Abstract
To investigate contributions of genetic and environmental risk factors and possible direction of causation for the relationship between symptoms of cannabis use disorders (CUD) and psychotic-like experiences (PLEs), a population-based sample of 2793 young adult twins (63.5% female, mean [range] age 28.2 [19–36] y) were assessed for symptoms of CUD and PLEs using the Composite International Diagnostic Interview. Latent risk of having symptoms of CUD or PLEs was modeled using Item Response Theory. Co-twin control analysis was performed to investigate effect of familiar confounding for the association between symptoms of CUD and PLEs. Biometric twin models were fitted to estimate the heritability, genetic and environmental correlations, and direction for the association. Lifetime use of cannabis was reported by 10.4 % of the twins, and prevalence of PLEs ranged from 0.1% to 2.2%. The incidence rate ratio of PLEs due to symptoms of CUD was 6.3 (95% CI, 3.9, 10.2) in the total sample and 3.5 (95% CI, 1.5, 8.2) within twin pairs. Heritability estimates for symptoms of CUD were 88% in men and women, and for PLEs 77% in men and 43% in women. The genetic and environmental correlations between symptoms of CUD and PLEs were 0.55 and 0.52, respectively. The model allowing symptoms of CUD to cause PLEs had a better fit than models specifying opposite or reciprocal directions of causation. The association between symptoms of CUD and PLEs is explained by shared genetic and environmental factors and direct effects from CUD to risk for PLEs.
Keywords: THC, psychosis, item response theory, direction of causation
Introduction
Use of cannabis occurs more frequently among patients with psychotic disorders than in the general population.1 Regular use of cannabis entails a 2- to 3-fold increased risk of subsequent non-affective psychotic disorders.2–4 Following the seminal registry-based follow-up study of Swedish conscripts in 1987,5 the association between cannabis use in adolescence and later risk of psychotic symptoms and psychotic disorders has been demonstrated in both clinical6 and general population settings.7–12 The mechanisms underlying this association are not fully understood, but some data indicate that cannabis is a causal factor for psychotic disorders.2,13
Age of cannabis initiation and the amount and frequency of use,14,15 genetic variation,16 and a family history of psychotic illness all seem to increase the risk for psychotic disorders.13 Evidence that cannabis use precedes onset of psychotic symptoms,9,17 and a dose-response relationship2,18 argue for a causal relationship.19 However the issue of reversed causality, ie, that experiencing psychotic symptoms or having an emerging psychotic disorder may increase the risk for cannabis use,20 and the fact that cannabis intoxication may lead to psychotic-like symptoms, must be carefully addressed.17 The relationship between cannabis use and psychosis may also be explained by shared genetic or environmental factors, or by gene-environment interaction.21–23 Results from previous analyses of genetically informative samples have lent support for a common etiology of psychotic disorders and cannabis use disorders (CUD), although the results are mixed.20,24–27
Based on twin studies, the point estimated heritability for liability to schizophrenia is 81%,28 and a mega-analysis of genome-wide association datasets revealed more than 100 genetic susceptibility loci.29 The heritability of CUD is about 40%–50%,30 but no genome-wide significant susceptibility loci have been established.31
Converging evidence from population-based studies provides empirical support for a psychosis continuum that range from infrequent subclinical manifestations of psychosis proneness via psychotic-like experiences (PLEs), to manifest psychotic symptoms and clinical psychotic disorders like schizophrenia.32 Based on data from structured interviews of more than 30 000 individuals from 18 countries, mean lifetime prevalence of psychotic experiences in the general population is 5.8%.33 However, the prevalence estimates vary considerably across studies.34 Premorbid environmental risk factors and affective symptomatology may differ between PLEs and psychotic disorders.35 Longitudinal studies have shown that presence of PLEs in adolescence increases the risk of psychotic disorder in adulthood,34 and twin studies have demonstrated genetic similarities between subclinical and clinical psychosis phenotypes.36,37 Estimating the effect of cannabis use on experiencing PLEs in the general population may thus be a more cost-efficient and valid alternative to studying the relationship in clinical psychosis patients, who are fewer and more susceptible to selection bias.
Prospective randomized control experiments are considered the best way to study causal relationships, but innovative statistical methods applied to cross-sectional data can be an alternative. One approach is to model causation based on pairs of genetically informative relatives measured on a single occasion.38–40 Under the assumptions that members of a twin pair do not have mutual effect on one another either within or across traits, the relationship between the 2 traits is equivalent for both members of a twin pair, the 2 traits have differing modes of inheritance, and there are no unmeasured variables that influence both measures and thereby inflate the correlations arising through the causal influence of one variable on the other,39 differences in the patterns of cross-twin cross-trait correlations can allow one to falsify hypotheses about the direction of causation (DOC) between 2 variables measured on a single occasion.
The phenotypic association between cannabis use and psychosis is well established,2 but the relative importance of common genetic and environmental factors is unclear. If the association is environmental or causal, reducing cannabis use in the population would lead to lower prevalence of PLEs and psychotic illness. On the other hand, if the association is entirely due to common genetic factors, the prevalence of psychotic disorders would be independent of level of cannabis use in the population.
The aim of the present study was to investigate the relative contribution of genetic and environmental risk factors, and DOC, for the putative association between symptoms of CUD and PLEs in a population-based sample of young adult Norwegian twins. More specifically, we aimed to estimate (1) to which degree familial factors shared by twins confound the putative phenotypic association between symptoms of CUD and PLEs, (2) the heritability of latent factors for symptoms of CUD and PLEs, and (3) the extent to which common genetic and environmental risk factors or direct causal effects may explain the co-occurrence of symptoms of CUD and PLEs.
Methods
Participants
The Norwegian Institute of Public Health Twin panel is a population-based cohort of twins born between 1967 and 1979.41 Since 1992, self-report questionnaire and face-to-face diagnostic interview data have been collected as part of a mental health research project. Details regarding recruitment and assessments have been published previously.42,43 Briefly, all complete pairs of 8045 twins who had completed a questionnaire in 1998 were invited to a diagnostic interview for DSM-IV mental disorders. Between 1999 and 2004, 2801 twins completed the interview, of which 2793 twins had full data on psychotic symptoms and 2469 twins had complete data on symptoms of CUD. The current sample thus includes information on 2793 twins (63.5% female, mean age 28.2 y, range 19–36 y). Zygosity classification was based on questionnaire data and validated by microsatellite analysis for 676 same-sex twin pairs, resulting in less than 1% misclassification.42 The current sample consisted of 898 monozygotic (MZ) females, 444 MZ males, 532 same-sex dizygotic (DZ) females, 235 same-sex DZ males, and 684 DZ twins from opposite-sex pairs (344 females and 340 males). Participation in the interview study was predicted by higher age and monozygosity, but not by any mental health indicator from previous questionnaire data.44
Procedures
All participants were assessed for lifetime DSM-IV axis I disorders using the Norwegian version of the computerized Munich Composite International Diagnostic Interview (M-CIDI).45 The interviewers were mostly psychology students in the final part of their training and psychiatric nurses trained by teachers certified by the World Health Organization and supervised closely during the data collection period. Most interviews were conducted face-to-face. For practical reasons, 231 (8.3%) of the interviews were conducted over telephone. Members of a twin pair were assessed by different interviewers.
The substance use module of the M-CIDI includes a screen for lifetime use of any substance of abuse. Lifetime ratings on all items concerning cannabis use were included in the analyses. The psychosis module of the M-CIDI includes a screen for 22 psychotic symptoms. Individuals endorsing at least one of the screen items were administered the full module. All items concerning delusions were rated with 2 thresholds, the first indicating that the item was endorsed, and the second that the content was definite psychotic based on responses to a probing question. When reviewing the verbal responses, 3 of the delusion items (“Have you ever believed people were spying on you?,” “Was there ever a time when you believed people were following you?,” and “Have you been convinced that people you saw talking to each other were talking about you or laughing at you?”) had a high number of false positive responses. Thus only definite psychotic responses were kept in the analyses for these items while responses to both thresholds were kept for the remaining items. The items kept in the analysis will in the following be referred to as PLEs.
The study was approved by the Norwegian Data Inspectorate and the Regional Committee for Medical and Health Research Ethics, and written informed consent was obtained from all participants after complete description of the study.
Statistical Analysis
Item Response Theory (IRT) was used to investigate the construct validity of a latent factor structure for symptoms of CUD and PLEs. Latent trait theory relates characteristics of items (ie, item parameters) and characteristics of individuals (ie, latent traits) to the probability of endorsing a particular response category.46 Responses on the M-CIDI were modeled directly using a factor analysis for categorical data using a logistic log link. The latent factor had a fixed mean of zero and variance of unity in males (ie, standard normal distribution), one threshold parameter for each response category within each item and a discrimination parameter, or slope, for each item, often referred to as the 2-parametric (2P) IRT model. The threshold parameter indicates the severity of a given symptom, and the discrimination parameter indicates how effectively a given item can discriminate between subjects with different severity of a mental disorder. If the discrimination parameter is equal for all items, ie, all items are equally reliable, a 1-parametric (1P) IRT-model is estimated, also known as the Rash model when used on dichotomous data.47 By the principle of parsimony, the 1P model should be preferred over the 2P model given a non-significant reduction of fit to the data. To allow for population heterogeneity, the latent mean and variance was estimated in females.
To obtain estimates of excess risk of PLEs given symptoms of CUD, and evidence for familial confounding of the association, co-twin control analyses were run based on derived ordinals from the IRT models. Lower risk estimates within twin pairs than in the total sample indicates familial confounding due to genetic and environmental factors shared by the twins. Lower risk estimates in MZ than in DZ twins indicates a strong genetic effect for the association. We used a mixture confirmatory factor analysis in which the parametric latent variable distribution was replaced by a non-parametric approach using a discretized or ordinalized representation of the distribution.48 We selected the best fitting mixture model using Bayesian Information Criterion (BIC). Based on the mixture confirmatory factor analysis we grouped the latent distributions of both symptoms of CUD and PLEs into 4 derived ordinal categories. The prevalences of the categories for symptoms of CUD risk were (from high to low) 1.3%, 3.1%, 2.9%, and 92.7%, and the prevalences of the categories for PLE risk were 0.2%, 1.3%, 6.9%, and 91.7%. Co-twin control analyses on the derived ordinal categories were run in STATA version 1449 by fitting conditional fixed-effects Poisson regression models. All other analyses were run in Mplus 7.3150 using Full Information Maximum Likelihood raw data methods.
Biometric twin modeling was used to estimate the heritability of symptoms of CUD and PLEs separately. Individual differences in liability are assumed to derive from 4 latent sources of risk: additive genetic (A), non-additive genetic (D), shared environmental (C), and individual-specific environmental factors (E), which includes all environmental factors that contribute to differences between the twins plus measurement error. Bivariate methods51 were used to exploit the expected genetic and environmental correlations between MZ and DZ twin pairs, and to estimate the genetic and environmental correlations between symptoms of CUD and PLEs. Because MZ twin pairs are genetically identical and DZ twin pairs share on average half of their genes, A contribute twice as much to the resemblance in MZ compared to DZ twins for a particular phenotype, and D contribute only one-fourth to the resemblance in DZ twins compared to MZ twins. Both MZ and DZ twins are assumed to share all their C factors and none of their E factors. In the present study we modeled symptoms of CUD and PLEs as latent traits without random measurement error. The genetic and environmental correlations are the proportional overlap in genetic and environmental risk factors for the 2 phenotypes. The sum of genetic (covG) and environmental covariance (covE) equals the phenotypic covariance (covP). The relative importance of covG and covE can be found by dividing covG and covE by covP.
Univariate biometric IRT models were compared in the following order: (1) 2P vs 1P, (2) genetic sex differences, and (3) significance of A, C and D effects. Since D and C effects are confounded in the classical twin design, we tested for C effects when the MZ correlation was less than 2 times the DZ correlation and for D effects when the MZ correlation was more than 2 times the DZ correlation. We tested 3 forms of genetic sex differences: (1) general sex limitation, where different genetic factors influence the trait in men and women, (2) common sex limitation, where the same genetic factors influence the trait in question, but with different effect in men and women, and (3) no sex-limitation, where the same genetic factors have the same effect across sex. Model comparisons were evaluated using the Akaike Information Criterion (AIC) and Sample Size Adjusted BIC (SSABIC).52,53 Both AIC and SSABIC provide a balance between model complexity and model or data misfit, with the SSABIC disfavoring model complexity more than AIC.
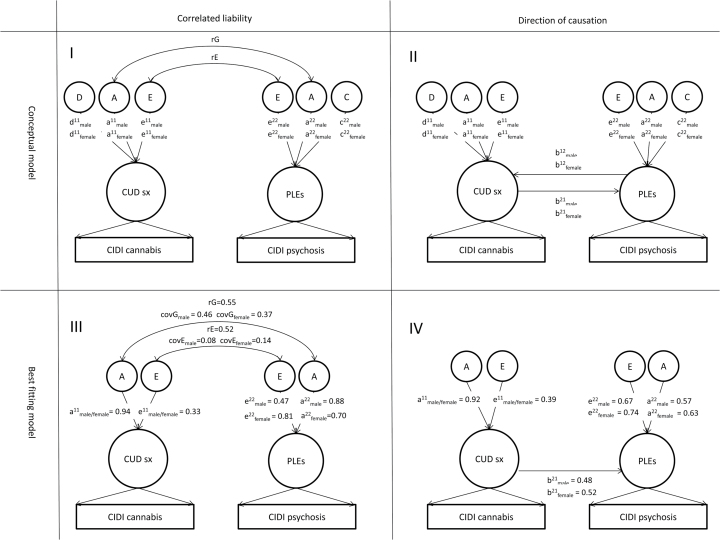
Bivariate twin models were fitted to compare the correlated liability model (figure 1-I) and the DOC model (figure 1-II).38 Phenotypes used in the bivariate models were the best fitting univariate biometric IRT models. While the phenotypes are not causally related in the correlated liability model, but influenced by common genetic and environmental risk factors, the phenotypes are allowed to reciprocally cause each other in the DOC model. We constrained the genetic and environmental correlations to be equal across sex in all bivariate models.54 All biometric models were run directly on the observed symptom data using IRT-models, and not on the derived ordinals used in the co-twin control analyses.
Fig. 1.
Biometric twin modeling of correlated liability and direction of causation for the association between symptoms of cannabis use disorder (CUD) and psychotic-like experiences (PLEs). The figure presents conceptual and best fitting models of the relative contribution of additive genetic (A), non-additive genetic (D), shared environmental (C), and individual-specific environmental effects in correlated liability (panel I and III) and direction of causation (panel II and IV) models for the association between symptoms of CUD and PLEs modeled as latent traits based on responses to questions in the Composite International Diagnostic Interview (CIDI). Circles represent latent variables and rectangles represent observed variables. Unidirectional paths represent regression coefficients. Unidirectional path estimates must be squared to obtain the proportion of variance explained by each component. rG denotes genetic correlation and rE denotes environmental correlation.
Results
A total of 289 subjects (10.4%) reported lifetime use of cannabis, and 201 (7.2%) had used cannabis more than 5 times. Prevalence of individual symptoms of CUD ranged between 0.1% (experienced an accident or injury when influenced by cannabis) and 1.3% (used cannabis more or for a longer period than planned; table 1). A total of 708 individuals (25.3%) were administered the full psychosis module in M-CIDI after endorsing 1 or more of the screening questions. Prevalence of individual PLEs ranged from 0.1% (delusions about thought insertion) and 2.2% (delusions of reference; table 2). The best fitting IRT model for symptoms of CUD was a 1P AE no sex limitation model, while the best fitting model for PLEs was a 1P AE common sex limitation model (table 3). All items regarding symptoms of CUD discriminated equally well between subjects with different severity of CUD, but they indexed different levels of risk. Similarly, all items regarding psychotic symptoms in M-CIDI, except the screening questions, discriminated equally well between subjects with different severity of psychosis risk, but they indexed different levels of risk. Further details regarding item characteristic curves for symptoms of CUD and PLEs are found in the supplementary material.
Table 1.
Prevalence of Endorsed Items From CIDI Regarding Cannabis Use and Symptoms of Cannabis Use Disorders Among 2793 Young Adult Norwegian Twins
| Item | Item Content | Response Categories | n | % |
|---|---|---|---|---|
| L3A1 | Have you ever taken cannabis? | 1 | 289 | 10.4 |
| L3B1 | Have you ever taken cannabis 5 times or more? | 1 | 201 | 7.2 |
| 1a | 46 | 1.7 | ||
| 2b | 28 | 1.0 | ||
| L7 | In the period when you were using cannabis most frequently, how often did you use it? | 3c | 31 | 1.1 |
| 4d | 38 | 1.4 | ||
| 5e | 57 | 2.0 | ||
| L9A | Have you ever felt such a strong desire or urge to use cannabis that you could not keep from using it; or did you ever want cannabis so badly, that you could not think of anything else? | 1 | 19 | 0.7 |
| L10A | Have you ever tried to stop taking cannabis altogether, but found you couldn’t or have you ever had the continual desire to reduce your consumption? | 1 | 17 | 0.6 |
| Have you already tried several times or had the continual desire to reduce your use of cannabis or stop taking it altogether but found you couldn’t? | 2 | 10 | 0.4 | |
| L11A | If you took less cannabis than you normally did or didn’t take any, did you have any of these problems (list of withdrawal symptoms)? | 1 | 35 | 1.3 |
| L11C | Did you ever take cannabis in order to make these problems (list of withdrawal symptoms) go away or to keep from having them? | 1 | 22 | 0.8 |
| L12A | Has there ever been a period when you spent a great deal of time using or getting over the effects of cannabis? | 1 | 20 | 0.7 |
| L13A | Have there often been times when you used more cannabis than you intended to or when you used cannabis for a much longer period than you intended to? | 1 | 35 | 1.3 |
| L14A | Did you ever find that you had to use much more cannabis than before to get the effect you wanted or did you ever find that the same amount had less of an effect on you than it once did? | 1 | 30 | 1.1 |
| L15A | Have you ever given up or greatly reduced important activities in order to get or to use cannabis – activities like sports, work or associating with friends? | 1 | 19 | 0.7 |
| L16A | Has taking cannabis ever caused you any medical problems? | 1 | 5 | 0.2 |
| L17A | Has taking cannabis ever caused you any emotional or psychological problems – such as being uninterested in your usual activities, being depressed, suspicious or distrustful of other people, or having strange thoughts? | 1 | 32 | 1.2 |
| L17C | Did you continue to use cannabis after you realized it was causing you any of these emotional problems? | 1 | 21 | 0.8 |
| L19E | Did you ever have considerable problems with your family or friends because of your taking cannabis [OR] Did your taking cannabis ever cause the break-up of a relationship you’re your partner, a family member or friend [OR] Did you ever get into financial difficulties because of your taking cannabis [OR] Have you ever attacked someone or injured them while under the influence of cannabis? | 1 | 16 | 0.6 |
| Did you ever have any of these kinds of problems more than once in connection with your taking cannabis? | 2 | 5 | 0.2 | |
| L20A | Have there been several times when you were under the influence of cannabis, or suffering from its after-effects when that increased your chances of getting hurt – for instance when riding a bicycle, driving a car or boat, operating a machine, or anything else? | 1 | 20 | 0.7 |
| L21A | Has taking cannabis ever caused considerable problems with your family, friends at work or at school (for example, repeatedly staying home from work/school, poor performance, expulsion from school, neglecting of children and the household)? | 1 | 18 | 0.6 |
| L22A | Did your use of cannabis ever lead to you getting into trouble with the police more than once, for example, for possession of drugs, theft in order to obtain the substance or driving under the influence? | 1 | 16 | 0.6 |
| L23A | Have you ever accidentally injured yourself, that is, had an accident or a bad fall while under the influence of cannabis? | 1 | 4 | 0.1 |
Note: CIDI, Composite International Diagnostic Interview.
aLess than 1 day a month.
b1–3 days a month.
c1 or 2 days a week.
d3 or 4 days a week.
eAlmost daily.
Table 2.
Prevalence of Endorsed Items From CIDI Regarding Psychotic-Like Experiences Among 2793 Young Adult Norwegian Twins
| Item | Item Content | Thresholda | n | % |
|---|---|---|---|---|
| G0 | Have you ever had any of the experiences on this list (22 screening items) | 1 | 708 | 25.4 |
| G1 | Have you ever believed people were spying on you? | 1 | 38 | 1.4 |
| 2 | 24 | 0.9 | ||
| G2 | Was there ever a time when you believed people were following you? | 1 | 87 | 3.1 |
| 2 | 36 | 1.3 | ||
| G2B | Have you been convinced that people you saw talking to each other were talking about you or laughing at you? | 12 | 25662 | 9.22.2 |
| G3 | Have you ever believed that you were being secretly tested or experimented on? | 1 | 6 | 0.2 |
| 2 | 3 | 0.1 | ||
| G4 | Have you ever believed that someone was plotting against you or trying to hurt you or poison you? | 12 | 117 | 0.40.3 |
| G5 | Have you ever been convinced that someone you had not met was in love with you? | 1 | 5 | 0.2 |
| G6 | Have you ever been unreasonably convinced that your spouse or partner was being unfaithful, although he/she told you that was not true? | 1 | 14 | 0.5 |
| G7 | Have you ever believed that someone was reading your mind? | 12 | 2513 | 0.90.5 |
| G8 | Have you ever been convinced you could actually hear what another person was thinking, even though he or she was not speaking? | 1 | 17 | 0.6 |
| G9 | Have you ever been convinced that others could hear your thoughts? | 1 | 19 | 0.7 |
| G10 | Have you ever been convinced that you were under the control of some power or force, so that your actions and thoughts were not your own? | 12 | 129 | 0.40.3 |
| G11 | Have you ever been convinced that strange thoughts, or thoughts that were not your own, were being put directly into your mind? | 1 | 12 | 0.4 |
| G12 | Have you ever been convinced that someone or something could take or steal your thoughts out of your mind? | 1 | 2 | 0.1 |
| G13 | Have you ever been convinced that you were being sent special messages through television or the radio, or that a program had been arranged just for you alone? | 1 | 6 | 0.2 |
| G13B | Have you felt that a book, or newspaper, or song was meant only for you and no one else? | 1 | 7 | 0.3 |
| 2 | 8 | 0.3 | ||
| G14 | Have you ever felt strange forces working on you, as if you were being hypnotized or magic was being performed on you, or you were being hit by x-rays or laser beams? | 1 | 8 | 0.3 |
| G15 | Other volunteered delusions | 1 | 29 | 1.0 |
| G17 | Have you ever seen something or someone that others who were present could not see - that is, had a vision\hallucination when you were completely awake? | 1 | 39 | 1.4 |
| G18 | Have you more than once heard things other people couldn’t hear, for example sounds or something like a voice? | 12 | 2118 | 0.80.6 |
| G19 | Did you ever hear voices others could not hear? | 1 | 18 | 0.6 |
| 2 | 3 | 0.1 | ||
| G20 | Have you ever been bothered by strange smells that nobody else seemed to be able to smell, perhaps even unusual odours coming from your own body? | 1 | 19 | 0.7 |
| G20C | Have you ever had strange tastes in your mouth that could not be explained by anything you had eaten or put in your mouth? | 12 | 112 | 0.40.1 |
| G21 | Have you ever had unusual feelings on your skin or inside your body - like being touched when nothing was there or feeling something moving inside your body? | 1 | 31 | 1.1 |
| G22 | Have you ever had a time when you were unable to move at all when it wasn’t due to a physical or other medical reason? | 12 | 279 | 1.00.3 |
| G22A | Have you ever had a time when you moved constantly and couldn’t stop when it wasn’t due to a physical or other medical reason? | 12 | 73 | 0.30.1 |
Note: aThreshold 1 reflects that the individual has the symptom, and threshold 2 reflects that the symptom has definite psychotic or bizarre content.
Table 3.
Model Fitting of Univariate Biometric IRT-Analyses for Latent Risk of Having Symptoms of Cannabis Use Disorders (CUD) or Psychotic-Like Experiences (PLEs) Among Young Adult Twins
| Model | Variance Components | Sex Effects | IRT Model | −2*ll | No. of Free Parameters | AIC | SSABIC | |
|---|---|---|---|---|---|---|---|---|
| Symptoms of CUD | c0 | ADE | GSL | 2p | 8423.1 | 54 | 8531.1 | 8643.0 |
| c1 | ADE | GSL | 1p | 8452.6 | 39 | 8530.6 | 8611.5 | |
| c2 | ADE | CSL | 1p | 8452.7 | 38 | 8528.7 | 8607.5 | |
| c3 | ADE | NSL | 1p | 8452.8 | 35 | 8522.8 | 8595.4 | |
| c4 | AE | NSL | 1p | 8453.1 | 34 | 8521.1 | 8591.6 | |
| PLEs | p0 | ACE | GSL | 2p | 11258.4 | 73 | 11404.4 | 11555.7 |
| p1 | ACE | GSL | 1p | 11285.3 | 49 | 11383.3 | 11484.9 | |
| p2 | ACE | CSL | 1p | 11285.3 | 48 | 11381.3 | 11480.8 | |
| p3 | ACE | NSL | 1p | 11292.6 | 45 | 11382.6 | 11475.9 | |
| p4 | CE | CSL | 1p | 11293.4 | 46 | 11385.4 | 11480.7 | |
| p5 | AE | CSL | 1p | 11286.6 | 46 | 11378.6 | 11474.0 |
Note: Bold font indicates best fitting model. IRT, item response theory; GSL, general sex limitation; CSL, common sex limitation; NSL, no sex limitation; AIC, Akaike Information Criterion; SSABIC, Sample Size Adjusted Bayesian Information Criterion.
When controlling for age and sex, the incidence rate ratio (IRR) for belonging to one higher derived ordinal category of latent risk for PLEs given one higher derived ordinal category of latent risk of symptoms of CUD was 6.3 (95% CI, 3.9–10.2) in the total sample. In the co-twin control analyses, the IRR was reduced to 3.5 (95% CI, 1.5–8.2) within twin pairs (MZ and DZ pairs combined).
Latent risk for symptoms of CUD was 88% heritable in both men and women, and latent risk for PLEs was 77% heritable in men and 43% heritable in women. The latent traits for symptoms of CUD and PLEs were correlated 0.54 in men and 0.47 in women. The proportion of the phenotypic covariance that was due to genetic factors was 85% in men and 73% in women (figure 1-III). The genetic and environmental correlations were 0.55 and 0.52, respectively. A model where comorbidity was entirely due to common genetic factors had an inferior fit to the data (table 4).
Table 4.
Bivariate Model Fitting for the Association Between Symptoms of CUD and PLEs Among Young Adult Twins
| Model | Covariance | −2ll | No. of Free Parameters | AIC | SSABIC | |
|---|---|---|---|---|---|---|
| Correlated liability | 1 | AE | 15358.0 | 78 | 15514.0 | 15675.7 |
| 2 | A | 15361.5 | 77 | 15515.5 | 15675.2 | |
| Direction of causation | 1 | Symptoms of CUD →← PLEs | 15354.1 | 78 | 15510.1 | 15671.8 |
| 2 | Symptoms of CUD → PLEs | 15359.2 | 76 | 15511.2 | 15668.8 | |
| 3 | Symptoms of CUD ← PLEs | 15371.8 | 76 | 15523.8 | 15681.3 |
Note: Bold font indicates best fitting model.
We compared 3 DOC-models: (1) Symptoms of CUD and PLEs reciprocally cause each other, (2) Symptoms of CUD cause PLEs, and (3) PLEs cause symptoms of CUD. Model 2 and 3 are more parsimonious than model 1, and we therefore compared model 2 and 3 with model 1. Model 3 fit the data significantly less well than the other 2 models, and model 2 had a better fit than model 1 (table 4). The best DOC model (Symptoms of CUD cause PLEs, figure1-IV) had a similar fit to the data as the best correlated liability model.
Discussion
In this population-based sample of 2793 young adult Norwegian twins we found that latent risk of PLEs was 6.3 times higher among individuals who had symptoms of CUD, but when controlling for genetic and environmental factors shared by twin pairs in co-twin control analyses, the excess risk was reduced to 3.5. The latent risk for symptoms of CUD and PLEs were highly heritable, and half of the genetic risk factors were common to both phenotypes, but common environmental factors could not be discarded. There was stronger support for a causal pathway from symptoms of CUD to PLEs when compared to the opposite or reciprocal pathways.
Previous family studies have predominantly demonstrated correlated liability for CUD and psychosis phenotypes,24,25,27 but some findings have supported causal effects,55 reversed causality,20 and shared environmental risk factors.26 In a Swedish population-based registry study, having a CUD diagnosis was associated with a 10-fold increase in risk for later receiving a schizophrenia diagnosis.27 However, the excess risk was gradually reduced by increasing time gap between first diagnosis of CUD and first diagnosis of schizophrenia, and by controlling for increasing degree of familial confounding. Nevertheless, there was still about 4 times higher risk for incident schizophrenia after a diagnosis of CUD when familial factors were controlled for. In the present study, we found 3.5 times higher risk of PLEs given symptoms of CUD when familial factors shared by MZ and DZ twins were controlled for. The similarity in findings indicate that the association between cannabis use and psychosis may not be restricted to clinical psychotic disorders, but may also include PLEs.
The heritability estimates for the latent factor of CUD in the present study (0.84) are higher than the 0.62–0.72 found in a US sample of female twins56 and 0.58–0.76 found in male twins,57 and substantially higher than the 0.45 found in an Australian,58 and 0.33 found in a US Vietnam Era twin sample.59 In the present study the heritability estimate for PLEs among men (0.77) was similar to the 0.81 reported from meta-analytic studies on clinical psychosis phenotypes,28,60 while studies of subclinical psychotic symptoms in adolescence have demonstrated considerably lower estimates (0.15–0.59).36,61 The higher heritability estimates for symptoms of CUD and PLEs in the present compared to previous studies may partly be explained by the latent trait model, which by definition excludes random measurement error from the individual-specific environmental risk factors, thus more accurately estimate genetic effects. Compatible with this explanation, in a study of CUD in male US twins, where a latent trait was modeled based on twin and co-twin ratings, the heritability estimate was 0.79,62 which is similar to the 0.84 found in the present study.
The heritability estimate for PLEs was lower among women (0.43) than among men (0.77) in the present study. Given that women have a later onset of psychotic disorder than men,63 and the sample was predominantly young adults, we may not have captured the true prevalence of psychotic symptoms among women, and therefore possibly underestimated the heritability in women.
The DOC results indicate that a portion of the phenotypic association between symptoms of CUD and PLEs may be accounted for by direct causal effects of cannabis. If cannabis were an independent risk factor for schizophrenia, the increasing use of cannabis, and especially the increasing availability of high potency cannabis,18 should be accompanied by an increase in the prevalence of schizophrenia. However, there are no indications of increased prevalence of schizophrenia.64 A putative explanation is that cannabis can trigger psychosis in the vulnerable brain and precipitate earlier onset of psychotic disorder.65
In the present study, 10% of the twins reported lifetime use of cannabis. In a telephone survey among 16–64-year old Norwegians in 2004, 16.2% reported lifetime cannabis use.66 The prevalence of cannabis use shows a wide geographical variation, ranging from only a few percent in some Eastern European countries to over 50% among US adults.67 A population-based interview study carried out in the 1990s in Norway demonstrated that lifetime prevalence of any illicit drug use disorder was 3.4% in the urban Oslo region68 and 0.4% in a rural Western coastal region.69 In contrast, population-based interview studies the last 2 decades have demonstrated lifetime prevalence of 10.3%–11.9% for any illicit drug use disorders in the United States.70,71 In support of the putative causal association between cannabis use and PLEs, in the US National Comorbidity Survey—Replication, visual and auditory hallucinations were reported by 6.3% and 4.0% of the respondents, respectively,72 while corresponding figures were 1.4% and 0.8%, respectively, in the present study.
Strengths and Limitations
The major strength of this study is the use of structured diagnostic interviews in a large population-based sample of twins. Members of each twin pair were assessed by independent interviewers to reduce bias, and the use of IRT allowed for investigation of the construct validity of the latent CUD and PLE traits. However, the results must be interpreted with some potential limitations in mind. First, the validity of psychotic symptoms as rated by lay interviewers has been questioned in previous studies using the CIDI72 The IRT modeling results indicate that the psychosis items in M-CIDI are reliable and demonstrate good construct validity in a non-clinical population setting, but it is not clear if the same pattern of results would be found in a clinical psychosis sample. Second, questions regarding use of cannabis and PLEs refer to lifetime occurrence without information about temporality. Third, there was not sufficient power to perform a co-twin control analysis within MZ and DZ twins separately. Fourth, only 43% of invited twins completed the diagnostic interview. Attrition was predicted by male sex, dizygosity, and lower level of education, and not by mental health indicators.44 Fifth, while the best fitting DOC model is consistent with a causal process whereby CUD increases the likelihood of PLEs, effects of other environmental factors such as stressful life events may still be of importance.
In conclusion, the present study demonstrates an association between symptoms of CUD and higher risk of PLEs in the general population. The twin modeling results indicate that the relationship may be explained by common genetic and environmental factors, but there is also support for a causal effect of symptoms of CUD on the risk of PLEs.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was funded by the Norwegian Institute of Public Health. Previous data collection and analyses of this material has been funded by grants from the National Institutes of Health (grant numbers MH-068643, 1R01DA037558-01A1, and 12R01DA018673); the Norwegian Research Council; the Norwegian Foundation for Health and Rehabilitation; the Norwegian Council for Mental Health; and the European Commission under the program “Quality of Life and Management of the Living Resources” of the Fifth Framework Program (grant number QLG2-CT-2002-01254).
Supplementary Material
Acknowledgments
We thank Espen Moen Eilertsen for preparing eFigure1 and eFigure 2. E.Y. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. Br J Psychiatry. 2005;187:306–313. [DOI] [PubMed] [Google Scholar]
- 2. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 3. Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. [DOI] [PubMed] [Google Scholar]
- 4. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. [DOI] [PubMed] [Google Scholar]
- 5. Andréasson S, Allebeck P, Engström A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. [DOI] [PubMed] [Google Scholar]
- 6. Arendt M, Rosenberg R, Foldager L, Perto G, Munk-Jørgensen P. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry. 2005;187:510–515. [DOI] [PubMed] [Google Scholar]
- 7. Henquet C, Krabbendam L, Spauwen J, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. [DOI] [PubMed] [Google Scholar]
- 9. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weiser M, Knobler HY, Noy S, Kaplan Z. Clinical characteristics of adolescents later hospitalized for schizophrenia. Am J Med Genet. 2002;114:949–955. [DOI] [PubMed] [Google Scholar]
- 11. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33:15–21. [DOI] [PubMed] [Google Scholar]
- 12. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michaels TI, Novakovic V. Can cannabis cause psychosis? Clin Neuropharmacol. 2015;38:63–64. [DOI] [PubMed] [Google Scholar]
- 14. Kuepper R, van Os J, Lieb R, Wittchen HU, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Andreasson S, Allebeck P. Cannabis, schizophrenia and other non-affective psychoses: 35 years of follow-up of a population-based cohort. Psychol Med. 2012;42:1321–1328. [DOI] [PubMed] [Google Scholar]
- 16. Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. [DOI] [PubMed] [Google Scholar]
- 17. Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction. 2005;100:354–366. [DOI] [PubMed] [Google Scholar]
- 18. Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radhakrishnan R, Wilkinson ST, D’Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Front Psychiatry. 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Power RA, Verweij KJ, Zuhair M, et al. Genetic predisposition to schizophrenia associated with increased use of cannabis. Mol Psychiatry. 2014;19:1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99:425–430. [DOI] [PubMed] [Google Scholar]
- 22. Nieman DH, Dragt S, van Duin ED, et al. COMT Val158Met genotype and cannabis use in people with an At Risk Mental State for psychosis: exploring Gene x Environment interactions. Schizophr Res. 2016;174:24–28. [DOI] [PubMed] [Google Scholar]
- 23. van Winkel R; GROUP Investigators Further evidence that cannabis moderates familial correlation of psychosis-related experiences. PLoS One. 2015;10:e0137625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veling W, Mackenbach JP, van Os J, Hoek HW. Cannabis use and genetic predisposition for schizophrenia: a case-control study. Psychol Med. 2008;38:1251–1256. [DOI] [PubMed] [Google Scholar]
- 25. Proal AC, Fleming J, Galvez-Buccollini JA, Delisi LE. A controlled family study of cannabis users with and without psychosis. Schizophr Res. 2014;152:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shakoor S, Zavos HM, McGuire P, Cardno AG, Freeman D, Ronald A. Psychotic experiences are linked to cannabis use in adolescents in the community because of common underlying environmental risk factors. Psychiatry Res. 2015;227:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychol Med. 2015;45:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. [DOI] [PubMed] [Google Scholar]
- 29. Ripke S, Neale BM, Corvin A, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verweij KJ, Zietsch BP, Lynskey MT, et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction. 2010;105:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verweij KJ, Vinkhuyzen AA, Benyamin B, et al. The genetic aetiology of cannabis use initiation: a meta-analysis of genome-wide association studies and a SNP-based heritability estimation. Addict Biol. 2013;18:846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39:179–195. [DOI] [PubMed] [Google Scholar]
- 33. McGrath JJ, Saha S, Al-Hamzawi A, et al. Psychotic experiences in the general population: a cross-national analysis based on 31,261 respondents from 18 countries. JAMA Psychiatry. 2015;72:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43:1133–1149. [DOI] [PubMed] [Google Scholar]
- 35. Binbay T, Drukker M, Elbi H, et al. Testing the psychosis continuum: differential impact of genetic and nongenetic risk factors and comorbid psychopathology across the entire spectrum of psychosis. Schizophr Bull. 2012;38:992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zavos HM, Freeman D, Haworth CM, et al. Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: a twin study of specific psychotic experiences in adolescence. JAMA Psychiatry. 2014;71:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lataster T, Myin-Germeys I, Derom C, Thiery E, van Os J. Evidence that self-reported psychotic experiences represent the transitory developmental expression of genetic liability to psychosis in the general population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1078–1084. [DOI] [PubMed] [Google Scholar]
- 38. Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. Am J Hum Genet. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- 39. Heath AC, Kessler RC, Neale MC, Hewitt JK, Eaves LJ, Kendler KS. Testing hypotheses about direction of causation using cross-sectional family data. Behav Genet. 1993;23:29–50. [DOI] [PubMed] [Google Scholar]
- 40. Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: theoretical and empirical considerations. Genet Epidemiol. 1994;11:483–502. [DOI] [PubMed] [Google Scholar]
- 41. Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health twin program of research: an update. Twin Res Hum Genet. 2006;9:858–864. [DOI] [PubMed] [Google Scholar]
- 42. Kendler KS, Aggen SH, Czajkowski N, et al. The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Arch Gen Psychiatry. 2008;65:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kendler KS, Aggen SH, Tambs K, Reichborn-Kjennerud T. Illicit psychoactive substance use, abuse and dependence in a population-based sample of Norwegian twins. Psychol Med. 2006;36:955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tambs K, Rønning T, Prescott CA, et al. The Norwegian Institute of Public Health twin study of mental health: examining recruitment and attrition bias. Twin Res Hum Genet. 2009;12:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wittchen HU, Pfister H. DIA-X Interviews (M-CIDI): Manual für Screening - Verfahren und Interview: Interviewheft Langsschnittuntersuchung (DIA-X-Lifetime); Erganzungsheft (DIA-X-Lifetime); Interviewheft Querschnittuntersuchung (DIA-X 12 Monate); Erganzungsheft (DIA-X 12 Monate); PC-Programm zur Durchführung des Interviews (Langs- und Querschnittuntersuchung); Auswertungsprogramm. Frankfurt, Germany: Swets & Zeitlinger; 1997. [Google Scholar]
- 46. Lord FM. Application of Item Response Theory to Practical Testing Problems. New York, NY: Routledge; 1980. [Google Scholar]
- 47. Rash G. Probabilistic Models for Some Intelligence and Attainment Tests. Chicago, IL: University of Chicago Press; 1960. [Google Scholar]
- 48. Muthén B. Latent variable hybrids: overview of old and new models. In: Hancock GR, Samuelsen KM, eds. Advances in Latent Variable Mixture Models. Charlotte, NC: Information Age Publishing, Inc; 2008. [Google Scholar]
- 49. Stata Statistical Software. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 50. Muthén LK, Muthén BO. Mplus User’s Guide. 7th ed. Los Angeles, CA: Muthén & Muthén; 1998. –2012. [Google Scholar]
- 51. Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. 1st ed. Dordrecht, Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 52. Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 53. Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- 54. Neale MC, Røysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G x E interaction. Twin Res Hum Genet. 2006;9:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67:440–447. [DOI] [PubMed] [Google Scholar]
- 56. Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155:1016–1022. [DOI] [PubMed] [Google Scholar]
- 57. Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57:261–269. [DOI] [PubMed] [Google Scholar]
- 58. Lynskey MT, Heath AC, Nelson EC, et al. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med. 2002;32:195–207. [DOI] [PubMed] [Google Scholar]
- 59. Tsuang MT, Lyons MJ, Eisen SA, et al. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–477. [DOI] [PubMed] [Google Scholar]
- 60. Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56:162–168. [DOI] [PubMed] [Google Scholar]
- 61. Hur YM, Cherny SS, Sham PC. Heritability of hallucinations in adolescent twins. Psychiatry Res. 2012;199:98–101. [DOI] [PubMed] [Google Scholar]
- 62. Ystrom E, Reichborn-Kjennerud T, Neale MC, Kendler KS. Genetic and environmental risk factors for illicit substance use and use disorders: Joint analysis of self and co-twin ratings. Behav Genet. 2014;44:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thorup A, Waltoft BL, Pedersen CB, Mortensen PB, Nordentoft M. Young males have a higher risk of developing schizophrenia: a Danish register study. Psychol Med. 2007;37:479–484. [DOI] [PubMed] [Google Scholar]
- 64. Suvisaari JM, Haukka JK, Tanskanen AJ, Lönnqvist JK. Decline in the incidence of schizophrenia in Finnish cohorts born from 1954 to 1965. Arch Gen Psychiatry. 1999;56:733–740. [DOI] [PubMed] [Google Scholar]
- 65. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. [DOI] [PubMed] [Google Scholar]
- 66. Bye EK, Amundsen EJ, Lund M. Bruk av tobakk, rusmidler og vanedannende legemidler i Norge - hovedfunn fra SIRUS’ befolkningsundersøkelse i 2012. Oslo, Norway: Norwegian Institute of Drug Research; 2013. [Google Scholar]
- 67. Hall W, Degenhardt L. Prevalence and correlates of cannabis use in developed and developing countries. Curr Opin Psychiatry. 2007;20:393–397. [DOI] [PubMed] [Google Scholar]
- 68. Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001;158:1091–1098. [DOI] [PubMed] [Google Scholar]
- 69. Kringlen E, Torgersen S, Cramer V. Mental illness in a rural area: a Norwegian psychiatric epidemiological study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:713–719. [DOI] [PubMed] [Google Scholar]
- 70. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 71. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.