about clerical staff or physical labor is needed.
The positive association of exercise with arginase activity in the cross-sectional study and decreased l-arginine, decreased diastolic blood pressure, and increased NO2− in the intervention study may suggest that exercise training supports the reduction of blood pressure via increase in NO; and the up-regulation of catalase, GPx1, and SIRT3 mRNA suggests exercise training is a prerequisite for the protection of cells from oxidative stress and the support of an elongated life span.
Acknowledgments
Many thanks for staff support and kind gifts of several chemicals from the OSK Company in Okayama City.
Funding Statement
This work was supported in part by JSPS KAKENHI Grant Number JP25870977.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovasc Res. 2011;89:499–506. doi: 10.1093/cvr/cvq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens. 2010;24:175–182. doi: 10.1038/jhh.2009.51. [DOI] [PubMed] [Google Scholar]
- 3.Duncan JJ, Farr JE, Upton SJ, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA. 1985;254:2609–2613. [PubMed] [Google Scholar]
- 4.Nelson L, Jennings GL, Esler MD, Korner PI. Effect of changing levels of physical activity on blood-pressure and haemodynamics in essential hypertension. Lancet. 1986;2:473–476. doi: 10.1016/s0140-6736(86)90354-5. [DOI] [PubMed] [Google Scholar]
- 5.Martinez DG, Nicolau JC, Lage RL, et al. Effect of long-term exercise training on autonomic control in myocardial infarction patients. Hypertension. 2011;58:1049–1056. doi: 10.1161/HYPERTENSIONAHA.111.176644. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S. Nitric oxide in the vasculature: physiolgy and pathophysiology. Ann N Y Acad Sci. 1977;811:60–67. doi: 10.1111/j.1749-6632.1997.tb51989.x. [DOI] [PubMed] [Google Scholar]
- 7.Nosarev AV, Smagliy LV, Anfinogenova Y, Popov SV, Kapilevich LV. Exercise and NO production: relevance and implications in the cardiopulmonary system. Front Cell Dev Biol. 2014;2:73. doi: 10.3389/fcell.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancia G, Bombelli M, Facchetti R, et al. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 9.Lenfant C, Chobanian AV, Jones DW, Roccella EJ, Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7): resetting the hypertension sails. Hypertension. 2003;41:1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 10.Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008;586:3511–3534. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyakova EY, Kapilevich LV, Shylko VG, Popov SV, Anfinogenova Y. Physical exercise associated with NO production: signaling pathways and significance in health and disease. Front Cell Dev Biol. 2015;3:19. doi: 10.3389/fcell.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furchgott RF, Zawadzki JV. The Obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Lugg JA, Gnozález-Cadavid NF, Rajfer J. The role of nitric oxid in erectile function. J Androl. 1995;16:2–4. [PubMed] [Google Scholar]
- 15.Kobizk L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 16.Kobizk L, Stringer B, Balligand JL, Reid MB, Stamler JS. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem Biophyc Res Commun. 1995;211:375–381. doi: 10.1006/bbrc.1995.1824. [DOI] [PubMed] [Google Scholar]
- 17.Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol (1985) 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- 18.Lira VA, Soltow QA, Long JH, Betters JL, Sellman JE, Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E1062–E1068. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- 19.Lira VA, Brown DL, Lira AK, et al. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588 (Pt 18):3551–3566. doi: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijkamp FP, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther. 1995;329:81–96. [PubMed] [Google Scholar]
- 21.Lambertucci RH, Silveira Ldos R, Hirabara SM, Curi R, Sweeney G, Pithoin-Curi TC. Effects of moderate electrical stimulation on reactive species production by primary rat skeletal muscle cells: cross talk between superoxide and nitric oxide production. J Cell Physiol. 2012;227:2511–2518. doi: 10.1002/jcp.22989. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Ming XF. Arginase: the emerging therapeutic target for vascular oxidative stress and inflammation. Front Immunol. 2013;4:149. doi: 10.3389/fimmu.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceylan E, Aksoy N, Gencer M, Vural H, Keles H, Selek S. Evaluation of oxidative-antioxidative status and the L-arginine-nitric oxide pathway in asthmatic patients. Respir Med. 2005;99:871–876. doi: 10.1016/j.rmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ogino K, Obase Y, Takahashi N, et al. High serum arginase I levels in asthma: its correlation with high-sensitivity C-reactive protein. J Asthma. 2011;48:1–7. doi: 10.3109/02770903.2010.528496. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Ogino K, Takemoto K, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L17–L24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- 26.Kövamees O, Shemyakin A, Checa A, et al. Arginase inhibition improves microvascular endothelial function in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:3952–3958. doi: 10.1210/jc.2016-2007. [DOI] [PubMed] [Google Scholar]
- 27.Leu SY, Wang SR. Clinical significance of arginase in colorectal cancer. Cancer. 1992;70:733–736. doi: 10.1002/1097-0142(19920815)70:4<733::aid-cncr2820700403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Kovameees O, Shemyakin A, Eriksson M, Angelin B, Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. J Intern Med. 2016;279:477–484. doi: 10.1111/joim.12461. [DOI] [PubMed] [Google Scholar]
- 29.Ogino K, Takahashi N, Takigawa N, Obase Y, Wang DH. Association of serum arginase I with oxidative stress in a healthy population. Free Radic Res. 2011;45:147–155. doi: 10.3109/10715762.2010.520318. [DOI] [PubMed] [Google Scholar]
- 30.Ogino K, Wang DH, Kubo M, et al. Association of serum arginase I with L-arginine, 3-nitrotyrosine, and exhaled nitric oxide in healthy Japanese workers. Free Radic Res. 2014;48:137–145. doi: 10.3109/10715762.2013.842979. [DOI] [PubMed] [Google Scholar]
- 31.Ogino K, Murakami I, Wang DH, et al. Evaluation of serum arginase I as an oxidative stress biomarker in a healthy Japanese population using a newly established ELISA. Clin Biochem. 2013;46:1717–1722. doi: 10.1016/j.clinbiochem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Cui W, Taub DD, Gardner K. qPrimerDepot: a primer database for quantitative real time PCR. Nucleic Acids Res. 2007;35:D805–D809. doi: 10.1093/nar/gkl767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40:D1144–D1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santhanam L, Lim HK, Lim HK, et al. Inducible No synthase depdendent S-nitrosylation and activation of arginase 1 contribute to age-related endothelial dusfuntion. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrin M, Miquet-Alfonsi C, Berthelot A, Mazzolai L, Laurant P. Long-term swimming exercise does not modulate the Akt-dependent endothelial nitric oxide synthase phosphorylation in healthy mice. Can J Physiol pharmacol. 2011;89:72–76. doi: 10.1139/y10-107. [DOI] [PubMed] [Google Scholar]
- 36.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 1985. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 37.Lefer DJ. Nitrite therapy for protection against ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2006;290:F777–F778. doi: 10.1152/ajprenal.00470.2005. [DOI] [PubMed] [Google Scholar]
- 38.Zweier JL, Wang P, Samouuilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 39.van Fassen EE, Bahrami S, Feelish M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingwell BA. Nitric oxide as a metabolic regulator during exercise: effects of training in health and disease. Clin Exp Pharmacol Physiol. 2000;27:239–250. doi: 10.1046/j.1440-1681.2000.03232.x. [DOI] [PubMed] [Google Scholar]
- 41.Langberg H, Born C, Boushel R, Hellsten Y, Kjaer M. Exercise-induced increase in interstitial bradykinin and adenosine concentrations in skeletal muscle and peritendinous tissue in humans. J Physiol. 2002;542 (Pt 3):977–983. doi: 10.1113/jphysiol.2002.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Persson MG, Wiklund NP, Gustafsson LE. Endogenous nitric oxide in single exhalations and change during exercise. Am Rev Respir Dis. 1993;148:1210–1214. doi: 10.1164/ajrccm/148.5.1210. [DOI] [PubMed] [Google Scholar]
- 43.Whyte JJ, Laughlin MH. The effects of acute and chronic exercise on the vasculature. Acta Physiol (Oxf) 2010;199:441–450. doi: 10.1111/j.1748-1716.2010.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumagai H, Zempo-Miyaki A, Yoshikawa T, Tsujimoto T, Yanaka K, Maeda S. Increased physical activity has a great effect than reduced energy intake on lifestyle modification-induced increases in testosterone. J Clin Biochem Nutr. 2016;58:84–89. doi: 10.3164/jcbn.15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janjic MM, Stojkov NJ, Andric SA, Kostic TS. Anabolic–androgenic steroids induce apoptosis and NOS2 (nitric–oxide synthase 2) in adult rat Leydig cells following in vivo exposure. Reprod Toxicol. 2012;34:686–693. doi: 10.1016/j.reprotox.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Puttabyatappa Y, Stallone JN, Erdul A, et al. Peroxynitrite mediates testosterone-induced vasodilation of microvascular resistance vessels. J Pharmacol Exp Ther. 2013;345:7–14. doi: 10.1124/jpet.112.201947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandaur J, Andersen MA, Kellezi H, et al. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front Physiol. 2015;6:85. doi: 10.3389/fphys.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibuya T, Kuburagi T, Nagai Y, Oshiro S. The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. J Clin Biochem Nutr. 2015;57:44–49. doi: 10.3164/jcbn.15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat Med. 2014;20:709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]