Abstract
Reduced expression of Brain-Derived Neurotrophic Factor (BDNF) has been implicated in the pathophysiology of schizophrenia. The BDNF Val66Met polymorphism, which results in deficient activity-dependent secretion of BDNF, is associated with clinical features of schizophrenia. We investigated the effect of this polymorphism on Prepulse Inhibition (PPI), a translational model of sensorimotor gating which is disrupted in schizophrenia. We utilized humanized BDNFVal66Met (hBDNFVal66Met) mice which have been modified to carry the Val66Met polymorphism, as well as express humanized BDNF in vivo. We also studied the long-term effect of chronic corticosterone (CORT) exposure in these animals as a model of history of stress. PPI was assessed at 30ms and 100ms interstimulus intervals (ISI). Analysis of PPI at the commonly used 100ms ISI identified that, irrespective of CORT treatment, the hBDNFVal/Met genotype was associated with significantly reduced PPI. In contrast, PPI was not different between hBDNFMet/Met and hBDNFVal/Val genotype mice. At the 30ms ISI, CORT treatment selectively disrupted sensorimotor gating of hBDNFVal/Met heterozygote mice but not hBDNFVal/Val or hBDNFMet/Met mice. Analysis of startle reactivity revealed that chronic CORT reduced startle reactivity of hBDNFVal/Val male mice by 51%. However, this was independent of the effect of CORT on PPI. In summary, we provide evidence of a distinct BDNFVal66Met heterozygote-specific phenotype using the sensorimotor gating endophenotype of schizophrenia. These data have important implications for clinical studies where, if possible, the BDNFVal/Met heterozygote genotype should be distinguished from the BDNFMet/Met genotype.
Keywords: brain-derived neurotrophic factor, BDNF, Val66Met, G196A, rs6265, schizophrenia, psychosis, prepulse inhibition, sensorimotor gating, stress, glucocorticoid hormones
Introduction
Brain-Derived Neurotrophic Factor (BDNF) is a key molecular mediator of neuronal development, differentiation and plasticity. Reduced expression of BDNF has been observed in the post-mortem brain1,2 and serum3–5 of schizophrenia patients, and the BDNF gene has been extensively screened for association with schizophrenia. One genetic alteration, termed the Val66Met polymorphism, results in deficient subcellular translocation and defective activity-dependent secretion of BDNF,6 reducing the secretion of BDNF by approximately 18% in cells transfected with one BDNF66Met allele and 29% in those transfected with 2.7 The BDNF66Met allele has been associated with impaired memory function and reduced hippocampal volumes,6–9 and has been implicated as a locus of risk for several psychiatric disorders including schizophrenia.10,11 Clinical studies have found that the BDNFMet/Met genotype is more frequent in patients reporting more positive than negative symptoms,12 while another study reported that schizophrenia patients of BDNFMet/Met genotype had significantly more delusional symptoms than patients carrying the BDNF66Val allele.13 However, the role of the BDNFVal66Met polymorphism within this disorder remains controversial.14
Sensorimotor gating refers to the latent ability to inhibit a motor response given a preceding sensory stimulus. Sensorimotor gating deficits are observed amongst schizophrenia patients15,16 and are typically quantified using the Prepulse Inhibition (PPI) paradigm; where a blunted startle response to an auditory pulse stimulus is observed when preceded by the presentation of a weaker auditory prepulse. As PPI deficits are observed during acute phases of illness17 and are a correlate of positive symptom presentation18 amongst schizophrenia patients, PPI is commonly viewed as an endophenotype of relevance to psychosis.19 PPI deficits are not necessarily specific to psychosis itself, as evidenced by their persistence during treatment20 and occasional observation amongst other psychiatric disorders,21 but their presence in unaffected relatives of schizophrenia patients22 classifies PPI as a schizophrenia endophenotype that can be used to dissect the molecular genetic architecture of the disorder. A strength of the PPI paradigm is its translational merit, with both rodent and human versions of the test available. This can be exploited for the preclinical modeling of schizophrenia endophenotypes,23 as genetic and environmental heterogeneity can be controlled for in rodents allowing for the detection of subtle genomic modifiers of PPI. While the Val66Met polymorphism’s regulation of PPI has not been assessed, studies in BDNF heterozygote knockout mice with a global reduction in BDNF expression of approximately 50%, have revealed reduced PPI,24,25 including sex-specific disruptions at varying prepulse intensities and interstimulus intervals (ISI).24
The aim of the current study was to assess the role of the BDNFVal66Met polymorphism in PPI. We utilized humanized BDNFVal66Met (hBDNFVal66Met) mice genetically modified to (1) carry the Val66Met polymorphism and (2) express human BDNF via endogenous mouse promoters. Given that the Val66Met genotype determines stress sensitivity,26 and that in healthy samples both mild social stress27 and childhood abuse28 result in an increase in the expression of psychosis-related symptom expression in BDNF66Met allele carriers, a secondary aim was to explore whether a chronic corticosterone (CORT) treatment administered in late adolescence/young adulthood, a critical period associated with the psychosis prodrome, may interact with BDNF genotype to determine adult PPI.
Method
hBDNFVal66Met Mice and Housing
hBDNFVal66Met mice were generated as previously described.29 Mice used in the present study were offspring from hBDNFVal/Met × hBDNFVal/Met breeding pairs. There were 12 groups comprising 3 genotypes (hBDNFVal/Val, hBDNFVal/Met and hBDNFMet/Met), 2 treatment groups (CORT and control) and 2 sexes (male and female). Group sizes ranged from 11 to 15 mice per group, for a total sample size of 152 mice. All animals were of a C57Bl/6 genetic background. Animals were group-housed with same-sex littermates (n = 2–6 per box) in individually-ventilated cages (IVC; Techniplast) under standard lighting conditions (ie, an automated 12-h light cycle), and had ad libitum access to standard pellet food and water. IVC cages contained woodchip bedding and enrichment in the form of tissue paper and a small amount of “furl.” Mice were checked daily by animal house staff, and cages were changed as necessary when soiled. Mice were observed and weighed at least weekly to track health, and the effects of our stress treatment. Housing and experimental procedures were carried out with approval from the Florey Institute of Neuroscience’s animal ethics committee.
Chronic CORT Protocol
Chronic adolescent stress was simulated by treating mice with 25mg/L of the mouse stress hormone, CORT, which was dissolved in the animal’s drinking water.30 Treatment occurred between weeks 6 to 9, a period we have previously established to mimic late adolescence/early adulthood31 that is also commonly associated with the emergence of the psychosis prodome. The CORT solution was changed every 3 days over the treatment period. Control animals received unaltered water over this period. A 2-week washout period followed the treatment period before behavioral experimentation commenced, so that long-term behavioral adaptation to the chronic CORT treatment could be examined.
Prepulse Inhibition Protocol
Automated SR-Lab startle boxes (San Diego Instruments) were used to assess PPI. The test session consisted of 104 stimulus presentations as previously described.32,33 Briefly, test sessions began and ended with a block of 8 presentations of a 40ms 115 dB pulse, while prepulse-pulse trials consisted of a single 115 dB pulse that was preceded 100ms or 30ms by a 20ms prepulse of variable intensity (2, 4, 8, or 16 dB over baseline). For all trials, 65 dB of background noise was used to prevent startle by exogenous stimuli. Prepulse inhibition was quantified as the difference between stimulus responses during prepulse-pulse and pulse-only trials, and expressed as a ratio of pulse-alone responses. Four blocks of 8 115 dB pulses of 40ms duration were used to generate a startle habituation curve.
Data Analysis
Data analysis was undertaken using the IBM Predictive Analytical Software (PASW) and Graphpad Prism packages. A 3 (genotype) × 2 (sex) × 2 (treatment) ANOVA was used to analyze data for within-subject and between-subject comparisons. Analyses were considered significant at P < .05. Sidak’s correction was applied to all post hoc comparisons, while the Greenhouse-Geisser correction was applied per Mauchley’s Test of Sphericity. If no significant interaction involving the sex of the animals was observed, then data from male and female mice were analyzed together to increase power. This analysis strategy is consistent with clinical research given the potentially small effect sizes generated by the Val66Met polymorphism,34 as well as our previous report utilizing this mouse line.30
Results
No Effect of hBDNFVal66Met Genotype on Body Weight Across Development
Body weight was recorded across development (weeks 4 to 14) and behavioral testing to rule out any confounding effect of genotype-mediated body weight differences on startle amplitudes. A mixed model ANOVA revealed a significant main effect of week (F(9,1368) = 1438.17, P < .001) and a week × sex interaction (F(9,1368) = 70.48, P < .001), reflecting that body weight increased between weeks 4 to 14 as a function of time and that female mice weighed less than males. There was no main effect of hBDNFVal66Met genotype, suggesting no major effect on food intake or metabolism.
hBDNFVal66Met Genotype Interacts With a History of CORT Exposure to Regulate PPI in an ISI-Specific Manner
A Mixed-Model ANOVA revealed a significant between-subject effect of hBDNFVal66Met genotype (F(2,140) = 5.2, P = .007), as well as a significant hBDNFVal66Met genotype × CORT interaction (F(2,140) = 3.68, P = .028) suggesting differences in PPI between the genotypes depending on CORT treatment. However, there was also a significant effect of ISI (F(1,140) = 22.57, P < .001), as well as an ISI × hBDNFVal66Met genotype × CORT treatment interaction (F(2,140) = 4.32, P = .015), reflecting that such genotype-dependent changes in PPI differed between the 100ms and 30ms ISI and as a function of CORT treatment. Because of this complex interaction, we split our PPI dataset according to ISI to further dissect the effect of hBDNFVal66Met genotype.
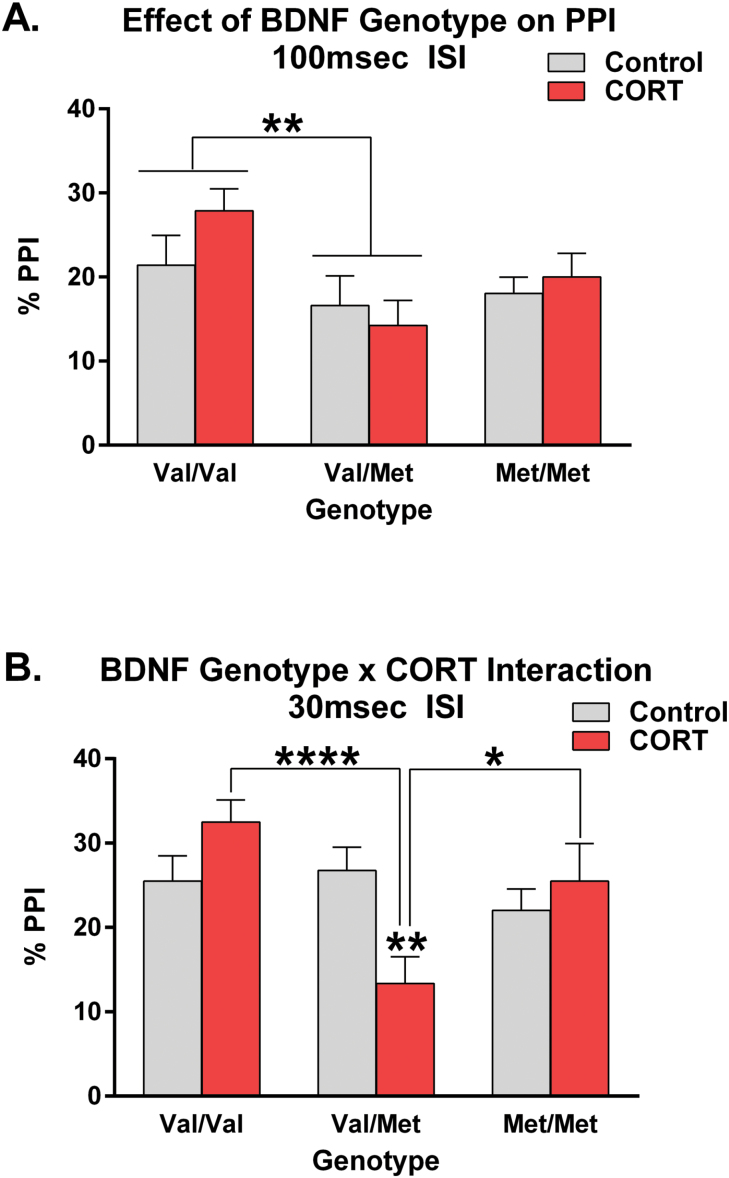
Analysis of PPI at the commonly used 100ms ISI, revealed a significant between-subjects main effect of genotype (F(2,140) = 4.94, P = .008), where the hBDNFVal/Met genotype was associated with lower PPI than the hBDNFVal/Val group (P < .01; figure 1A). The hBDNFVal/Val and hBDNFMet/Met genotypes did not significantly differ from one another. No other between-subjects or repeated-measures main effects or interactions emerged from this analysis implying that PPI did not significantly differ between groups in its direction across PP intensities (supplementary figure 1) nor did it depend on sex or CORT treatment (figure 1A).
Fig. 1.
Human Brain-Derived Neurotrophic Factor (BDNF) variant Val66Met modulates Prepulse Inhibition (PPI). At the 100ms interstimulus interval (ISI) (panel A), no effect or interaction comprising corticosterone (CORT) treatment was observed but the hBDNFVal/Met genotype group had significantly lower PPI than the hBDNFVal/Val “wildtype” group. However, at the 30ms ISI (panel B), a significant hBDNFVal66Met genotype × CORT treatment interaction was observed, where chronic glucocorticoid hormone exposure unmasked an effect of the hBDNFVal/Met heterozygote genotype on PPI. Specifically, CORT-treated hBDNFVal/Met mice had significantly lower PPI than hBDNFVal/Val and hBDNFMet/Met homozygote mice. No sex differences were detected. All data presented as mean ± SEM; *P < .05, **P < .01, ****P < .0001, corrected for multiple comparisons. Per group, n = 23–27.
Analysis of PPI at the 30ms ISI yielded a similar but divergent behavioral outcome. Specifically, a significant main effect of hBDNFVal66Met genotype was once more detected (F(2,140) = 4.02, P = .02), independent of sex. However, unlike at the 100ms ISI, a significant hBDNFVal66Met genotype × CORT interaction (F(2,140) = 5.93, P = .003) was found at this ISI (figure 1B). Further analysis of this interaction revealed that following CORT exposure in late adolescence, hBDNFVal/Met mice had significantly lower PPI than both hBDNFVal/Val (P < .0001) and hBDNFMet/Met (P < .05) homozygotes, who did not significantly differ from one another (figure 1B). Analysis of control-treated mice alone failed to reveal an effect of genotype, further implicating that a phenotype at this ISI only emerges following CORT treatment.
Aside from these between-subject effects, ANOVA also revealed a significant interaction comprising hBDNFVal66Met genotype and PP Intensity at the 30ms ISI (F(5.09,356.302) = 2.36, P = .039). Splitting the analysis by prepulse intensity revealed that there was an inhibitory effect of CORT treatment on the hBDNFVal/Met genotype group (F(1,196) = 19.20, P < .0001) across the PP intensities studied. Analyzing for genotype-mediated differences across individual PP intensities identified a significant effect of hBDNFVal66Met genotype at PP intensities of 4 db (F(2,140) = 5.23, P = .006) and 16 db (F(2,140) = 6.45, P = .002), which was underscored by the deficient PPI of the hBDNFVal/Met group. Interactions between hBDNFVal66Met genotype and chronic CORT treatment were also observed at PP intensities of 2db (F(2,140) = 4.73, P = .01) and 8db (F(2,140) = 5.26, P = .006), which once more reflected a selective effect of prior CORT treatment on the PPI of the hBDNFVal/Met group (supplementary figure 1).
Corticosterone Interacts With hBDNFVal66Met Genotype to Regulate Startle
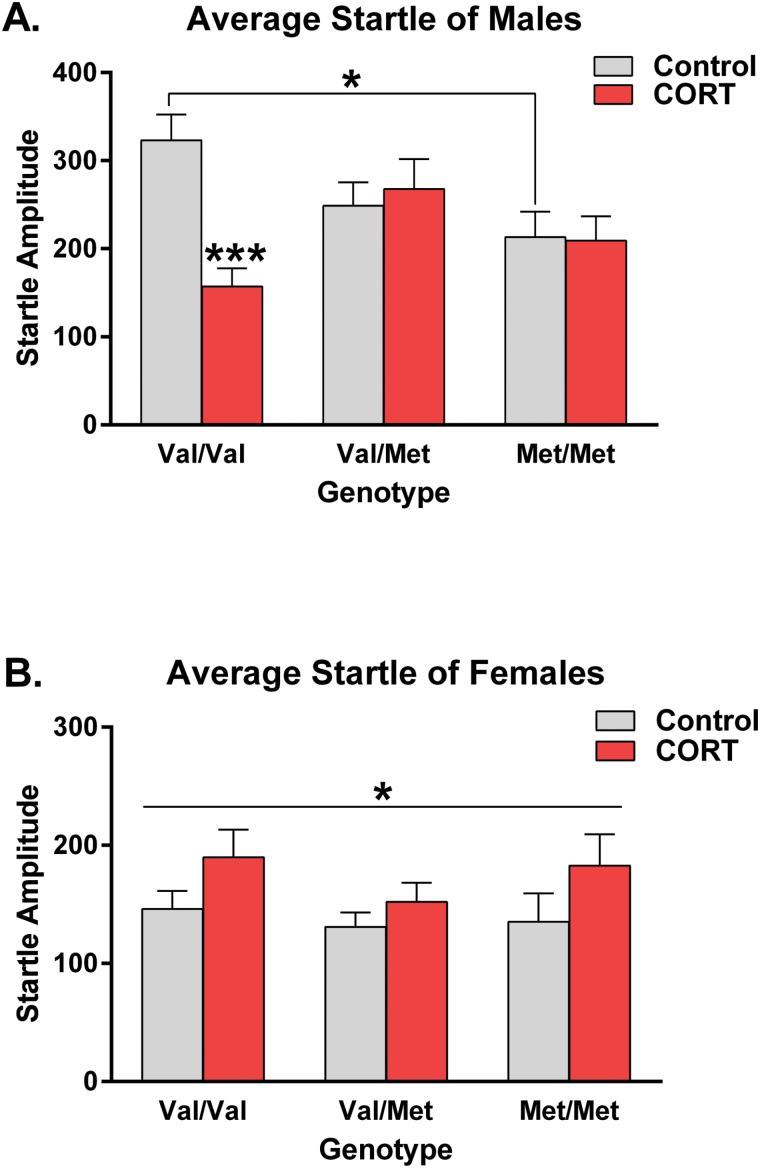
A Mixed-Model ANOVA revealed a strong trend for a hBDNFVal66Met genotype × CORT interaction (F(2,140) = 3.01, P = .053) for startle reactivity. Because of a significant hBDNFVal66Met genotype × CORT × sex interaction (F(2, 140) = 4.37, P = .014), we split our post hoc analysis according to sex. Amongst the male-only data (figure 2A), a hBDNFVal66Met genotype × CORT interaction was once more observed (F(2,72) = 5.63, P = .005). hBDNFMet/Met male mice had significantly lower startle amplitudes than hBDNFVal/Val “wildtype” male mice at baseline (P < .05). Splitting the post-hoc analysis for an effect of chronic adolescent CORT exposure revealed that the hBDNFVal/Val group was the only genotype group to significantly respond to such (P < .01). Specifically, chronic adolescent CORT exposure was found to reduce the average startle amplitude of this group by 51.4% when compared to nontreated controls (figure 2A). In contrast, analysis of the female-only dataset revealed that chronic CORT slightly increased startle amplitudes irrespective of hBDNFVal66Met genotype (F(1,68) = 4.06, P = .048; figure 2B). This result suggests that in a sex-dependent fashion, hBDNFVal66Met genotype confers vulnerability to alterations in startle reactivity both at baseline and following exposure to chronic CORT in adolescence.
Fig. 2.
hBDNFVal66Met genotype, history of corticosterone (CORT) exposure and sex interact to determine startle reactivity. Analysis of average startle revealed a significant hBDNFVal66Met genotype × adolescent CORT treatment × sex interaction. Splitting the analysis according to sex revealed that chronic adolescent CORT exposure reduced startle reactivity in hBDNFVal/Val male mice by 51.4% (A). In the female dataset (B), chronic CORT increased startle reactivity irrespective of hBDNFVal66Met genotype. All data presented as mean ± SEM; *P < .05, ***P < .001, corrected for multiple comparisons. Per group, n = 11–15.
We next verified whether alterations in startle reactivity mediate the effects of CORT and hBDNFVal66Met genotype on PPI—especially amongst our hBDNFVal/Val genotype group. To explore this hypothesis we ran an ANCOVA, with average startle amplitude included as our primary covariate. After controlling for average startle, the multivariate model retained the hBDNFVal66Met × chronic CORT interaction observed in the overall analysis (F(2,139) = 4.30, P = .015), as well as at the 30ms ISI (F(2,139) = 6.44, P = .002). These results confirm that, irrespective of any changes in average startle amplitude between groups, the alterations in PPI induced by the hBDNFVal66Met genotype × CORT interaction represent changes that are specific to the sensorimotor gating circuitry.
As expected, mice habituated to repeated startle stimulus presentations (F(3,2.73) = 23.68, P < .001). However, analysis with a Mixed-Model ANOVA, with startle block included as a repeated-measures factor, failed to produce evidence of an effect of hBDNFVal66Met genotype either alone or via interaction with adolescent CORT treatment or sex (supplementary figure 2). This result implicates that the hBDNFVal66Met genotype × CORT × sex interaction observed for average startle is a change in overall amplitude and is not a secondary effect of a genotype-mediated difference in habituation.
Discussion
The current study sought to determine the role of the BDNFVal66Met polymorphism in sensorimotor gating, a translational endophenotype of schizophrenia. We report evidence of a distinct PPI deficit amongst hBDNFVal/Met mice that is sensitive to chronic CORT treatment, and that startle reactivity is regulated via complex interactions including susceptibility to glucocorticoid stress hormones and sex of the animals. These results have important implications for the clinical schizophrenia literature focused on BDNF, which has implicated that the BDNF66Met allele may alter clinical features such as the positive symptoms of schizophrenia.14
Translational Merit and Model Validity
To study the effect of the Val66Met polymorphism on PPI, we utilized a novel transgenic mouse line that carries this gene variant, as well as an extended sequence which humanizes the BDNF peptide in vivo.29 This pre-clinical, bottom-up, approach was used due to the documented effects of ethnicity, sampling bias, medication history and other environmental factors which may confound clinical investigations of BDNF functionality.10,14 In support of the construct and predictive validity of Val66Met transgenic mice, both humans and mice show deficient hippocampus-dependent memory function,6,7,30 extinction learning,35 as well as altered anxiety-related behavior in mice7 and traits in humans.36 In this respect, BDNFVal66Met knock-in mice arguably have face, construct, and predictive validity10 making preclinical investigations that utilize this model inherently of translational value. On the other hand, chronic CORT exposure is an established model of chronic stress that targets glucocorticoid receptors independent of other factors involved in the stress response. A major advantage of this simulation of chronic stress is that it removes the need for animal handling, movement or novel environment exposure, which may impact later behavior. Low doses of chronic CORT (25–100mg/L) in drinking water have been shown to induce a long-lasting anhedonia-like phenotype in mice that persists following treatment termination but can be rescued by antidepressant therapeutics.37 Likewise, low-dose chronic CORT has also been shown to mimic the long-term decrease in hippocampal phosphorylation of CREB and BDNF’s cognate receptor, TrkB, much like environment-based models of stress.37 In this respect, chronic CORT as a simulation of chronic stress also has face, construct and predictive validity, and confers numerous methodological advantages in modeling psychiatric endophenotypes.
Defining a Novel hBDNFVal/Met PPI Phenotype
Adapting these genetic and stress models revealed a deficient PPI phenotype selectively amongst the hBDNFVal/Met genotype group at the commonly used 100ms ISI in the total dataset, and a modulatory effect of glucocorticoid stress hormones on the PPI of this genotype at the 30ms ISI. Collectively, these experimental results suggest that hBDNFVal/Met mice, in general, have poor information handling and relay efficiency of sensory inputs similar to what is observed in schizophrenia patients. Interestingly, no significant PPI deficit was found amongst hBDNFMet/Met mice in any of our PPI analyses. While the 66Met allele has been previously shown to exert a gene-dosage effect on hippocampus-dependent behavior,7 the data presented here do not support that this gene-dosage effect occurs for the deficient sensorimotor gating endophenotype. Similar heterozygote-specific effects have been reported for the COMT Val158Met variant in the regulation of PPI amongst schizophrenia patients,38 as well as several other gene variants in affective disorders39 and for antidepressant response.40 Importantly, there is evidence to suggest that the BDNFVal/Met heterozygote genotype may also elicit other biological effects that are gene dosage-independent. Specifically, the BDNFVal/Met genotype may alter gray matter volumes,41 cortical morphology,42 and brain development43 to produce distinct phenotypic divergence from both BDNFVal/Val wildtype and BDNFMet/Met homozygotes. Our data build upon these reports and reinforce the need for clinical research to sample and stratify all 3 BDNF genotypes where possible.10
The mechanism underscoring this heterozygote-specific PPI phenotype, however, remains unclear. The underlying PPI circuitry includes a number of brainstem and pontine nuclei, which receive modulatory input from several forebrain regions.44–46 While it is likely that there are partly independent pathways for PPI at short vs long ISI delays in mice, an exact definition of each respective pathway remains elusive.45,47 In previous studies, we and others have observed differential effects of treatments depending on the ISI,32,48–50 similar to the effect of the hBDNFVal/Met genotype and chronic CORT treatment in this study. A mechanism for this effect, given a lack of defined circuitry, remains difficult to assess. However, a biochemical clue is a recent report which shows that the 66Met-containing BDNF prodomain binds with altered affinity to the SorCS2 receptor51—which plays an important role in dopaminergic wiring52—to elicit biological effects.53 Given this role of SorCS2 on the development of dopaminergic innervation51,52 in the brain, and the established role of dopamine in schizophrenia54 and on PPI,55–57 it is possible that hBDNFVal/Met mice show altered catecholamine innervation of the PPI circuitry, rendering the PPI performance of this genotype group more vulnerable to environmental factors such as stress. The hBDNFMet/Met group may be spared from this phenotype due to intrinsic compensation given the greater degree of perturbed activity-dependent BDNF release.6 Further research to explore this hypothesis is, however, required and will be the topic of further mechanistic investigation.
Role of the BDNFVal66Met Variant on Startle Reactivity
Aside from PPI, an effect of both glucocorticoid stress hormone exposure and hBDNFVal66Met genotype also emerged for startle reactivity via a modulatory effect of sex of the animals. Amongst females, no effect of genotype emerged but prior CORT treatment was found to slightly increase startle reactivity. Contrary to this, amongst male mice, a significant genotype × CORT treatment interaction was observed for startle amplitudes. It should be noted that this sex-specific divergence in phenotype—or rather lack of genotype or interaction effect amongst female mice—may be influenced by a relative floor effect in range of startle amplitudes, as female mice have much lower startle amplitudes than male mice. In any case, amongst male mice, it was observed that, following chronic CORT exposure, the startle reactivity of the hBDNFVal/Val group was markedly reduced. Given that our previous studies utilizing BDNF+/− heterozygous mice have failed to detect CORT-induced reductions in startle amongst wildtype littermates,25 the effect of chronic CORT treatment on the startle amplitudes of hBDNFVal/Val mice suggests that this phenotype is likely specific to this genotype and transgenic line and should therefore be the subject of further investigation—especially given the relevance of startle reactivity to other psychiatric conditions such as post-traumatic stress disorder.
Conclusion
The genomic influence of the BDNFVal66Met polymorphism in schizophrenia has been a controversial topic within the clinical literature. Our study provides support for reports that the BDNF66Met allele may play a role in the pathophysiology of schizophrenia, specifically stress-sensitive clinical aspects such as positive-related symptomology12,13 neurocognitive function58,59 and age of onset,60–63 and that some of this involvement is likely to occur as a result of differential susceptibility to glucocorticoid signaling during critical periods such as adolescence/young adulthood. Clinical studies assessing a genomic role of BDNF in schizophrenia should therefore consider stratifying their analyses for all 3 Val66Met genotypes, as well as for an effect of adversity, early life stress, trauma or related measures of stress exposure, which may play a role in phenotype determination.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Project support was provided by the National Health and Medical Research Council (NHMRC) of Australia (grant number 1044777). M.J.N. was supported by an Australian Postgraduate Award, R.A.H. by a NHMRC Career Development Fellowship, and M.vdB. by a NHMRC Senior Research Fellowship.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi M, Shirakawa O, Toyooka K, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5:293–300. [DOI] [PubMed] [Google Scholar]
- 3. Toyooka K, Asama K, Watanabe Y, et al. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. [DOI] [PubMed] [Google Scholar]
- 4. Xiu MH, Hui L, Dang YF, et al. Decreased serum BDNF levels in chronic institutionalized schizophrenia on long-term treatment with typical and atypical antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1508–1512. [DOI] [PubMed] [Google Scholar]
- 5. Rizos E, Rontos I, Laskos E, et al. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1308–1311. [DOI] [PubMed] [Google Scholar]
- 6. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. [DOI] [PubMed] [Google Scholar]
- 7. Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. [DOI] [PubMed] [Google Scholar]
- 9. Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress & controversy. Mol Psychiatry. 2015;20:916–930. [DOI] [PubMed] [Google Scholar]
- 11. Hong CJ, Liou YJ, Tsai SJ. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res Bull. 2011;86:287–297. [DOI] [PubMed] [Google Scholar]
- 12. Zhai J, Yu Q, Chen M, et al. Association of the brain-derived neurotrophic factor gene G196A rs6265 polymorphisms and the cognitive function and clinical symptoms of schizophrenia. Int J Clin Exp Pathol. 2013;6:1617–1623. [PMC free article] [PubMed] [Google Scholar]
- 13. Han DH, Park DB, Choi TY, et al. Effects of brain-derived neurotrophic factor-catecholamine-O-methyltransferase gene interaction on schizophrenic symptoms. Neuroreport. 2008;19:1155–1158. [DOI] [PubMed] [Google Scholar]
- 14. Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51:15–30. [DOI] [PubMed] [Google Scholar]
- 15. Parwani A, Duncan EJ, Bartlett E, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. [DOI] [PubMed] [Google Scholar]
- 16. Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. [DOI] [PubMed] [Google Scholar]
- 17. Meincke U, Mörth D, Voss T, Thelen B, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis–a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2004;254:415–421. [DOI] [PubMed] [Google Scholar]
- 18. Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 2014;156:596–602. [DOI] [PubMed] [Google Scholar]
- 19. van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncan EJ, Szilagyi S, Efferen TR, et al. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology (Berl). 2003;167:63–71. [DOI] [PubMed] [Google Scholar]
- 21. Kohl S, Heekeren K, Klosterkötter J, Kuhn J. Prepulse inhibition in psychiatric disorders–apart from schizophrenia. J Psychiatr Res. 2013;47:445–452. [DOI] [PubMed] [Google Scholar]
- 22. Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42:588–594. [DOI] [PubMed] [Google Scholar]
- 23. Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl). 2008;199:331–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manning EE, van den Buuse M. BDNF deficiency and young-adult methamphetamine induce sex-specific effects on prepulse inhibition regulation. Front Cell Neurosci. 2013;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klug M, Hill RA, Choy KH, Kyrios M, Hannan AJ, van den Buuse M. Long-term behavioral and NMDA receptor effects of young-adult corticosterone treatment in BDNF heterozygous mice. Neurobiol Dis. 2012;46:722–731. [DOI] [PubMed] [Google Scholar]
- 26. Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 2012;32:4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simons C, Wichers M, Derom C, et al. Subtle gene–environment interactions driving paranoia in daily life. Genes Brain Behav. 2009;8:5–12. [DOI] [PubMed] [Google Scholar]
- 28. Alemany S, Arias B, Aguilera M, et al. Childhood abuse, the BDNF-Val66Met polymorphism and adult psychotic-like experiences. Br J Psychiatry. 2011;199:38–42. [DOI] [PubMed] [Google Scholar]
- 29. Cao L, Dhilla A, Mukai J, et al. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol. 2007;17:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Notaras M, Hill R, van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol Psychiatry. 2016;21:730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill RA, Wu YW, Kwek P, van den Buuse M. Modulatory effects of sex steroid hormones on brain-derived neurotrophic factor-tyrosine kinase B expression during adolescent development in C57Bl/6 mice. J Neuroendocrinol. 2012;24:774–788. [DOI] [PubMed] [Google Scholar]
- 32. van den Buuse M, Becker T, Kwek P, Martin S, Ruimschotel E, Risbrough V. Disruption of prepulse inhibition by 3,4-methylenedioxymethamphetamine (MDMA): comparison between male and female wild-type and 5-HT(1A) receptor knockout mice. Int J Neuropsychopharmacol. 2011;14:856–861. [DOI] [PubMed] [Google Scholar]
- 33. van den Buuse M, Gogos A. Differential effects of antipsychotic drugs on serotonin-1A receptor-mediated disruption of prepulse inhibition. J Pharmacol Exp Ther. 2007;320:1224–1236. [DOI] [PubMed] [Google Scholar]
- 34. Mandelman SD, Grigorenko EL. BDNF Val66Met and cognition: all, none, or some? A meta-analysis of the genetic association. Genes Brain Behav. 2012;11:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010;179:86–90. [DOI] [PubMed] [Google Scholar]
- 37. Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci. 2009:9:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quednow BB, Wagner M, Mössner R, Maier W, Kühn KU. Sensorimotor gating of schizophrenia patients depends on Catechol O-methyltransferase Val158Met polymorphism. Schizophr Bull. 2010;36:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Erhardt A, Czibere L, Roeske D, et al. TMEM132D, a new candidate for anxiety phenotypes: evidence from human and mouse studies. Mol Psychiatry. 2011;16:647–663. [DOI] [PubMed] [Google Scholar]
- 40. Horstmann S, Lucae S, Menke A, et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology. 2010;35:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu ME, Huang CC, Chen MH, et al. Effect of the BDNF Val66Met polymorphism on regional gray matter volumes and cognitive function in the Chinese population. Neuromolecular Med. 2014;16:127–136. [DOI] [PubMed] [Google Scholar]
- 42. Forde NJ, Ronan L, Suckling J, et al. Structural neuroimaging correlates of allelic variation of the BDNF val66met polymorphism. Neuroimage. 2014;90:280–289. [DOI] [PubMed] [Google Scholar]
- 43. Hashimoto T, Fukui K, Takeuchi H, et al. Effects of the BDNF Val66Met polymorphism on gray matter volume in typically developing children and adolescents. Cerebral Cortex. 2016;26:1795–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campbell L, Huges M, Budd T, et al. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur J Neurosci. 2008;26:2327–2333. [DOI] [PubMed] [Google Scholar]
- 45. Fendt M, Liang L, Yeoman J. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology 2001;156:216–224. [DOI] [PubMed] [Google Scholar]
- 46. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001;156:234–258. [DOI] [PubMed] [Google Scholar]
- 47. Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2011;156:194–215. [DOI] [PubMed] [Google Scholar]
- 48. Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. [DOI] [PubMed] [Google Scholar]
- 49. Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology. 2008;33:1680–1689. [DOI] [PubMed] [Google Scholar]
- 50. Brosda J, Hayn L, Klein C, et al. Pharmacological and parametrical investigation of prepulse inhibition of startle and prepulse elicited reactions in Wistar rats. Pharmacol Biochem Behav. 2011;99:22–28. [DOI] [PubMed] [Google Scholar]
- 51. Rezgaoui M, Hermey G, Riedel IB, Hampe W, Schaller HC, Hermans-Borgmeyer I. Identification of SorCS2, a novel member of the VPS10 domain containing receptor family, prominently expressed in the developing mouse brain. Mech Dev. 2001;100:335–338. [DOI] [PubMed] [Google Scholar]
- 52. Glerup S, Olsen D, Vaegter CB, et al. SorCS2 regulates dopaminergic wiring and is processed into an apoptotic two-chain receptor in peripheral glia. Neuron. 2014;82:1074–1087. [DOI] [PubMed] [Google Scholar]
- 53. Anastasia A, Deinhardt K, Chao MV, et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. 2013;4:2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. [DOI] [PubMed] [Google Scholar]
- 55. Bubser M, Koch M. Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology (Berl). 1994;113:487–492. [DOI] [PubMed] [Google Scholar]
- 56. Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology (Berl). 1990;100:413–416. [DOI] [PubMed] [Google Scholar]
- 57. Powell SB, Geyer MA, Preece MA, Pitcher LK, Reynolds GP, Swerdlow NR. Dopamine depletion of the nucleus accumbens reverses isolation-induced deficits in prepulse inhibition in rats. Neuroscience. 2003;119:233–240. [DOI] [PubMed] [Google Scholar]
- 58. Tan YL, Zhou DF, Cao LY, Zou YZ, Wu GY, Zhang XY. Effect of the BDNF Val66Met genotype on episodic memory in schizophrenia. Schizophr Res. 2005;77:355–356. [DOI] [PubMed] [Google Scholar]
- 59. Rybakowski JK, Borkowska A, Skibinska M, et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin Neurosci. 2006;60:70–76. [DOI] [PubMed] [Google Scholar]
- 60. Decoster J, van Os J, Kenis G, et al. Age at onset of psychotic disorder: cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:363–369. [DOI] [PubMed] [Google Scholar]
- 61. Gourion D, Goldberger C, Leroy S, Bourdel MC, Olié JP, Krebs MO. Age at onset of schizophrenia: interaction between brain-derived neurotrophic factor and dopamine D3 receptor gene variants. Neuroreport. 2005;16:1407–1410. [DOI] [PubMed] [Google Scholar]
- 62. Numata S, Ueno S, Iga J, et al. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism in schizophrenia is associated with age at onset and symptoms. Neurosci Lett. 2006;401:1–5. [DOI] [PubMed] [Google Scholar]
- 63. Chao HM, Kao HT, Porton B. BDNF Val66Met variant and age of onset in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.