Abstract

Mercury (Hg) isotopic signatures were characterized in polished rice samples from China, U.S., and Indonesia (n = 45). Hg isotopes were also analyzed in paired hair samples for participants from China (n = 21). For the latter, we also quantified the proportion of methylmercury intake through rice (range: 31–100%), and the weekly servings of fish meals (range: 0–5.6 servings/weekly). For these participants, 29% (n = 6) never ingested fish, 52% (n = 11) ingested fish < twice/weekly, and 19% (n = 4) ingested fish ≥ twice/weekly. In rice and hair, both mass-dependent fractionation (MDF, reported as δ202Hg) and mass-independent fractionation (MIF, reported as Δ199Hg) of Hg isotopes were observed. Compared to rice, hair δ202Hg values were enriched on average (±1 standard deviation) by 1.9 ± 0.61‰, although the range was wide (range: 0.45‰, 3.0‰). Hair Δ199Hg was significantly inversely associated with %methylmercury intake from rice (Spearman’s rho = −0.61, p < 0.01, n = 21), i.e., as the proportion of methylmercury intake from rice increased, MIF decreased. Additionally, hair Δ199Hg was significantly higher for participants ingesting fish ≥ twice/weekly compared to those who did not ingest fish or ingested fish < twice/weekly (ANOVA, p < 0.05, n = 21); Overall, results suggest that Hg isotopes (especially MIF) in human hair can be used to distinguish methylmercury intake from rice versus fish.
1. Introduction
Mercury (Hg) is a global pollutant and potent neurotoxin.1 In the environment and during metabolism, Hg undergoes transformations that modify its toxicity.1 Hg is comprised of seven stable isotopes (196–204 amu), which can be used to elucidate processes governing Hg transformations.2,3 All Hg isotopes are subject to mass-dependent fractionation (MDF, reported as δ202Hg), while the highest degree of mass-independent fractionation (MIF) occurs for two odd-isotopes (reported as Δ199Hg and Δ201Hg). MDF has been observed for various abiotic/biotic transformations.4−6 In environmental samples, the MIF isotopic signature is most likely obtained during methylmercury (MeHg) photodegradation or Hg(II) photoreduction.2,3,7
Hg isotopes have been used to track MeHg trophic transfer in aquatic food webs,8−16 and in human fish-eating populations.17−21 Among fish consumers, hair δ202Hg was enriched by ∼2‰ compared to seafood, suggesting MDF occurred during MeHg metabolism.17−21 However, no significant MIF was observed during trophic transfer because photochemical reactions are the primary cause for MIF, as noted above.2,3,7 The absence of MIF during metabolic processes suggests MIF may be used as a tool to trace MeHg sources in food webs.
Fish ingestion is considered the primary exposure pathway for MeHg; however, rice ingestion is also an important dietary source of MeHg.22−26 To the best of our knowledge, just two studies reported Hg stable isotopes in rice, and both were for rice samples from Wanshan, China.27,28 Compared to fish tissue, rice δ202Hg and Δ199Hg values were more negative, as follows. The maximum Δ199Hg value reported for rice was +0.06‰,27 compared to +5.73‰ for freshwater fish,12 while the maximum value for rice δ202Hg was −0.48‰, compared to approximately +1.5‰ for freshwater fish.3 Higher δ202Hg and Δ199Hg values for fish are attributed to enhanced photochemical degradation of Hg in the water column, which differs from flooded paddy soil.2,3,27,28
In the present study, we characterized Hg isotopes in polished rice samples from three countries, including China, U.S., and three artisanal and small-scale gold mining (ASGM) locations in Indonesia. In addition, Hg isotopes are reported for hair samples from 21 pregnant mothers in China, who also donated rice samples from home, i.e., hair/rice samples were paired. For the entire cohort of pregnant mothers, we previously reported rice ingestion comprised on average 79% (median: 88%) of dietary MeHg intake, while fish ingestion comprised on average 21% (median: 12%) of MeHg intake (n = 398 mothers).23 Total Hg (THg) and/or MeHg concentrations for rice were previously reported for locations in China23,29 and the U.S..30
For Hg isotopes, we hypothesize that rice Hg MDF will differ between ASGM and non-ASGM sites due to differences in the environmental Hg sources. For hair and rice MDF, we hypothesize that trophic transfer may differ from other studies among seafood consumers17−21 due to potential differences in Hg speciation in rice and seafood, and/or differences in the metabolism of rice and seafood.24 Lastly, we hypothesize that hair MIF may distinguish the proportion of MeHg intake from rice versus fish.
2. Materials and Methods
2.1. Rice Collection and Polishing
Rice samples were from Daxin, China (n = 21),23 Wanshan, China (n = 8),29 Arkansas, U.S. (n = 3),30 and three ASGM (n = 13) locations in Indonesia (n = 45 total rice samples). Daxin is located in Guangxi province, and this area is considered noncontaminated for Hg.23 Wanshan is located in Guizhou province, China, the site of the former Wanshan Hg mine, which officially closed in 2002.29 Rice was also cultivated at the University of Arkansas Rice Research and Extension Center.30 There were no local Hg point sources; rice samples for this analysis were harvested from rice fields, which were continuously flooded (n = 2) or drained one time early in the rice cultivation season (n = 1).30 Thirteen rice samples from Indonesia were harvested from villages located in Bombana (n = 1), Cisitu (n = 6), and Pangkal Jaya Village (n = 6). ASGM has been documented in all three sites, including the use of ball-mills, where liquid Hg(0) is mixed with crushed ores to recover gold.31 In two Indonesian sites (Bombana and Cisitu), rice grain was collected from households located approximately 5–7 km from ASGM activities, and these sites are hereafter referred to as the Indonesian background sites. In Pangkal Jaya Village, rice grain was collected directly from paddy fields located next to ASGM activities; hereafter referred to as the Indonesian ASGM site. In all sites, rice samples were retained in this analysis if the rice THg concentration was >10 ng/g, measured using 202Hg isotopic signals (see Section 2.4).
Rice samples from Arkansas and the ASGM site in Indonesia were dehulled and polished as previously described,30 using different polishing discs for high- and low-Hg rice. Rice samples from China and the Indonesian background sites were already hulled and polished. All rice samples were ground to a powder, using two different coffee grinders for high- and low-Hg rice. In addition, the polisher and grinders were cleaned between samples with ethanol to prevent carry-over of Hg.
2.2. Hair Collection and Washing
In Daxin, China, hair samples were paired with rice samples collected from the same participants (n = 21) (see section 2.1). The following protocols were reviewed and approved by the Institutional Review Boards at the University of South Carolina and XinHua Hospital (China). Pregnant women were recruited at parturition at the Maternal and Child Health Hospital in Daxin county, China. After providing informed consent, mothers donated a hair sample, and a family member brought a rice sample from home. The hair sample was cut from the occipital region using stainless steel scissors, the proximal end was tied with dental floss, and the sample was stored in a plastic bag at room temperature. Rice samples were stored frozen (−26 °C), and then at −80 °C. For the present study, the portion of hair corresponding to the second trimester was analyzed.32 There was insufficient volume of hair to analyze MeHg.
Prior to Hg analysis, hair samples were washed to remove exogenous Hg, using methods previously described.23 Briefly, porcelain dishes were soaked overnight in 1.2 N hydrochloric acid (HCl), then triple-rinsed in double-distilled H2O (DDI-H2O) (>18.0 MΩ cm–1). Hair samples were weighed into acid-washed porcelain dishes, 50 mL of 0.1% (v/v) 2-mercaptoethanol were added, samples were gently shaken for 1 h, triple-rinsed using DDI-H2O, air-dried overnight in a biosafety cabinet equipped with a HEPA (high efficiency particulate air) filter (Baker Company, Sanford, ME), and then double-bagged to prevent further Hg contamination.
2.3. Food Frequencies and Dietary MeHg Intake
During their hospital stay, mothers filled out a 102-item semiquantitative food frequency questionnaire including categories for rice, pork, other meats, eggs, fruits, vegetables, and seven varieties of fish (ocean fish, freshwater fish, shrimp, eel, other shellfish, snails, and crab), reflecting food intake during the third trimester.33 Mothers chose from eight options ranging from “never” to “≥ twice per day”, which were converted to servings/day, as previously described.23 Mothers selected the portion size (g/serving) for rice from three pictures containing known quantities of rice and/or actual bowls. The portion size for ocean fish and freshwater fish was 170 g/serving, while the portion size for other fish/shellfish varieties was 100 g/serving.23 Rice MeHg concentrations were determined (see section 2.4). THg concentrations were quantified for freshwater fish tissue purchased in Daxin markets (n = 13) (see section 2.4), and fish tissue THg concentrations were determined for the other six varieties of fish/shellfish from a comprehensive literature search (SI Table S1).23 Rice MeHg intake and fish MeHg intake were calculated using the following equations; for eq 2, we assumed fish tissue THg was approximately equivalent to fish tissue MeHg.1
| 1 |
| 2 |
Total dietary MeHg intake (μg/day) was determined by adding eqs 1 and (2); the proportion of dietary MeHg intake attributed to rice or fish was also obtained.
2.4. Hg Analyses
THg and MeHg
THg concentrations for fish, hair, and most rice samples (n = 34/45) were measured using U.S. Environmental Protection Agency (EPA) Method 7473,34 including thermal decomposition, amalgamation, and quantification by atomic absorption spectrophotometry (Lumex Model RA-915+/PYRO-915+, St. Petersburg, Russia). A subset of rice samples (n = 11) were measured using cold acid digestion (EPA 1631), as follows.35 Rice samples (0.5 g) were digested overnight in 40 mL borosilicate glass bottles with Teflon-lined lids using 2.5 mL of HCl and nitric acid (HNO3) (4:1 HCL:HNO3 v/v). Then samples were oxidized overnight using 0.2 N bromine monochloride (BrCl) (0.5%). The following day, hydroxylamine hydrochloride (0.050 mL) was added to neutralize BrCl, then Hg(II) was reduced to Hg(0) using stannous chloride, and Hg was purged onto gold traps. THg concentrations were quantified using the Merx-T and cold vapor atomic fluorescence spectrometry (CVAFS) (Brooks Rand Instruments, Seattle, WA).35
Rice MeHg was extracted using methods from Liang et al.36 and extracts were analyzed using EPA Method 1630.37 Briefly, ∼0.5 g rice were weighed into a 50 mL polypropylene vial and digested in 2 mL of 25% (w/v) potassium hydroxide-methanol in a 75 °C oven. Then 6 mL of dichloromethane and 1.5 mL of HCl were added, samples were shaken, centrifuged (4000 rpm = 3000g, 30 min), and phases were separated. The organic layer was transferred to a preweighed 50 mL polypropylene vial, then 35 mL of DDI-H2O were added, and vials were heated for 1.5 h at 60–70 °C to remove dichloromethane. MeHg extracts were analyzed following ethylation with sodium tetraethylborate, purge and trap onto Tenax traps, and quantification by gas chromatography-CVAFS (Brooks Rand Instruments, Seattle, WA).37
For quality assurance/quality control for all data sets, see SI Table S2. For THg, recovery of five certified reference materials averaged 74–110% (n = 35) and the relative percent difference between replicates averaged 8.5% (n = 87). For MeHg, the average recovery of matrix spikes and two certified reference materials ranged from 69 to 96% (n = 103), and the relative percent between replicates averaged 13% (n = 74). The minimum detection levels were 0.0095 μg/g for hair THg, 0.002 ng/g for rice MeHg, 0.5 ng/g for rice THg (using EPA 7473), and 0.01 ng/g for rice THg (using EPA 1631). All results exceeded the detection levels.
Hg Isotopes
Rice (∼0.4 g) and hair samples (∼0.015 g) were digested in aqua regia [rice: 5 mL HNO3, hair: 1.5 mL of HNO3:HCl (3:1 v/v)] in a water bath at 85–95 °C for 1.5–2.5 h,17,28 then 0.25 mL of 0.2 N BrCl were added at least 12 h before analysis to convert all Hg to Hg(II). Rice and hair digests were diluted using DDI-H2O to a final concentration of 0.3–1.0 ng/mL and ∼1 ng/mL, respectively, with 5–20% acid concentration. Just before analysis, hydroxylamine hydrochloride (0.05 mL) was added to remove excessive BrCl. Standard reference materials [IAEA-086 (human hair) and TORT-2 (lobster)] were prepared and analyzed using appropriate protocols, described above. THg concentrations were monitored by multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS) using 202Hg signals, which yielded mean recoveries for rice, hair, and standard reference materials of 100% (median: 98%, range: 77–134%, n = 73). The sensitivity for 202Hg was 0.5–0.6 V per ng/mL Hg, and the 202Hg signals were <0.04 V for blank solutions.
Hg isotopes were analyzed using a Neptune Plus MC-ICP-MS, as described by Yin et al.38 Briefly, Hg(II) extracts were continuously mixed and reduced to Hg(0) with 3% tin chloride using a cold vapor generator, and volatile Hg(0) was separated by a frosted glass phase separator and introduced to the MC-ICP-MS with argon gas. Instrumental mass bias was corrected using an internal thallium (Tl) standard (NIST SRM 997, 20 ng/g Tl in 3% HCl) and sample-standard bracketing. The Hg concentrations and acid matrices of the bracketing standard (NIST SRM 3133) differed by <10% compared to the neighboring samples. MDF is expressed using the δ202Hg notation (eq 3), while MIF is expressed as the difference between the measured δxxxHg value, the value predicted based on MDF, and the δ202Hg value (eqs 4-6).39
| 3 |
| 4 |
| 5 |
| 6 |
The UM-Almadén secondary standard solutions with similar Hg concentrations (0.3, 0.5, and 1.0 ng/mL) and acid matrices (10%) were measured once every 10 samples. Data uncertainties reflect the larger values of either the external precision of the replication of the UM-Almadén standard or the measurement uncertainty of standard reference materials. For UM-Almadén (n = 18) and standard reference materials, the overall average and uncertainty (±2 SD) agreed with previously reported results.15,17,18,20,39 See SI Table S3 for all Hg isotope data.
Rice and hair THg and MeHg analyses were completed at the University of South Carolina, fish tissue THg was analyzed at Beijing Lumex Analytical Co. Ltd., China, and stable Hg isotopes were analyzed at the University of Wisconsin-Madison’s State Laboratory of Hygiene.
2.5. Statistics
Bivariate associations between continuous variables were determined using Spearman’s correlation or Pearson’s correlation; for the latter, a log10-transformation was applied if the data elements were right-skewed. Differences between groups were compared using the Kruskal–Wallis test (for skewed variables) or one-way analysis of variance (ANOVA) (for normally distributed variables). Following ANOVA, pairwise differences were assessed using Sidak’s test for multiple comparisons, and these p-values were reported in the text. Simple linear regression was used to assess the strength of the relationship between Δ199Hg and Δ201Hg.2 An alpha-level of 0.05 was chosen as guide for significance. Stata 9.2 (College Station, Texas) and the R-platform were used for all statistical analyses.
3. Results and Discussion
3.1. Rice Hg
Concentrations of rice THg, rice MeHg, and rice percent MeHg (of THg) are reported in Table 1 and SI Table S3. Rice THg concentrations in the Indonesian ASGM site averaged 6.1–10 times higher compared to the four other sites, including two Indonesian background sites (Kruskal–Wallis, p < 0.01). Although ASGM was practiced at all three Indonesian sites, THg concentrations were elevated for rice harvested from paddies next to ASGM activities, and not from paddies located approximately 5–7 km away, suggesting contamination of rice paddies was somewhat localized. Rice MeHg concentrations averaged 1.5–3.5 times higher in Arkansas, compared to the other four sites (Kruskal–Wallis, p < 0.05). In addition, rice %MeHg (of THg) averaged 1.2–9.7 times higher in Arkansas compared to the other four sites (Kruskal–Wallis, p < 0.001). Although flooding-reflooding may result in higher soil MeHg,24 the Arkansas rice samples were from fields that were continuously flooded or drained one time early in the season (Section 2.1), which was similar to the hydrology used in China and Indonesia. Instead, higher rice MeHg in Arkansas possibly reflected differences in soil organic content, iron content, or other environmental factors that influenced microbial Hg methylation.24 Rice THg and MeHg were positively correlated using Spearman’s correlation (Spearman’s rho = 0.41, p < 0.01, n = 45), and using Pearson’s correlation, when variables were log10-transformed (Pearson’s rho = 0.37, p = 0.02, n = 45).
Table 1. Summary Statistics for Mercury Concentrations in Polished Rice (n = 45) and Hair (n = 21)a.
| sample size (n) | mercury sources | THg (ng/g) Mean ±1 SD (range) | MeHg (ng/g) Mean ±1 SD (range) | %MeHg (of THg) Mean ±1 SD (range) | ||
|---|---|---|---|---|---|---|
| rice | all | 45 | NA | 32 ± 46 | 8.6 ± 4.5 | 49 ± 28 |
| (8.2–200) | (1.8–22) | (4.8–96) | ||||
| rice | Daxin, Chinab | 21 | background | 14 ± 2.8 | 8.4 ± 2.7 | 64 ± 21 |
| rice | Wanshan, Chinab | 8 | former Hg mine | (9.5–21) | (5.3–15) | (28–96) |
| 20 ± 9.5 | 8.0 ± 3.6 | 42 ± 13 | ||||
| rice | Bombana and Cisitu, Indonesia | 7 | background | (10–38) | (2.9–13) | (23–60) |
| 15 ± 5.6 | 4.6 ± 3.8 | 28 ± 14 | ||||
| (8.9–26) | (1.8–12) | (13–46) | ||||
| rice | Pangkal Jaya Village, Indonesia | 6 | ASGM | 140 ± 44 | 11 ± 5.2 | 8.1 ± 4.7 |
| rice | Arkansas, U.S.b | 3 | background | (100–200) | (5.7–18) | (4.8–17) |
| 18 ± 8.8 | 16 ± 7.2 | 91 ± 5.3 | ||||
| (8.2–25) | (7.8–22) | (86–96) | ||||
| hair | Daxin, China | 21 | background | 1500 ± 570 | NA | NA |
| (trimester 2) | (1030–3050) |
Excluding the Indonesian ASGM site, all rice THg concentrations were within the range reported for global nonpolluted sites (range: 1.0–45 ng/g),24 including rice samples from Wanshan, China (Table 1). Many studies from Wanshan reported higher rice THg concentrations; however, most rice samples were collected from paddies near the former Hg mine or near active Hg smelters.24 Rice samples included in this analysis were originally from a feasibility pilot among pregnant women, who lived throughout Wanshan District, including less-contaminated areas.29 For all rice samples, 24% (=11/45) of the rice MeHg concentrations were within the range reported for global nonpolluted sites (range: 0.86–5.8 ng/g),24 including one sample from the Indonesian ASGM site.
3.2. Rice MDF and MIF
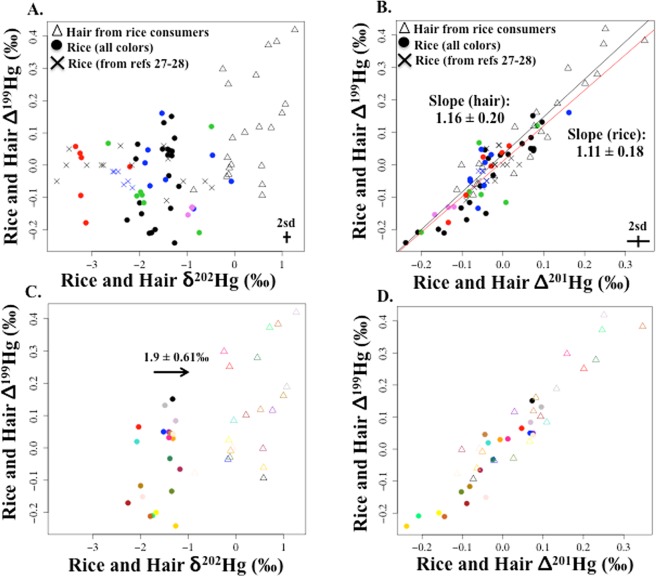
Rice δ202Hg averaged (±1 SD) −1.69 ± 0.54‰ (range: −3.3‰, −0.07‰, n = 45) (Figure 1ac, SI Table S3). Rice Δ199Hg and Δ201Hg averaged (±1 SD) −0.04 ± 0.11‰ and −0.05 ± 0.09‰, respectively (range for both: −0.24‰, 0.16‰, n = 45) (Figure 1bd). The range for MIF (0.40‰) was narrow compared to the range for δ202Hg (3.23‰). No significant MIF of Δ200Hg was observed for rice (average ±1 SD: 0.00 ± 0.05‰). Rice Hg isotopes from this study were comparable to values for rice from Feng et al.27 and Yin et al.,28 which were included in Figures 1ab.
Figure 1.
For rice and hair samples: (a) Δ199Hg versus δ202Hg and (b) Δ199Hg versus Δ201Hg, and simple linear regression (slope ±2 standard error) for rice (red line) and hair (black line) from this study. In graphs (a) and (b), rice from this study = closed circles (n = 45) and hair from this study = open triangles (n = 21). Legend for closed circles: black (Daxin, China), blue (Wanshan, China), green (Indonesian background sites), red (Indonesian artisanal and small scale gold mining sites), and pink (Arkansas, U.S.). Rice Hg isotope values from other studies include black ×’s27 and blue ×’s.28 Representative values for 2 standard deviations (sd) of analytical uncertainty measured for this study are shown in (a) and (b). Figures (c) and (d) are for the same parameters as in (a) and (b), respectively, including just the paired rice samples (closed circles) and hair samples (open triangles) from Daxin, China (n = 21 pairs) with corresponding colors for each pair. Figure 1c includes the difference between hair and rice δ202Hg (average ±1 SD = 1.9 ± 0.61‰).
Rice samples from the Indonesian ASGM site had significantly lower δ202Hg values (mean ±1SD: −3.1 ± 0.43‰, range: −3.3‰, −2.2‰, n = 6), compared to the other four sites, including the Indonesian background sites (mean ±1SD: −1.5 ± 0.54‰, range: −2.7‰, −0.07‰, n = 39) (ANOVA, p < 0.0001 for all pairwise associations), while no significant differences were observed between sites for both Δ199Hg and Δ201Hg (ANOVA, p = 0.42–1.0 for all pairwise associations). δ202Hg values observed in this study for the Indonesian ASGM site were similar to δ202Hg values for rice samples from two active Hg mining sites in Wanshan, China.27
MDF occurs during incorporation of Hg by rice;28 however, uptake of Hg was not expected to differ between these locations. Instead, significantly lower δ202Hg values in the Indonesian ASGM rice samples suggested higher incorporation of Hg(0) by two potential pathways, i.e., through the atmosphere or through the soil, as follows. ASGM miners use liquid Hg(0) to amalgamate gold particles, and the Hg-gold amalgamate is heated at a high temperature to release Hg(0). Rice paddies located next to ASGM activities were also possibly irrigated with Hg-laden runoff. Estrade et al.4 reported heating of liquid Hg(0) volatilized the lighter Hg isotopes, i.e., for vapor collected following evaporation of liquid Hg(0), δ202Hg values averaged (±2 SE) −6.65 ± 0.28‰ at 22 °C, while at 100 °C δ202Hg values for vapor averaged (±2 SE) −0.79 ± 0.22‰. Significantly lower rice δ202Hg values in the ASGM sites compared to the other four locations suggested higher incorporation of liquid Hg(0), which was not likely incinerated, and instead, was accumulated from the paddy soil. Alternatively, significantly lower δ202Hg values possibly reflected higher incorporation of atmospheric Hg. Lower δ202Hg values were reported in precipitation collected near coal-fired power plants compared to distant sites,40 suggesting more negative δ202Hg values in polluted air. Most Hg (∼80%) in rice seeds is accumulated from the soil, while a smaller fraction of Hg originates from the atmosphere.28 Both pathways possibly contributed to significantly lower δ202Hg values in the ASGM site compared to the other locations.
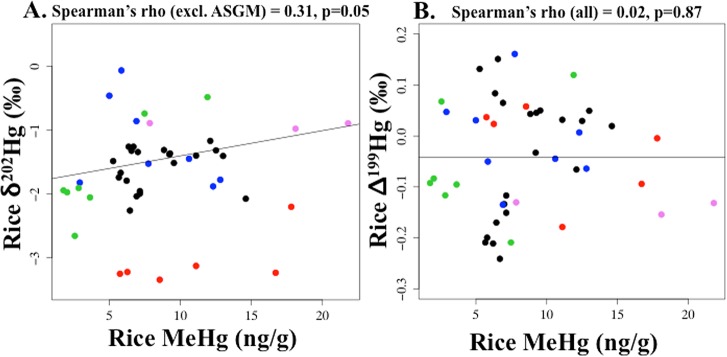
Rice δ202Hg was positively correlated with rice MeHg, excluding rice from the ASGM Indonesian site (Figure 2a, n = 39). When the ASGM site was included, Spearman’s correlation was attenuated from 0.31 (p = 0.05, n = 39) to 0.16 (p = 0.30, n = 45). This positive association (excluding the ASGM site) was possibly due to fractionation of δ202Hg during microbial Hg(II) methylation, reflecting preferential microbial methylation of lighter isotopes.6,41,42 Conversely, rice Δ199Hg was not correlated with rice MeHg (Figure 2b). This was not surprising because MIF is not produced by biotransformations, including microbial methylation/demethylation.5,13,41 Using rice %MeHg (of THg) instead of rice MeHg, a positive correlation was observed between rice δ202Hg and rice %MeHg (of THg) (when all data were included), while there was no correlation between Δ199Hg and rice %MeHg (of THg) (SI Figure S1), similar to Feng et al.27
Figure 2.
(a) Rice δ202Hg versus rice methylmercury (MeHg) and (b) Rice Δ199Hg versus rice methylmercury (MeHg). For rice: black (Daxin, China), blue (Wanshan, China), green (background sites in Indonesia), red [artisanal and small scale gold mining (ASGM) sites in Indonesia], and pink (Arkansas, U.S.). In Figure 2a, when rice from ASGM sites were included, Spearman’s rho = 0.16, p = 0.30, n = 45.
In environmental samples, the MIF isotopic signature is mainly obtained through MeHg photodegradation or Hg(II) photoreduction.2 The strength of the relationship between 199Hg and 201Hg is used to distinguish between the two mechanisms.2 In the present study, the rice Δ199Hg:Δ201Hg slope (±2 SE) was 1.11 ± 0.18 (r-squared = 0.78, n = 45) (Figure 1b), which was similar to the slope reported for Hg(II) photoreduction experiments (mean ±2 SE: 1.00 ± 0.02).2 This differs from the slope for experimental MeHg photodegradation (1.36 ± 0.02‰, 2 SE),2 which was also observed in fish tissue and biota (Δ199Hg:Δ201Hg slope: 1.26–1.32).2,8,15,16 Results suggested that Hg accumulated in rice grain had undergone Hg(II) photoreduction rather than MeHg photodegradation, which was also reported for rice paddy soil, rice roots, leaves, stems, and seeds (Δ199Hg:Δ201Hg mean: ∼ 1.0) by Yin et al.28
3.3. Hair THg
For 21 pregnant mothers in Daxin, hair THg (trimester 2) averaged (±1SD) 1.5 ± 0.57 μg/g (range: 1.03 μg/g, 3.05 μg/g), which was higher than hair THg (trimester 3) reported for the entire cohort (0.48 ± 0.26 μg/g, range: 0.08 μg/g, 1.70 μg/g, n = 398).23 For this analysis, we included rice samples with THg concentrations >10 ng/g, and therefore retained participants with higher hair THg. Hair THg concentrations for the second and third trimesters were significantly positively correlated, when log10-transformed (Pearson’s rho = 0.47, p < 0.05, n = 21). Using Spearman’s correlation, hair THg concentrations were positively correlated, but not significantly (Spearman’s rho = 0.40, p = 0.08, n = 21).
3.4. Rice and Fish MeHg Intake
For these 21 mothers from Daxin, rice was the main but not exclusive dietary source for MeHg. Most mothers (86%) ate rice daily, averaging 1.8 meals/daily (median: 2.5 servings/daily, range: 0.08–2.5 servings/daily), while mothers ingested on average 1.0 fish meal/weekly (median: 0.21 meals/weekly, range: 0–5.6 meals/weekly), including six mothers (29%) who never ate fish, 11 mothers (52%) who ingested fish < twice/weekly, and four mothers (19%) who ingested fish ≥ twice/weekly (SI Table S3). In this rural inland region, freshwater fish, and shrimp were ingested most frequently (weekly ingestion by 13 and five mothers, respectively), while ocean fish, crab, and snails were ingested weekly by one mother each, and eel was never consumed. Using eqs 1 and (2), the average %MeHg intake from rice was 80% (median: 87%, range: 31–100%), while the average %MeHg intake from fish was 20% (median: 13%, range: 0–69%).
3.5. Hair MDF and MIF
Hair δ202Hg averaged (±1 SD) 0.32 ± 0.54‰ (range: −0.86‰, 1.27‰) (Figure 1a, SI Table S3). Hair Δ199Hg and Δ201Hg averaged (±1 SD) 0.12 ± 0.16‰ (range: −0.09‰, 0.42‰) and 0.07 ± 0.13‰ (range: −0.11‰, 0.35‰), respectively (see Figure 1 for hair Δ199Hg). The range for hair δ202Hg was 2.13‰, while the range for hair Δ199Hg and Δ201Hg was narrow (0.51‰ and 0.46‰, respectively). No significant MIF of 200Hg was observed for hair samples (average ±1 SD: 0.00 ± 0.05‰).
In previous studies among fish consumers, researchers utilized δ202Hg in human biomarkers to investigate biotransformation and accumulation of MeHg.17−21 For δ202Hg, approximately +2‰ increase was reported in hair δ202Hg relative to the dominant seafood for several cohorts, including Bolivian Esse Ejjas native people (offset: 2.0 ± 0.2‰),17 a French cohort (2.2 ± 0.8‰),18 U.S. dentists (∼2‰),20 Faroese whalers (1.75‰),19 and Gulf of Mexico anglers who predominantly consumed ocean fish (offset: 1.98–2.30‰).19 In the latter study, the δ202Hg offset varied from 1.4 to 3.2‰ for consumers of coastal fish, freshwater fish, and shellfish; the authors suggested this range of values potentially reflected differences in MeHg metabolism, discrepancies is dietary recall, or lower %MeHg (of THg) for these varieties of seafood compared to ocean fish or pilot whale.19
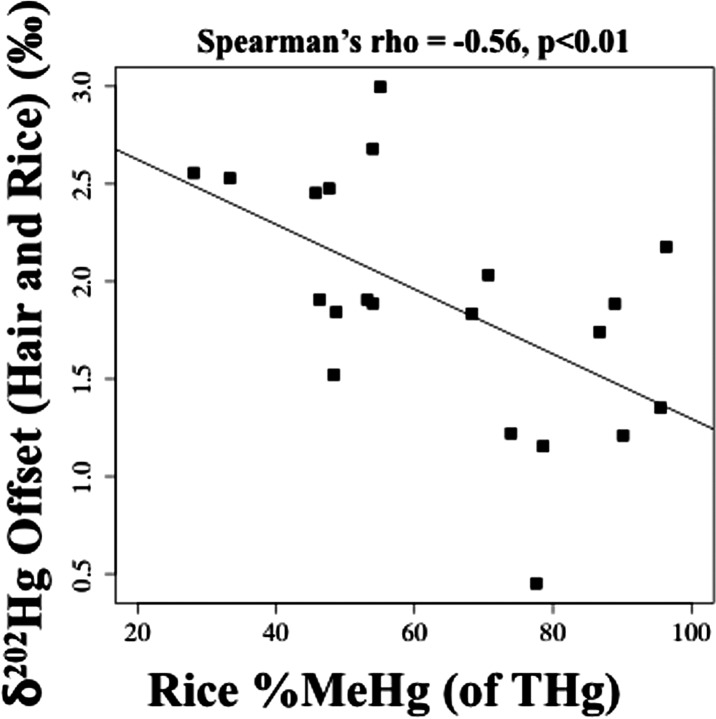
In the present study, the mean difference (±1 SD) in δ202Hg values between paired hair and rice samples was 1.9 ± 0.61‰ (range: 0.45‰, 3.0‰) (Figure 1c). The offset range was wider than observed for most previous studies among seafood consumers,17−20 which possibly reflected ingestion of fish, in addition to rice. However, for mothers reporting no fish consumption (n = 6), the δ202Hg offset averaged 1.7 ± 0.91% and the range did not change (range: 0.45‰, 3.0‰). Variability in the δ202Hg offset possibly reflected differences in rice %MeHg (of THg), which ranged from 28 to 96% for Daxin (SI Table S3), as previously suggested.19 From Figure 2a, δ202Hg increased as rice MeHg increased; therefore higher rice %MeHg (of THg) would likely be more enriched δ202Hg. We found the δ202Hg offset and rice %MeHg (of THg) were significantly inversely correlated (Spearman’s rho = −0.56, p < 0.01, n = 21) (Figure 3). This was consistent with the premise that MeHg is more enriched in δ202Hg compared to inorganic Hg. Similar results were reported for tissues in whales and seals,13 and for invertebrates in upland forest sites.43 Preferential uptake of MeHg and excretion of inorganic Hg combined with in vivo demethylation of MeHg in the human body may lead to the larger offset in δ202Hg values observed here among consumers that have higher inorganic Hg in their rice.
Figure 3.
δ202Hg offset (=hair δ202Hg - rice δ202Hg) versus rice %methylmercury (MeHg) [of total mercury (THg)] (n = 21).
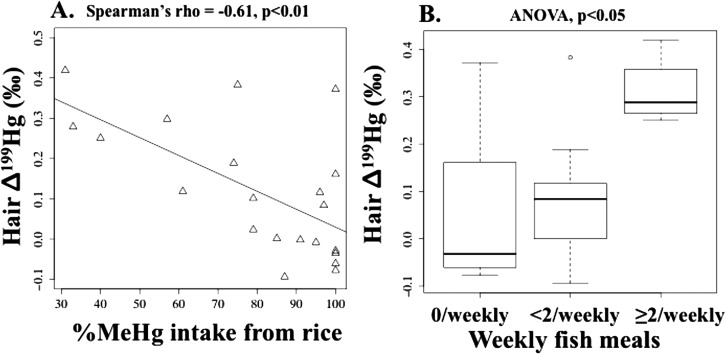
For Δ199Hg (as well as Δ201Hg), researchers reported no significant differences between the MIF signature of hair and the dominant seafood, and suggested the MIF isotopic signature was conserved during trophic transfer between seafood and seafood consumers.17−21 However, from Figure 1c, six participants had higher Δ199Hg values for hair compared to rice. Of the six participants, four ingested fish ≥ twice/weekly, one ingested fish < twice/weekly, and one did not ingest fish. We did not retain fish tissue for measurement of Hg isotopes; however, as noted in the Introduction, the magnitude for Δ199Hg values in fish tissue is much higher compared to rice (maximum Δ199Hg for rice: + 0.16‰ from this study, SI Table S3; maximum for freshwater fish: + 5.73‰12). Using the proportion of MeHg intake from rice, we found that hair Δ199Hg was significantly inversely correlated with the %MeHg intake from rice (Spearman rho = −0.61, p < 0.01, n = 21) (Figure 4a). Using the number of fish meals, we also found participants consuming fish ≥ twice/weekly had hair Δ199Hg values that were significantly higher compared to most mothers who did not consume fish or ingested fish less often (ANOVA, p < 0.05 for both, n = 21) (Figure 4b). To interpret, as the proportion of dietary MeHg intake from rice increased, hair MIF decreased; similarly, for mothers ingesting fish ≥ twice/weekly, hair MIF increased compared to mothers ingesting less fish or no fish. Results suggest that Hg isotopes (especially MIF) in human hair can be used to distinguish MeHg intake from rice versus fish. We also considered whether the variability in hair Δ199Hg was due to differences in rice %MeHg (of THg); however, this bivariate association was inverse but nonsignificant (Spearman’s rho = −0.30, p = 0.19, n = 21).
Figure 4.
(a) Hair Δ199Hg versus %methylmercury (MeHg) [of total mercury (THg)] intake from rice, and (b) Hair Δ199Hg versus number of fish meals/weekly (0/weekly, < 2/weekly, ≥ 2/weekly).
In conclusion, stable Hg isotopes were measured in rice and hair samples. Although rice MeHg concentrations are lower compared to fish, rice ingestion is an important dietary source of MeHg.24 The Hg isotopic composition in rice differs from fish, reflecting different Hg accumulation pathways.28 In this study, rice δ202Hg values were significantly lower for rice from the Indonesian ASGM site compared to other locations, including other Indonesian sites 5–7 km away, potentially reflecting uptake of Hg(0) through the soil or atmosphere. For rice consumers, the average offset (1.9‰) between rice and hair δ202Hg was similar to other studies among seafood consumers.17−20 However, the offset range (range: 0.45‰, 3.0‰) was wider in our study, which was likely due in part to the range of values for rice %MeHg (of THg) (range: 28–96%). In addition, Δ199Hg was inversely correlated with the %MeHg intake from rice, and significantly higher for participants ingesting fish meals ≥ twice/weekly, compared to participants who did not ingest fish or ingested fish less frequently, suggesting the MIF isotopic signature was conserved, which was also reported for seafood consumers.17−20 Therefore, the Hg MIF isotopic signature may be used to distinguish between these two dietary sources of MeHg (rice and fish). These results may be useful for future studies concerning MeHg exposure among rice consumers.
Acknowledgments
We thank Dr. Merle Anders from the University of Arkansas Rice Research & Extension Center for assistance with rice cultivation, as well as three anonymous reviewers, who provided constructive comments. This research was supported in part by grants to S.E.R. from the U.S. National Institute of Environmental Health Sciences (R15 ES022409 and R21 ES026412) and the U.S. National Institutes of Health Loan Replacement Program (L30 ES023165). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health. The study sponsors did not play a role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. The authors declare no competing financial interest.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.7b01039.
Fish tissue THg (Table S1), quality assurance for THg and MeHg measurements (Table S2), Hg isotopes for rice and hair (Table S3), and bivariate scatterplots for Hg isotopes (rice δ202Hg and rice Δ199Hg) versus rice %MeHg (of THg) (Figure S1) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Clarkson T. W.; Magos L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36 (8), 609–662. 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Bergquist B. A.; Blum J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 318 (5849), 417–420. 10.1126/science.1148050. [DOI] [PubMed] [Google Scholar]
- Blum J. D.; Sherman L. S.; Johnson M. W. Mercury isotopes in Earth and environmental sciences. Annu. Rev. Earth Planet. Sci. 2014, 42, 249–269. 10.1146/annurev-earth-050212-124107. [DOI] [Google Scholar]
- Estrade N.; Carignan J.; Sonke J. E.; Donard O. F. X. Mercury isotope fractionation during liquid-vapor evaporation experiments. Geochim. Cosmochim. Acta 2009, 73 (10), 2693–2711. 10.1016/j.gca.2009.01.024. [DOI] [Google Scholar]
- Krittee K.; Barkay T.; Blum J. D. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta 2009, 73 (5), 1285–1296. 10.1016/j.gca.2008.11.038. [DOI] [Google Scholar]
- Rodriguez-Gonzalez P.; Epov V. N.; Bridou R.; Tessier E.; Guyoneaud R.; Monperrus M.; Amouroux D. Species-specific stable isotope fractionation of mercury during Hg(II) methylation by an anaerobic bacteria (Desulfobulbus propionicus) under dark conditions. Environ. Sci. Technol. 2009, 43 (24), 9183–9188. 10.1021/es902206j. [DOI] [PubMed] [Google Scholar]
- Sonke J. E. A global model of mass independent mercury stable isotope fractionation. Geochim. Cosmochim. Acta 2011, 75 (16), 4577–4590. 10.1016/j.gca.2011.05.027. [DOI] [Google Scholar]
- Donovan P. M.; Blum J. D.; Singer M. B.; Marvin-DiPasquale M. C.; Tsui M. T. K. Isotopic composition of inorganic mercury and methylmercury downstream of a historical gold mining region. Environ. Sci. Technol. 2016, 50 (4), 1691–1702. 10.1021/acs.est.5b04413. [DOI] [PubMed] [Google Scholar]
- Gehrke G. E.; Blum J. D.; Slotton D. G.; Greenfield B. K. Mercury isotopes link mercury in San Francisco Bay forage fish to surface sediments. Environ. Sci. Technol. 2011, 45 (4), 1264–1270. 10.1021/es103053y. [DOI] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Carvan M. J.; Basu N.; Head J. A.; Madenjian C. P.; David S. R. Absence of fractionation of mercury isotopes during trophic transfer of methylmercury to freshwater fish in captivity. Environ. Sci. Technol. 2012, 46 (14), 7527–7534. 10.1021/es300794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Chirby M. A.; Chesney E. J. Application of mercury isotopes for tracing trophic transfer and internal distribution of mercury in marine fish feeding experiments. Environ. Toxicol. Chem. 2013, 32 (10), 2322–2330. 10.1002/etc.2313. [DOI] [PubMed] [Google Scholar]
- Perrot V.; Pastukhov M. V.; Epov V. N.; Husted S.; Donard O. F.; Amouroux D. Higher Mass-independent isotope fractionation of methylmercury in the pelagic food web of Lake Baikal (Russia). Environ. Sci. Technol. 2012, 46 (11), 5902–5911. 10.1021/es204572g. [DOI] [PubMed] [Google Scholar]
- Perrot V.; Masbou J.; Pastukhov M. V.; Epov V. N.; Point D.; Berail S.; Becker P. R.; Sonke J. E.; Amouroux D. Natural Hg isotopic composition of different Hg compounds in mammal tissues as a proxy for in vivo breakdown of toxic methylmercury. Metallomics 2016, 8 (2), 170–178. 10.1039/C5MT00286A. [DOI] [PubMed] [Google Scholar]
- Senn D. B.; Chesney E. J.; Blum J. D.; Bank M. S.; Maage A.; Shine J. P. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the northern Gulf of Mexico. Environ. Sci. Technol. 2010, 44 (5), 1630–1637. 10.1021/es902361j. [DOI] [PubMed] [Google Scholar]
- Sherman L. S.; Blum J. D. Mercury stable isotopes in sediments and largemouth bass from Florida lakes, USA. Sci. Total Environ. 2013, 448 (SI), 163–175. 10.1016/j.scitotenv.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Yin R.; Feng X.; Zhang J.; Pan K.; Wang W.; Li X. Using mercury isotopes to understand the bioaccumulation of Hg in the subtropical Pearl River Estuary, South China. Chemosphere 2016, 147, 173–179. 10.1016/j.chemosphere.2015.12.100. [DOI] [PubMed] [Google Scholar]
- Laffont L.; Sonke J. E.; Maurice L.; Hintelmann H.; Pouilly M.; Sanchez Bacarreza Y.; Perez T.; Behra P. Anomalous mercury isotopic compositions of fish and human hair in the Bolivian Amazon. Environ. Sci. Technol. 2009, 43 (23), 8985–8990. 10.1021/es9019518. [DOI] [PubMed] [Google Scholar]
- Laffont L.; Sonke J. E.; Maurice L.; Monrroy S. L.; Chincheros J.; Amouroux D.; Behra P. Hg speciation and stable isotope signatures in human hair as a tracer for dietary and occupational exposure to mercury. Environ. Sci. Technol. 2011, 45 (23), 9910–9916. 10.1021/es202353m. [DOI] [PubMed] [Google Scholar]
- Li M.; Sherman L. S.; Blum J. D.; Grandjean P.; Mikkelsen B.; Weihe P.; Sunderland E. M.; Shine J. P. Assessing sources of human methylmercury exposure using stable mercury isotopes. Environ. Sci. Technol. 2014, 48 (15), 8800–8806. 10.1021/es500340r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. S.; Blum J. D.; Franzblau A.; Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 2013, 47 (7), 3403–3409. 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Sherman L. S.; Blum J. D.; Basu N.; Rajaee M.; Evers D. C.; Buck D. G.; Petrlik J.; DiGangi J. Assessment of mercury exposure among small-scale gold miners using mercury stable isotopes. Environ. Res. 2015, 137, 226–234. 10.1016/j.envres.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Feng X.; Li P.; Qiu G.; Wang S.; Li G.; Shang L.; Meng B.; Jiang H.; Bai W.; Li Z.; Fu X. Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ. Sci. Technol. 2008, 42 (1), 326–332. 10.1021/es071948x. [DOI] [PubMed] [Google Scholar]
- Hong C.; Yu X.; Liu J.; Cheng Y.; Rothenberg S. E. Low-level methylmercury exposure through rice ingestion in a cohort of pregnant mothers in rural China. Environ. Res. 2016, 150, 519–527. 10.1016/j.envres.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg S. E.; Windham-Myers L.; Cresswell J. E. Rice methylmercury exposure and mitigation: a comprehensive review. Environ. Res. 2014, 133, 407–423. 10.1016/j.envres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg S. E.; Yu X.; Liu J.; Biasini F. J.; Hong C.; Jiang X.; Nong Y.; Cheng Y.; Korrick S. A. Maternal methylmercury exposure through rice ingestion and offspring neurodevelopment: a prospective cohort study. Int. J. Hyg. Environ. Health 2016, 219 (8), 832–842. 10.1016/j.ijheh.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Feng X.; Larssen T.; Qiu G.; Vogt R. D. In inland China, rice, rather than fish is the major pathway for methylmercury exposure. Environ. Health Perspect. 2010, 118 (9), 1183–1188. 10.1289/ehp.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C.; Pedrero Z.; Li P.; Du B.; Feng X.; Monperrus M.; Tessier E.; Berail S.; Amouroux D.. Investigation of Hg uptake and transport between paddy soil and rice seeds combining Hg isotopic composition and speciation. Elem. Sci. Anth. 2016, 4 ( (87), ), DOI 000087. 10.12952/journal.elementa.000087. [DOI] [Google Scholar]
- Yin R.; Feng X.; Meng B. Stable mercury isotope variation in rice plants (Oryza sativa L.) from the Wanshan mercury mining district, SW China. Environ. Sci. Technol. 2013, 47 (5), 2238–2245. 10.1021/es304302a. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. E.; Yu X.; Zhang Y. Prenatal methylmercury exposure through maternal rice ingestion: Insights from a feasibility pilot in Guizhou Province, China. Environ. Pollut. 2013, 180, 1–8. 10.1016/j.envpol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. E.; Anders M.; Ajami N. J.; Petrosino J. F.; Balogh E. Water management impacts rice methylmercury and the soil microbiome. Sci. Total Environ. 2016, 572, 608–617. 10.1016/j.scitotenv.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose-O’Reilly S.; Scheirl R.; Nowak D.; Siebert U.; William J. F.; Owi F. T.; Ismawati Y. A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environ. Res. 2016, 149, 274–281. 10.1016/j.envres.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Loussouarn G.; El Rawadi C.; Genain G. Diversity of hair growth profiles. Int. J. Dermatol. 2005, 44 (Suppl. 1), 6–9. 10.1111/j.1365-4632.2005.02800.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Dibley M. J.; Zhang X.; Zeng L.; Yan H.. Assessment of dietary intake among pregnant women in a rural area of western China. BMC Public Health 2009, 9 ( (222), ), DOI: 10.1186/1471-2458-9-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Method 7473, Mercury in solids and solutions by thermal decomposition, amalgamation and atomic absorption spectrophotometry. Washington, D.C., 2007, www.epa.gov/sites/production/files/2015-12/documents/7473.pdf.
- U.S. Environmental Protection Agency (USEPA). Method 1631, Revision E: Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Washington, D.C., 2002, https://www.epa.gov/sites/production/files/2015-08/documents/method_1631e_2002.pdf.
- Liang L.; Horvat M.; Cernichiari E.; Gelcin B.; Balogh S. Simple solvent extraction technique for elimination of matrix interferences in the determination of methylmercury in environmental and biological samples by ethylation-gas chromatography-cold vapor atomic fluorescence spectrometry. Talanta 1996, 43 (11), 1883–1888. 10.1016/0039-9140(96)01964-9. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Method 1630, Methyl mercury in water by distillation, aqueous ethylation, purge and trap, and cold vapor atomic spectrometry. Washington, D.C., 2001, www.gesamp.org/data/gesamp/files/file_element/a0fb543065c7cbb316e83a54489c0ab2/EPA_1630.pdf.
- Yin R.; Krabbenhoft D.; Bergquist B. A.; Zheng W.; Lepak R. F.; Hurley J. P. Effects of mercury and thallium concentrations on high precision determination of mercury isotope composition by Neptune Plus multiple collector inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2016, 31 (10), 2060–2068. 10.1039/C6JA00107F. [DOI] [Google Scholar]
- Blum J. D.; Bergquist B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 2007, 388 (2), 353–359. 10.1007/s00216-007-1236-9. [DOI] [PubMed] [Google Scholar]
- Sherman L. S.; Blum J. D.; Keeler G. J.; Demers J. D.; Dvonch J. T. Investigation of local mercury deposition from a coal-fired power plant using mercury isotopes. Environ. Sci. Technol. 2011, 46 (1), 382–390. 10.1021/es202793c. [DOI] [PubMed] [Google Scholar]
- Janssen S. E.; Schaefer J. K.; Barkay T.; Reinfelder J. R. Fractionation of mercury stable isotopes during microbial methylmercury production by iron- and sulfate-reducing bacteria. Environ. Sci. Technol. 2016, 50 (15), 8077–8083. 10.1021/acs.est.6b00854. [DOI] [PubMed] [Google Scholar]
- Perrot V.; Bridou R.; Pedrero Z.; Guyoneaud R.; Monperrus M.; Amouroux D. Identical Hg isotope mass dependent fractionation signature during methylation by sulfate-reducing bacteria in sulfate and sulfate-free environment. Environ. Sci. Technol. 2015, 49, 1365–1373. 10.1021/es5033376. [DOI] [PubMed] [Google Scholar]
- Tsui M. T. K.; Blum J. D.; Kwon S. Y.; Finlay J. C.; Balogh S. J.; Nollet Y. H. Sources and transfers of methylmercury in adjacent river and forest food webs. Environ. Sci. Technol. 2012, 46, 10957–10964. 10.1021/es3019836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.