Abstract
Background:
Meehl regarded schizotypy as a categorial liability for schizophrenia that is the product of genes, environment, and gene-environment interactions. We sought to test whether schizophrenia-related genotypes and environmental risk factors predict membership in classes defined by taxometric analyses of positive (cognitive-perceptual), negative (interpersonal), and disorganized schizotypy.
Methods:
Participants (n = 500) completed the Schizotypal Personality Questionnaire (SPQ) and provided information on the following risk factors: cannabis use, pregnancy and obstetric complications, social adjustment, and family history of psychosis. Saliva samples were obtained so that the frequency of single-nucleotide polymorphism (SNP) alleles associated with risk for developing schizophrenia could be determined. Genotyped SNPs were rs1625579 (MIR137), rs7004633 (MMP16), rs7914558 (CNNM2), and rs12966547 (CCDC68). Sets of SPQ items were subject to multiple coherent cut kinetic (CCK) analyses, including mean-above-minus-below-a-cut, maximum covariance, maximum eigenvalue, and latent modes analyses.
Results:
CCK analyses indicated latent taxonicity of schizotypy across the 3 item sets. The cognitive-perceptual class had a base rate of 25%, and membership was predicted by the rs7004633 SNP (odds ratio = 2.33, 95% confidence interval = 1.15–4.72 in adjusted analyses). Poor social adjustment predicted memberships in the interpersonal (16%) and disorganized (21%) classes. Classes were found not to be mutually exclusive.
Conclusions:
Schizotypy is taxonic and schizotypy class membership is predicted by genetic and environmental factors that predict schizophrenia. The findings hold the promise that a more complete understanding of schizotypy as a schizophrenia liability state will come from investigation of other genes and environmental factors associated with schizophrenia.
Keywords: schizophrenia, maximum covariance analysis, psychosis, class structure, taxometrics, MMP16, social adjustment
Introduction
Meehl1,2 conceived of schizotypy as the liability for schizophrenia. Schizotypy is the product of complex gene-environment interactions: A heritable neurointegrative defect, schizotaxia, interacts with the environment, generating outcomes in broad domains of function, including neurological and cognitive performance, social behavior, and perceptual and emotional experience. Within this framework, which is not universally held,3 clinical schizophrenia is a disease end-state phenotype that conceals an enduring subclinical phenotype characterized by poor interpersonal relationships, oddness in expression, perceptual distortions, odd or magical beliefs, suspiciousness of others, and blunted expression of emotion. The latter has also been referred to as schizotypal personality (in contrast to schizotypal personality disorder), psychosis proneness,4schizotypic psychopathology,5,6 and latent schizophrenia.7
Meehl2 regarded schizotaxia as a binary outcome with a general population prevalence of 10%. That is, he attributed the heterogeneity of schizotypy within the general population to an underlying taxonic or qualitative population-level process, not an underlying dimensional or quantitative population-level process.8 The weight of evidence from multivariate taxometric analyses, which pit taxonic models against dimensional ones, strongly favors that schizotypy is taxonic with prevalence rates of 8.5%–10.5% in normal population samples.9–14 Whereas a lot of this evidence derives from measures of self-reported schizotypy, heterogeneity within observational ratings and cognitive measures obtained from biological offspring and within attention and eye-tracking endophenotypes also conforms to a taxonic structure.15–17 Additionally, taxonicity is evident within mixed psychiatric samples,8 including samples without psychosis.18
There is accumulating evidence on the validity of taxonic schizotypy as liability for schizophrenia. Schizotypy class membership has been associated with memory difficulties,8 impaired attention,8,19 increased rates of psychiatric illness in relatives,20,21 family history of schizophrenia,17 and psychological distress.8,19 However, important questions on genetic and environmental predictors of taxonic schizotypy have not been addressed.
Numerous environmental variables predict schizophrenia. Validated risk factors include cannabis use,22–24 pregnancy and obstetric complications,25–29 personality traits including social aversiveness,30–32 and genetic liability. Family history of psychotic illness has been linked to risk for developing schizophrenia,33–36 and it is widely accepted that common alleles have an additive effect in contributing to risk for disorder.6,37–39 Insofar as schizotypy is a liability state for schizophrenia, alleles and environmental factors associated with schizophrenia should predict schizotypy class membership.
Therefore, we sought to test hypotheses on the association of schizotypy class membership with validated environmental and genetic variables including cannabis use, pregnancy and obstetric complications, poor social adjustment, family history of psychosis, and schizophrenia-related genotypes. Genotyping focused on 4 single-nucleotide polymorphisms (SNPs) strongly associated with schizophrenia: rs1625579 (located on MIR137), rs7004633 (MMP16), rs7914558 (CNNM2), and rs12966547 (CCDC68).40 These hypotheses were tested after first testing for taxonicity of positive, interpersonal, and disorganized facets of schizotypy.
Method
Participants
Participants were 500 undergraduates (age M = 20.29, SD = 3.27; 25.20% males) enrolled in introductory courses in psychology with normal or corrected-to-normal vision. Sample characteristics are given in table 1. Having completed participation, volunteers could learn about the study purpose and design and obtain extra course credit based on assessment of this learning. The University of Otago Human Ethics Committee reviewed and approved the study.
Table 1.
Final Sample (n = 430) Demographic and Descriptive Information
| Variable | n | % |
|---|---|---|
| Male | 107 | 24.88 |
| Race | ||
| Caucasian | 377 | 87.67 |
| Asian | 59 | 13.72 |
| Māori/Pacific | 37 | 8.60 |
| African | 2 | 0.47 |
| M | SD | |
| Age | 20.27 | 3.31 |
| Raw SPQ cognitive-perceptual score | 30.01 | 14.34 |
| Raw SPQ interpersonal score | 33.70 | 14.69 |
| Raw SPQ disorganized score | 24.45 | 10.47 |
| Cannabis use | 1.09 | 1.95 |
| Pregnancy and obstetric complications | 0.54 | 0.96 |
| Number of relatives with treated psychosis | 0.08 | 0.29 |
| Social adjustment average score | 1.72 | 0.29 |
| Frequency score for genetic risk | 0.55 | 0.18 |
| rs1625579 | 1.63 | 0.54 |
| rs7004633 | 0.45 | 0.62 |
| rs7914558 | 1.18 | 0.69 |
| rs12966547 | 1.13 | 0.68 |
Note: SPQ, Schizotypal Personality Questionnaire. Participants were able to report membership to more than 1 ethnic group. SPQ scores were calculated without suspiciousness items.
Measures
Schizotypy was assessed using the Likert version of the Schizotypal Personality Questionnaire (SPQ).41,42 Whereas the SPQ was constructed on the basis of DSM-III-R43 Schizotypal Personality Disorder, it is not a diagnostic instrument and is often used for the assessment of schizotypy.44 SPQ item content resembles that of other schizotypy measures, captures many of the behavioral, social, and perceptual phenotypes that characterize schizotypy, can be used to detect latent taxonicity,45 and has fewer items than alternative multifaceted assessment options. The 74 items are rated on a 5-point ordinal scales (strongly disagree to strongly agree) and comprise 3 factor scales as described in the SPQ manual.46 The cognitive-perceptual factor comprises items addressing ideas of reference, odd beliefs, unusual perceptual experiences, and suspiciousness; the interpersonal factor comprises items addressing lack of close friendships, constricted affect, social anxiety, and suspiciousness; and the disorganized factor comprises items addressing eccentric behavior and odd speech. Compared to the binary version of the SPQ,41 the Likert version better captures variability within phenotypes47 and its subscales have higher internal consistency (0.77–0.90 vs 0.58–0.90 in the binary version).42 The Likert SPQ subscales correlate highly with interviewer ratings of schizotypy (0.55–0.80).41 The 2-month test-retest reliability of the total score was reported to be 0.82.41 There is evidence the binary SPQ has a 4-factor structure.48,49 Importantly, taxometric analyses were applied to item-level data, not scale scores to which these psychometric properties relate.
Disingenuous or inattentive responding was assessed with 12 items dispersed across several self-report questionnaires in the study protocol, including the three in the SPQ. In these 12 items, participants were instructed to provide designated responses (eg, Respond to this question by selecting number 4). Noncomplying responses (≥2 of 12) were interpreted as evidence a participant was not reading items or was responding randomly.
Validation Measures
Cannabis Use.
History of cannabis use was assessed by self-report drug use questionnaire. The dependent measure was the number of times used (How many times have you used cannabis?), rated on a 7-point Likert scale (0–1, 2–5, 6–10, 11–15, 16–20, 21–30, 31–40, 41+).
Pregnancy and Obstetric Complications.
Measured complications included gestational bleeding, rhesus incompatibility, diabetes, or preeclampsia during pregnancy; uterine atony, emergency cesarean, or asphyxia during birth; low birth weight (<2500g); short gestation period (<37 wks); and whether the participant had a twin. The presence of any complication was coded 1, with possible scores ranging between 0 and 10.
Family History.
History of psychosis among first- and second-degree biological relatives was assessed with a self-report family history questionnaire. The dependent measure from the family history questionnaire was the number (0–3) of first- or second-degree biological relatives with treated psychosis (schizophrenia, bipolar disorder, and other psychoses).
Social Adjustment.
The Social Adjustment Scale-Self Report (SAS-SR)50 was administered to assess social functioning. Participants responded to a maximum of 34 items addressing their role as a student, use of spare time, familial relationships, and current romantic (close) relationships. Responses are made on varying 5-point Likert scales, and a single 6-point anchored scale. A high score is indicative of poor social adjustment. The SAS-SR has acceptable to good psychometric properties.50–52 The dependent measure was the average score for items on which responses were made.
Genetic Risk.
Genetic risk for schizophrenia was assessed directly through the measurement of 4 SNPs strongly associated with schizophrenia: rs1625579, rs7004633, rs7914558, and rs12966547.40 For each SNP, participants were assigned a risk score of 2 if they were homozygous for the risk allele, 1 if heterozygous, or 0 if homozygous for the nonrisk allele. The final genetic risk was the proportion of risk alleles present (0–1). It was required that participants have data for at least 2 SNPs for their final risk score to be calculated.
General Procedure and Genotyping
Individualized assessments were undertaken in the context of a larger study on schizotypy that included self-report, cognitive, neurophysiological, and interview measures. Having provided written informed consent, participants attended 2 appointments of 40- and 120-minute duration, respectively, completing the self-report questionnaires and providing demographic information in the latter. Where participants reported less than 80% confidence in their responses on the pregnancy and obstetric complications or family history questionnaire, consent was obtained to contact a parent or caregiver who was requested to provide written responses to the same questionnaire. Participants provided saliva samples using an Oragene-DNA OG-500 self-collection tube.
The 4 selected SNPs (rs1625579, rs7004633, rs7914558, and rs12966547) were genotyped using the TaqMan genotyping assays C_8946584_20, C_29048976_10, C_31978821_10, and C_152930_10, respectively. Assays were performed in a total reaction volume of 5 μl. This contained 2.73 μl of 2 × TaqMan Universal Master Mix, 2.13 μl of ddH2O, 0.14 μl of 40 × working mix of SNP genotyping assay, and 10ng of genomic DNA. The PCR was performed on an ABI PRISM 7000 Sequence Detection System, with an activation step of 10 minutes at 95°C, followed by 40 cycles of denaturation of 15 seconds at 95°C, and then annealing and extension for 1 minute at 60°C. Information on the genotype distribution and success can be found in online supplementary material.
Data Cleaning and Statistical Analyses
Analysis proceeded in 5 key stages: data cleaning, item selection, maximum covariance (MAXCOV) analysis, consistency testing, and class validation.
Data Cleaning.
Participants were excluded from analyses if there were 2 or more error responses on items that detected disingenuous or inattentive responding, if they had missing or erroneous data on measures contained in the larger study, or if they were univariate or multivariate outliers. Univariate outliers were identified by visual analysis of histograms whereas multivariate outliers were identified using Cook’s distance and leverage.53 Individual item scores were standardized within sexes prior to analyses.
Item Selection.
Items within each factor were screened to ensure that those included in the analyses did not violate assumptions of taxometric procedures.18,54,55 Screening involved the removal of items that did not conform with the anticipated latent structure (ie, evidence of a small risk class) in mean-above-minus-below-a-cut (MAMBAC) analyses,18 ensuring monotonicity of items,55,56 and removing items that had either very high or very low correlations with other items.57 Items were also read to identify overlapping content. Where present, redundant items were removed.54 Further information on these criteria can be found in the online supplementary material.
MAXCOV Analysis.
MAXCOV analysis relies on the general covariance mixture theorem.58 A key proposition of this theorem is that, given multivariate data from 2 separated classes, the covariance within the commingled data set is determined by the relative sizes of the classes and the degree of their separation, provided within-class covariance is zero. Therefore, in a population with a latent 2-class structure, the covariances within ordered subsets of a population sample will vary from subset to subset in a predictable manner: rising as the relative representation of classes within subsets approaches 1:1, and falling either side of this point. An underlying dimensional structure also yields a predictable pattern in covariance: Covariances from ordered subsets will be approximately equal. That is, MAXCOV analysis involves finding the pattern of covariance across ordered subsets of a population sample. When this pattern is peaked, the underlying structure is likely taxonic; when flat, the latent structure is likely dimensional. Detailed descriptions of the technical aspects of MAXCOV analysis are provided elsewhere.11,55,57–59
MAXCOV analysis was run in R 3.0.160 using a program based on algorithms described by Meehl and Yonce11 and code provided by Grove.61 MAXCOV analysis was applied iteratively to item-level data. Across iterations, indicators yielding flat covariance curves were removed. Low variance of smoothed covariance estimates also indicated nontaxonicity. The analysis was repeated until either only taxonic items remained or there were insufficient items remaining to continue. The analysis involved loess smoothing and slabs of n ≥ 20. Final results were corroborated by base rate variance and class membership probability estimates.
MAXCOV analysis was performed on 3 indicator sets: cognitive-perceptual, interpersonal, and disorganized. Items belonging to more than one set were not analyzed. Analyses were not performed on subscale or factor scores because item parcels (subscale or factor scores) are likely to be less sensitive to latent structure as meaningful variation on the constituent phenotypes is likely to be dampened in the composite score.62,63
Consistency Testing.
The maximum eigenvalue (MAXEIG) inchworm consistency test and latent modes (L-Mode) analyses were conducted to test the consistency of the MAXCOV analysis results. Consistency testing was completed using R 3.0.1.
MAXEIG analysis is a multivariate approach to MAXCOV analysis, wherein covariation is calculated as the primary eigenvalue. The inchworm consistency test involves increasing the number of overlapping windows across several MAXEIG analyses. As the number of windows increases, a taxonic latent structure will produce graphical output that peaks on the right (inching upward); a continuous structure will resemble a squashed inchworm (as described by Waller and Meehl).58 In addition to graphical output, the inchworm consistency test provides a base rate estimate. All indicators were standardized prior to analysis, and windows had 90% overlap. The maximum number of windows was determined by requiring that n per window was not less than 10 times the number of indicators in the analysis.
L-Mode is a factor-analytic procedure. It involves the estimation of latent structure and taxon base rates from the factor-score distribution obtained from a single-factor model of the data.58 Data are standardized and factor-analyzed, and a factor-score density plot is obtained. It is assumed that the primary factor revealed will be that of the underlying latent structure (eg, schizotypy). If this is taxonic, the output will be bimodal. The position of the modes is used to determine the underlying base rate by way of an average. L-Mode output additionally provides an estimate of class membership that can be directly compared with that derived in the MAXCOV analysis. The case-removal-consistency test was employed to determine the consistency of the L-Mode results. The removal of half of the lowest scoring complement members should double the estimated base rate. The analysis is deemed consistent if base rate estimates are within 5% of the expected base rate.64
Class Validation.
Logistic regression was completed to determine the odds of belonging to a single schizotypy class, relative to nonclass members, in the presence of investigated risk factors. This was completed in 3 stages: unadjusted, adjusted, and fully adjusted. Unadjusted analyses included no concurrent predictors beside the risk factor. For adjusted regression analyses, sex (male) and ethnicity (Caucasian and Asian) were included as predictor variables, along with scores from a single risk factor measure. For the fully adjusted analysis, sex and ethnicity, and scores from all risk factors were included. Adjusted analyses were undertaken in order determine that observed effects were likely specific and not attributable to common shared effects. A number of participants were excluded due to missing data (eg, family history data being rated with less than 80% confidence, or the participants or relatives not consenting to provide this information).
Results
Data Cleaning
Case-wise exclusions arose from disingenuous or inattentive responding (n = 35), missing or erroneous data (n = 28), and univariate or multivariate outliers on cognitive and other measures not included in this report (n = 7). The final sample had n = 430 (table 1).
Item Selection
Following indicator screening, the 25 cognitive-perceptual items were reduced to 16. Those remaining were SPQ items 3, 4, 13, 22, 28, 30, 31, 39, 40, 47, 48, 55, 56, 60, 61, and 64. The 25 interpersonal items were also reduced to 16: 2, 6, 8, 11, 17, 24, 26, 35, 41, 43, 49, 51, 62, 66, 68, and 73. Five of the original 16 disorganized items survived the screening process: 32, 34, 50, 72, and 74. The primary reason for item exclusion was failure to pass MAMBAC screening criteria (online supplementary material).
MAXCOV Analysis
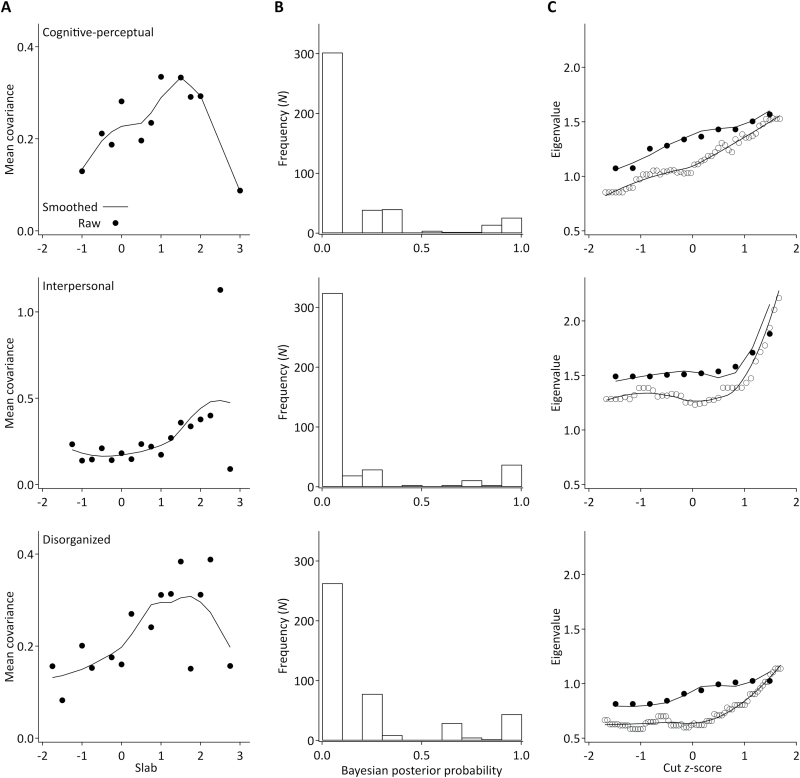
Evidence of latent taxonicity was identified across analyses (figure 1). The MAXCOV analysis outputs consistently show right-sided peaks, indicating the existence of a small risk class. This pattern is corroborated by the backward-J shape distributions of the posterior probabilities (figure 1).
Fig. 1.
Output of taxometric analyses for cognitive-perceptual (upper), interpersonal (middle), and disorganized (lower) item sets. (A) MAXCOV analysis output. Cusped peaks are indicative of latent taxonicity. (B) class membership probabilities. Backward J-shaped distributions corroborate evidence of small base rate class structures. (C) MAXEIG analysis output plots for analyses run with the lowest (10; dotted line) and highest number of windows (52, 38, and 70 for cognitive-perceptual, interpersonal, and disorganized item sets; solid line). Right-sided upward peaks are consistent with the MAXCOV analysis results across item sets.
Cognitive-Perceptual Items.
Following 10 iterations, 7 items remained (SPQ items 13, 22, 30, 40, 48, 60, and 64). The mean base rate was 24.56% (SD = 10.55%). The mean validity coefficient was k = 1.43 (SD = 0.45), and within-class correlations were r = −.01 and r = .24 for the taxon and complement, respectively. Posterior probabilities (figure 1) gave a class size of n = 48 participants (11.16%).
Interpersonal Items.
MAXCOV analysis of interpersonal items concluded after 8 iterations, with 9 items remaining (8, 11, 35, 41, 43, 49, 62, 66, and 68). The mean base rate was 16.11% (SD = 11.21%) and the mean validity k = 1.35 (SD = 0.42). Within-class correlations were minimal, r = .09 for the taxon and r = .19 for the complement. Posterior probabilities classified n = 54 participants (12.56%) as belonging to the interpersonal class.
Disorganized Items.
A single MAXCOV iteration was run with the 5 remaining disorganized items. This provided evidence of latent taxonicity, with a mean base rate of 21.20% (SD = 9.46%). Mean validity coefficient was k = 1.16 (SD = 0.36), and within-class correlations were r = .08 and r = .28 for the taxon and complement, respectively. Bayesian probabilities classified n = 80 participants (18.60%) as belonging to the disorganized class.
Overlap in Class Membership.
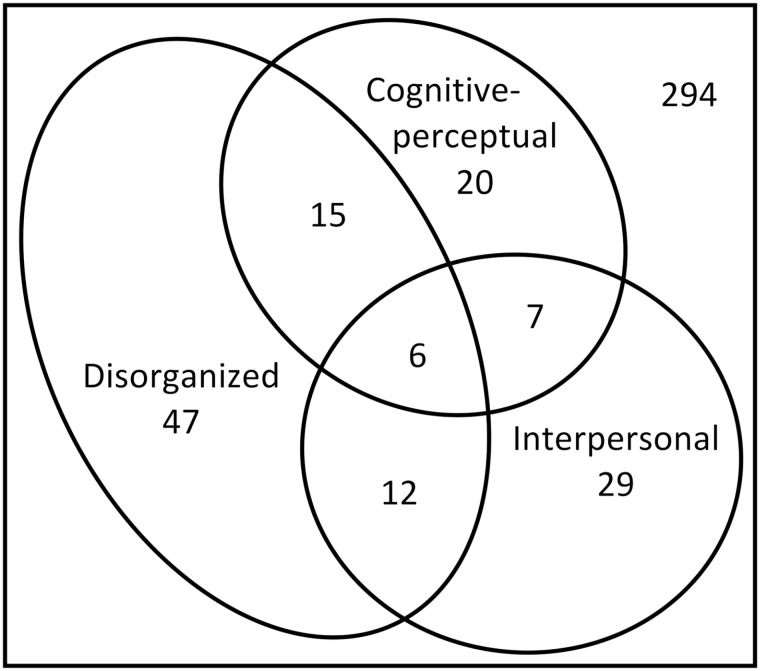
Fisher’s exact test revealed significant associations between class membership across all 3 class pairings: cognitive-perceptual and interpersonal P = .004, cognitive-perceptual and disorganized P < .001, and interpersonal and disorganized P = .005. Six participants were classified as belonging to all 3 classes (figure 2). These findings indicate that, while there is significant overlap in class membership, schizotypy features are not necessarily co-occurring.
Fig. 2.
Venn diagram showing the overlap in class membership based on Bayesian probabilities from the MAXCOV analysis. Proportional Venn diagram constructed using eulerAPE.65
Consistency Testing
The inchworm consistency test involved running consecutive MAXEIG analyses with sliding windows. An upward peak on the right-hand side of the plot indicates latent taxonicity. The clearest evidence of this was found with the interpersonal items (mean base rate 9.08%, figure 1). The cognitive-perceptual and disorganized outputs were more ambiguous, with peaks rising to a lesser degree. The estimated base rates for these analyses were 21.17% and 5.56%, respectively.
L-Mode analyses provided evidence of discontinuity underlying all data sets (supplementary figure S1). The average base rate estimate for the cognitive-perceptual items was 13.88%, and the probability estimate was 24.19% (n = 104). For the interpersonal items, the average base rate was estimated as 5.56%, with 9.30% of participants (n = 40) classified as class members. The average base rate for interpersonal items was 8.63%, and the probability estimate was 13.03% (n = 56). Fisher’s exact test revealed that individuals classified as belonging to a specific class by the MAXCOV and L-Mode analyses were all very similar, P < .001. Case-removal-consistency testing confirmed the interpersonal and disorganized results; base rate estimates were within 5% of the anticipated values. The cognitive-perceptual items did not provide consistent evidence of taxonicity.
Class Validation
Participants classified by Bayesian probabilities from the MAXCOV analysis as belonging to a single schizotypy class (cognitive-perceptual n = 20, interpersonal n = 29, and disorganized n = 47) were compared to participants that belonged to no risk class (n = 294). Measured risk factors failed to predict cognitive-perceptual class membership across unadjusted and adjusted models. There was, however, a consistent trend for scores and class membership to be positively associated, and when genetic risk was measured by individual SNP scores (range 0–2), the unadjusted model in which rs7004633 was a sole predictor was significantly better than chance (likelihood ratio χ2 = 4.81, P = .028, pseudo-R2 = .04, n = 291). This SNP remained a significant predictor of cognitive-perceptual class membership in the adjusted model also (table 2).
Table 2.
Odds Ratio (95% Confidence Intervals) of Membership in Feature-Specific Schizotypy Classes
| Cognitive-Perceptual | Interpersonal | Disorganized | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Unadjusted | Adjusted | Fully Adjusted | Unadjusted | Adjusted | Fully Adjusted | Unadjusted | Adjusted | Fully Adjusted |
| Cannabis use | 1.06 (0.85–1.32) | 1.06 (0.84–1.34) | 1.13 (0.88–1.44) | 1.06 (0.88–1.27) | 1.05 (0.87–1.28) | 0.96 (0.75–1.24) | 1.08 (0.93–1.25) | 1.15 (0.98–1.35) | 1.06 (0.88–1.29) |
| Pregnancy and obstetric | 1.33 (0.91–1.95) | 1.33 (0.91–1.95) | 1.22 (0.78–1.92) | 0.93 (0.60–1.46) | 0.95 (0.60–1.49) | 0.97 (0.62–1.53) | 0.77 (0.50–1.19) | 0.74 (0.48–1.35) | 0.77 (0.49–1.21) |
| Family history | 1.43 (0.36–5.64) | 1.48 (0.37–5.83) | 1.47 (0.34–6.43) | 1.97 (0.70–5.53) | 1.97 (0.70–5.54) | 1.56 (0.45–5.42) | 1.89 (0.78–4.58) | 1.96 (0.78–4.92) | 1.99 (0.74–5.34) |
| Poor social adjustment | 3.36 (0.73–15.36) | 3.64 (0.77–17.17) | 3.94 (0.77–20.12) | 14.60*** (4.01–53.15) | 14.66*** (4.02–53.51) | 13.05*** (3.37–50.90) | 9.68*** (3.08–30.48) | 13.55*** (3.98–46.17) | 9.10** (2.41–34.36) |
| Genetic risk score | 8.94 (0.59–134.95) | 12.76 (0.84–194.69) | 8.07 (0.43–150.30) | 5.82 (0.58–57.92) | 6.03 (0.60–60.48) | 4.43 (0.32–60.94) | 1.99 (0.33–12.18) | 3.05 (0.47–19.68) | 1.18 (0.14–10.29) |
| rs1625579 | 0.81 (0.36–1.84) | 0.92 (0.40–2.12) | — | 1.14 (0.53–2.44) | 1.16 (0.54–2.47) | — | 0.93 (0.52–1.67) | 0.99 (0.55–1.78) | — |
| rs7004633 | 2.20* (1.11–4.38) | 2.33* (1.15–4.72) | — | 0.93 (0.47–1.84) | 0.93 (0.46–1.87) | — | 0.79 (0.45–1.40) | 0.88 (0.49–1.57) | — |
| rs7914558 | 0.98 (0.45–2.10) | 0.96 (0.45–2.08) | — | 1.86 (0.92–3.75) | 1.85 (0.91–3.76) | — | 1.48 (0.89–2.47) | 1.61 (0.93–2.77) | — |
| rs12966547 | 1.46 (0.72–2.95) | 1.53 (0.74–3.18) | — | 1.40 (0.78–2.51) | 1.39 (0.77–2.49) | — | 1.43 (0.89–2.27) | 1.47 (0.91–2.39) | — |
Note: Adjusted, sex and ethnicity included; fully adjusted, sex and ethnicity and both risk factors included; unadjusted, risk factor only. The fully adjusted models were run with the 5 original risk measures, and not individual SNPs.
*P < .05, **P < .01, ***P < .001.
For both interpersonal and disorganized items, poor social adjustment consistently predicted class membership. The unadjusted (χ2 = 17.39, P < .001, pseudo-R2 = .09, n = 322) and adjusted (χ2 = 17.51, P = .002, pseudo-R2 = .09, n = 322) models predicted interpersonal class membership at a rate better than chance, and poor social adjustment remained a significant predictor in the fully adjusted model (table 2). The same pattern occurred with the unadjusted (χ2 = 15.88, P < .001, pseudo-R2 = .06, n = 340), adjusted (χ2 = 35.55, P < .001, pseudo-R2 = .13, n = 340), and fully adjusted models in the prediction of disorganized class membership. There was again a trend for schizophrenia liability to be positively associated with measured risk factors. Pregnancy and obstetric complications did not follow this trend.
Discussion
Latent classes for all item sets emerged fairly consistently across analyses. MAXCOV analysis provided estimated base rates of 25%, 16%, and 21% for cognitive-perceptual, interpersonal, and disorganized items, respectively. There was significant overlap in class membership, although important differences were also identified. Cognitive-perceptual class membership was predicted by a single SNP (rs7004633) associated with liability for developing schizophrenia, and membership to both the interpersonal and disorganized classes was predicted by poor social adjustment.
Administration of the SPQ afforded the opportunity to investigate a range of schizotypy features. The identification of a small schizotypy class across both negative and positive schizotypy is consistent with Meehl’s1,2 schizotaxia-schizotypy model, and builds on previous reports in which the SPQ has been employed. Keller et al66 identified a class structure for negative attributes only (base rates 11%–13%), while Bove and Epifani20 identified a class structure (11%–19%) underlying only positive attributes. Linscott45 reported taxonicity across all attributes (attribute-specific and combined), base rates that varied between 5% and 10%, and identified classes that were not independent. Whereas the observed base rates for cognitive-perceptual and disorganized classes were somewhat higher than averages reported in reviews,11 rates of ~25% have been found with analyses of measures of perceptual distortions67 and schizophrenia endophenotypes.17
The relationships among class memberships found here and by Linscott45 raises questions regarding whether schizotypy comprises 1 or multiple constructs.68 Whereas overlap of classes may imply a single entity or process, departure from redundancy of classification and differential associations of class membership with risk factors suggest multiple processes may be involved. The present research is the first known attempt to validate schizotypy class membership with genetic risk, and the significant association of cognitive-perceptual class membership with the SNP rs7004633 provides some evidence of a valid demarcation of liability. Separate to this, poor social adjustment was associated with membership to both the interpersonal and disorganized classes. Investigating the association between risk factors and membership to all classes may be an important step in understanding more about the latent structure of schizophrenia liability. A larger sample size is required for this, however.
The observed relationship between rs7004633 and the cognitive-perceptual taxon complements a larger body of evidence on the relationship of genotypes with continuous or quasi-continuous measures of schizotypy.69 Much of this has not involved analyses of SNPs identified through genome-wide association studies (GWAS). However, in several studies that have examined the association of psychosis experience with polygenic risk scores, inverse associations have been found,70,71 particularly as the statistical criterion for detection has increased.71 Some have raised the possibility that SNPs associated with schizophrenia may not contribute directly to schizophrenia-specific causal mechanisms but to non-specific precursors of morbidity (eg, poor resilience). Unfortunately, we were not able to explore this possibility.
If a latent schizotypy class exists, it should be identifiable from analysis of different schizotypy attributes and with different analysis methods.57–59 In the present research, taxonicity was consistently identified across MAXCOV, MAXEIG, and L-Mode analyses for interpersonal features, with some ambiguity emerging in the analysis of cognitive-perceptual and disorganized features. There remains a clear need for replication and stronger consistency in results as well as analyses of indicators spanning multiple levels of observation (eg, self-report and performance measures).
Several limitations affect interpretation of the findings. Results here are based on ratings from a single self-report questionnaire45 obtained from an undergraduate convenience sample. However, undergraduate participants are likely to experience fewer psychiatric difficulties than the general population and, in particular, some features and correlates of schizotypy (eg, suspiciousness, social anxiety, IQ) may reduce the likelihood that schizotypes will pursue university education.72,73 Reliance on self-report measures is regarded by some as a limitation. However, as subjective experience can only be understood via self-report, interview-based observational ratings can introduce additional sources of error or bias that may affect latent structure findings.5,63,74 In contrast, self-report can be more sensitive to diverse etiological processes than clinician ratings.75
The sample size was modest. Although each identified class was associated with a measured risk factor, there were fewer significant findings than anticipated. With a larger sample, more robust comparisons of taxon and complement members can be achieved. Given limits on the number of alleles we could study, and the modest sample size for studying a simple gene load score, we were concerned not to wash out effects by collapsing schizotypy phenotypes together. Nevertheless, the findings hold the promise that a more complete picture of the relationship between schizotypy and schizophrenia-related alleles will come through better-resourced studies.
The number of decision points in the taxometric analysis approach was such that it was not feasible to consider all possible permutations of decisions (eg, the sequence of removal of items from items in MAXCOV iterations, removal of outliers). With respect to outliers, it may have been that these were not spurious but genuine—or, as Lenzenweger5 stated, “it makes considerable sense to think that schizotypes may actually live far out in the tails of a distribution; they do not represent mistakes or anomalies . . . . nature delivers up messy data” (p. 76). Had they been genuine cases, we would have anticipated slightly larger schizotypy classes.
Finally, many SPQ items were not retained in the final taxometric iterations. It is unclear whether exclusion of items reflects idiosyncratic properties of items within this data collection instance or differential sensitivity of items to the latent structure. This notwithstanding, several points should be kept in mind. First, Meehl developed MAXCOV and other taxometric approaches as search methods for the identification of valid indicators of schizotypy.76 That is, Meehl anticipated the elimination of inappropriate indicators and the refinement of the schizotaxia-schizotypy construct.77 In such circumstances, removal of irrelevant items does not diminish the validity of the remaining items and judgments of content validity based on conventions on the assessment of schizotypy become moot.78 Second, search and elimination through taxometric method does not create taxonicity any more than large sample sizes create genotype associations with schizophrenia in GWAS.
The present research adds to the body of work showing that indicators of schizotypy are taxonic,9–11 consistent with a latent taxonicity in schizotaxia. Importantly, it shows for the first time that schizotypy class membership is predicted by genetic and environmental factors that predict schizophrenia. At the same time, considerable work remains to be undertaken to meet Meehl’s challenge to refine the schizotaxia construct77 and to replicate and understand the differential relationships that genetic and environmental risk variables have with differently defined schizotypy classes. Work is also required to better understand the nature of the latent discontinuity that underlies evidence of taxonicity. For example, taxonicity of schizotypy reported here and elsewhere is often assumed to emanate from a dichotomous latent process. However, our findings could equally be attributable to some sort of nonlinear step, sigmoid, or threshold function, such as a polygenic threshold effect.79 Finally, despite evidence in favor of taxonicity, many regard schizotypy as having a dimensional structure or a structure that is both dimensional and taxonic.80 Relatedly, there is an important school of thought (eg, represented by Claridge3 and others81) that schizotypy is not inherently pathological but a fully continuous individual difference variable that incorporates important adaptive potential. Resolution of these continuum-versus-taxon and personality-versus-pathology controversies will require continued thoughtful analysis and examination.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
New Zealand Schizophrenia Research Group (NZSRG) Schizophrenia Research Award to R.J.L.
Supplementary Material
Acknowledgments
We are grateful to Charlotte Levings, Hannah Macgregor-Wolken, Rosie Marsh, Danielle McHardy, Buaphrao Raphiphatthana, and Ellen Warhurst, who assisted with data collection. The authors declare that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 2. Meehl PE. Toward an integrated theory of schizotaxia, schizotypy, and schizophrenia. J Pers Disord. 1990;4:1–99. [Google Scholar]
- 3. Claridge G. Single indicator of risk for schizophrenia: probable fact or likely myth? Schizophr Bull. 1994;20:151–168. [DOI] [PubMed] [Google Scholar]
- 4. Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. [DOI] [PubMed] [Google Scholar]
- 5. Lenzenweger MF. Schizotypy and Schizophrenia: The View From Experimental Psychopathology. New York, NY: Guilford Press; 2010. [Google Scholar]
- 6. Lenzenweger MF. Thinking clearly about schizotypy: hewing to the schizophrenia liability core, considering interesting tangents, and avoiding conceptual quicksand. Schizophr Bull. 2015;41(suppl 2):S483–S491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bleuler E. Dementia Praecox or the Group of Schizophrenias. Translated by Zinkin J. New York, NY: International Universities Press; 1911/1950. [Google Scholar]
- 8. Everett KV, Linscott RJ. Dimensionality vs taxonicity of schizotypy: some new data and challenges ahead. Schizophr Bull. 2015;41(suppl 2):S465–S474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korfine L, Lenzenweger MF. The taxonicity of schizotypy: a replication. J Abnorm Psychol. 1995;104:26–31. [DOI] [PubMed] [Google Scholar]
- 10. Lenzenweger MF, Korfine L. Confirming the latent structure and base rate of schizotypy: a taxometric analysis. J Abnorm Psychol. 1992;101:567–571. [DOI] [PubMed] [Google Scholar]
- 11. Linscott RJ, Lenzenweger MF, van Os J. Continua or classes? Vexed questions on the latent structure of schizophrenia. In: Gattaz WF, Busatto GF, eds. Advances in Schizophrenia Research 2009. New York, NY: Springer; 2010:333–355. [Google Scholar]
- 12. Fossati A, Lenzenweger MF. Natura facit saltus: discontinuities in the latent liability to schizophrenia and their implications for clinical psychiatry. Giorn Ital Psicopat. 2009;15:219–230. [Google Scholar]
- 13. Beauchaine TP, Lenzenweger MF, Waller NG. Schizotypy, taxometrics, and disconfirming theories in soft science. Comment on Rawlings, Williams, Haslam, and Claridge. Pers Individ Differ. 2008;44:1652–1662. [Google Scholar]
- 14. Haslam N, Holland E, Kuppens P. Categories versus dimensions in personality and psychopathology: a quantitative review of taxometric research. Psychol Med. 2012;42:903–920. [DOI] [PubMed] [Google Scholar]
- 15. Erlenmeyer-Kimling L, Golden RR, Cornblatt BA. A taxometric analysis of cognitive and neuromotor variables in children at risk for schizophrenia. J Abnorm Psychol. 1989;98:203–208. [DOI] [PubMed] [Google Scholar]
- 16. Tyrka AR, Cannon TD, Haslam N, et al. The latent structure of schizotypy: I. Premorbid indicators of a taxon of individuals at risk for schizophrenia-spectrum disorders. J Abnorm Psychol. 1995;104:173–183. [DOI] [PubMed] [Google Scholar]
- 17. Lenzenweger MF, McLachlan G, Rubin DB. Resolving the latent structure of schizophrenia endophenotypes using expectation-maximization-based finite mixture modeling. J Abnorm Psychol. 2007;116:16–29. [DOI] [PubMed] [Google Scholar]
- 18. Golden RR, Meehl PE. Detection of the schizoid taxon with MMPI indicators. J Abnorm Psychol. 1979;88:217–233. [DOI] [PubMed] [Google Scholar]
- 19. Linscott RJ. The latent structure and coincidence of hypohedonia and schizotypy and their validity as indices of psychometric risk for schizophrenia. J Pers Disord. 2007;21:225–242. [DOI] [PubMed] [Google Scholar]
- 20. Bove EA, Epifani A. From schizotypal personality to schizotypal dimensions: a two-step taxometric study. Clin Neuropsychiatry. 2012;9:111–122. [Google Scholar]
- 21. Cohen AS, Emmerson LC, Mann MC, Forbes CB, Blanchard JJ. Schizotypal, schizoid and paranoid characteristics in the biological parents of social anhedonics. Psychiatry Res. 2010;178:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hambrecht M, Häfner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–475. [DOI] [PubMed] [Google Scholar]
- 24. Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. [DOI] [PubMed] [Google Scholar]
- 25. Bakan P, Peterson K. Pregnancy and birth complications: a risk factor for schizotypy. J Pers Disord. 1994;8:299–306. [Google Scholar]
- 26. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. [DOI] [PubMed] [Google Scholar]
- 27. Foerster A, Lewis SW, Owen MJ, Murray RM. Low birth weight and a family history of schizophrenia predict poor premorbid functioning in psychosis. Schizophr Res. 1991;5:13–20. [DOI] [PubMed] [Google Scholar]
- 28. Lahti J, Raïkkönen K, Sovio U, et al. Early-life origins of schizotypal traits in adulthood. Br J Psychiatry. 2009;195:132–137. [DOI] [PubMed] [Google Scholar]
- 29. Walshe M, McDonald C, Taylor M, et al. Obstetric complications in patients with schizophrenia and their unaffected siblings. Eur Psychiatry. 2005;20:28–34. [DOI] [PubMed] [Google Scholar]
- 30. Boydell J, van Os J, McKenzie K, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323:1336–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cannon M, Caspi A, Moffitt TE, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. [DOI] [PubMed] [Google Scholar]
- 32. van Os J, Driessen G, Gunther N, Delespaul P. Neighbourhood variation in incidence of schizophrenia. Evidence for person-environment interaction. Br J Psychiatry. 2000;176:243–248. [DOI] [PubMed] [Google Scholar]
- 33. Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014;40:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159:539–545. [DOI] [PubMed] [Google Scholar]
- 35. Craddock N, O’Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. J Med Genet. 2005;42:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. [DOI] [PubMed] [Google Scholar]
- 37. Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fanous AH. Can genomics help usher schizophrenia into the age of RDoC and DSM-6? Schizophr Bull. 2015;41:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 42. Wuthrich V, Bates TC. Reliability and validity of two Likert versions of the Schizotypal Personality Questionnaire (SPQ). Pers Individ Differ. 2005;8:1543–1548. [Google Scholar]
- 43. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed., revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 44. Mason OJ. The assessment of schizotypy and its clinical relevance. Schizophr Bull. 2015;41(suppl 2):S374–S385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Linscott RJ. The taxonicity of schizotypy: does the same taxonic class structure emerge from analyses of different attributes of schizotypy and from fundamentally different statistical methods? Psychiatry Res. 2013;210:414–421. [DOI] [PubMed] [Google Scholar]
- 46. Raine A. Manual for the Schizotypal Personality Questionnaire (SPQ and SPQ-B) Vol 2003 Los Angeles, CA: Department of Psychology, University of Southern California; http://www-rcf.usc.edu/~raine/ Accessed January 22, 2003. [Google Scholar]
- 47. Strauss JS. Hallucinations and delusions as points on continua function. Rating scale evidence. Arch Gen Psychiatry. 1969;21:581–586. [DOI] [PubMed] [Google Scholar]
- 48. Stefanis NC, Smyrnis N, Avramopoulos D, Evdokimidis I, Ntzoufras I, Stefanis CN. Factorial composition of self-rated schizotypal traits among young males undergoing military training. Schizophr Bull. 2004;30:335–350. [DOI] [PubMed] [Google Scholar]
- 49. Compton MT, Goulding SM, Bakeman R, McClure-Tone EB. Confirmation of a four-factor structure of the Schizotypal Personality Questionnaire among undergraduate students. Schizophr Res. 2009;111:46–52. [DOI] [PubMed] [Google Scholar]
- 50. Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33:1111–1115. [DOI] [PubMed] [Google Scholar]
- 51. Edwards DW, Yarvis RM, Mueller DP, Zingale HC, Wagman WJ. Test-taking and the stability of adjustment scales: can we assess patient deterioration? Eval Q. 1978;2:275–291. [Google Scholar]
- 52. Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychol Med. 2004;34:643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed. Boston, MA: Pearson Education; 2007. [Google Scholar]
- 54. Gangestad S, Snyder M. To carve nature at its joints: on the existence of discrete classes in personality. Psychol Rev. 1985;92:317–349. [Google Scholar]
- 55. Meehl PE. Clarifications about taxometric method. Appl Prev Psychol. 1999;8:165–174. [Google Scholar]
- 56. Maraun MD, Slaney K. An Analysis of Meehl’s MAXCOV-HITMAX Procedure for the Case of Continuous Indicators. Multivar Behav Res. 2005;40:489–518. [DOI] [PubMed] [Google Scholar]
- 57. Meehl PE. Factors and taxa, traits and types, differences of degree and differences in kind. J Pers. 1992;60:117–174. [Google Scholar]
- 58. Waller NG, Meehl PE. Multivariate Taxometric Procedures: Distinguishing Types From Continua. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- 59. Meehl PE, Yonce LJ. Taxometric analysis: II. Detecting taxonicity using covariance of two quantitative indicators in successive intervals of a third indicator (MAXCOV procedure). Psychol Rep. 1996;78:1091–1227. [Google Scholar]
- 60. R Core Team. R: a language and environment for statistical computing [computer program]. Version 3.1.2 Pumpkin Helmet. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 61. Grove WM. MAXCOVwmg taxometric procedure [computer program] Version February 17, 2004. www.psych.umn.edu/faculty/grove/ Accessed August 1, 2005.
- 62. Bandalos DL, Finney SJ. Item parceling issues in structural equation modeling. In: Marcoulides GA, Schumacker RE, eds. New Developments and Techniques in Structural Equation Modeling. Mahwah, NJ: Lawrence Erlbaum; 2001:269–296. [Google Scholar]
- 63. Linscott RJ, Allardyce J, van Os J. Seeking verisimilitude in a class: a systematic review of evidence that the criterial clinical symptoms of schizophrenia are taxonic. Schizophr Bull. 2010;36:811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ruscio J. Taxometric analysis with dichotomous indicators: the modified MAXCOV procedure and a case-removal consistency test. Psychol Rep. 2000;87:929–939. [DOI] [PubMed] [Google Scholar]
- 65. Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9:e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Keller F, Jahn T, Klein C. Anwendung von taxometrischen methoden und von mischverteilungsmodellen zur erfassung der schizotypie. In: Andresen B, Maß R, eds. Schizotypie: Psychometrische Entwicklungen und Biopsychologische Forschungsansätze. Göttingen, Germany: Hogrefe; 2001:391–412. [Google Scholar]
- 67. Lenzenweger MF, Moldin SO. Discerning the latent structure of hypothetical psychosis proneness through admixture analysis. Psychiatry Res. 1990;33:243–257. [DOI] [PubMed] [Google Scholar]
- 68. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 69. Barrantes-Vidal N, Grant P, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015;41(suppl 2):S408–S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stefanis NC, Hatzimanolis A, Avramopoulos D, et al. Variation in psychosis gene ZNF804A is associated with a refined schizotypy phenotype but not neurocognitive performance in a large young male population. Schizophr Bull. 2013;39:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jones HJ, Stergiakouli E, Tansey KE, et al. Phenotypic Manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meehl PE. Manual for Use With Checklist of Schizotypic Signs. Minneapolis, MN: Psychiatric Research Unit, University of Minnesota Medical School; 1964. [Google Scholar]
- 73. Newman DL, Moffitt TE, Caspi A, Silva PA. Comorbid mental disorders: implications for treatment and sample selection. J Abnorm Psychol. 1998;107:305–311. [DOI] [PubMed] [Google Scholar]
- 74. Beauchaine TP, Waters E. Pseudotaxonicity in MAMBAC and MAXCOV analyses of rating-scale data: turning continua into classes by manipulating observer’s expectations. Psychol Methods. 2003;8:3–15. [DOI] [PubMed] [Google Scholar]
- 75. Kendler KS, Myers J, Torgersen S, Neale MC, Reichborn-Kjennerud T. The heritability of cluster A personality disorders assessed by both personal interview and questionnaire. Psychol Med. 2007;37:655–665. [DOI] [PubMed] [Google Scholar]
- 76. Meehl PE. MAXCOV-HITMAX: a taxonomic search method for loose genetic syndromes. In: Meehl PE, ed. Psychodiagnosis: Selected Papers. Minneapolis, MN: University of Minnesota Press; 1973:200–224. [Google Scholar]
- 77. Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46:935–944. [DOI] [PubMed] [Google Scholar]
- 78. Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychol Bull. 1955;52:281–302. [DOI] [PubMed] [Google Scholar]
- 79. Gottesman II, Shields J, Hanson DR. Schizophrenia: The Epigenetic Puzzle. Cambridge, UK: Cambridge University Press; 1982. [Google Scholar]
- 80. Mason OJ. The duality of schizotypy: is it both dimensional and categorical? Front Psychiatry. 2014;5:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mohr C, Claridge G. Schizotypy—do not worry, it is not all worrisome. Schizophr Bull. 2015;41:S436–S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.