ABSTRACT
Professional phagocytes continuously extend dynamic, actin-driven membrane protrusions. These protrusions, often referred to as membrane ruffles, serve a critical role in the essential phagocyte processes of macropinocytosis and phagocytosis. Small GTPases, such as RAC1/2, spatially and temporally regulate membrane ruffle formation. We have recently shown that extracellular calcium regulates the elaboration of membrane ruffles primarily through the synthesis of phosphatidic acid (PtdOH) at the plasma membrane. RAC1/2 guanine nucleotide exchange factors harbouring polybasic stretches are recruited by PtdOH to sites of ruffle formation. Here we discuss our findings and offer perspectives on how the regulation of dynamic actin structures at the plasma membrane by small GTPases is a critical component of phagocyte function.
KEYWORDS: actin, calcium, CaSR, dendritic cells, macrophages, macropinocytosis, membrane ruffling, phagocytosis, phosphatidic acid, RAC
Macrophages and immature dendritic cells (iDCs) routinely execute a wide range of immunological and tissue remodeling tasks. In their capacity as immune sentinels, these professional phagocytes continuously search for danger signals by emitting actin-driven membrane extensions that sample the cellular microenvironment. Such ongoing and dynamic protrusions enable the ruffling of membranes that accompanies fluid-phase uptake by macropinocytosis,1 as well as the initial capture of particulate targets that precedes phagocytosis.2 This tightly coordinated remodeling of the filamentous actin (F-actin) architecture at sites of membrane ruffle formation is instrumental for the uptake of both soluble and particulate antigen by macrophages and iDCs.
Following the internalization of foreign and/or potentially pathogenic material, professional phagocytes orchestrate inflammatory responses through 2 critical avenues. First, they elicit antigen-specific immune defenses by presenting pathogen-derived antigenic determinants to the adaptive arm of immunity.3,4 Second, they adopt transcriptional profiles that support the secretion of pro-inflammatory and chemotactic cytokines, which in turn mediate the homing of effector cells to sites of tissue damage. For instance, phagocytosis of fungal or bacterial trespassers by macrophages triggers Toll-like receptor (TLR)-mediated biosynthesis of TNF-α.5
In addition to their critical functions in host defense, professional phagocytes also play instrumental roles in overall tissue homeostasis and metazoan development; even in the absence of overt malignancy or infection, it has been estimated that over 1 million old, excess or simply damaged cells are targeted for degradation every second in the human body.6 This staggering number adds up to approximately 250 billion apoptotic cells (more than 2 times the number of galaxies in the observable universe!) being deleted in a regulated manner on a monthly basis. Professional and non-professional phagocytes cooperate in the detection, internalization and ultimately in the clearance of these apoptotic cells.
As such, professional phagocytes play vital and multifaceted roles in metazoans by directly fighting infection, bridging innate and adaptive immunity, and supporting developmental programs. These diverse tasks involve a wide range of combinations between phagocytic targets and their receptors, and therefore may seem unrelated at first glance. However, all of these responses are in fact unified by an absolute requirement for professional phagocytes to be capable of finding, recognizing and engulfing their cognate targets–processes that are intimately driven by a finely tuned and dynamic actin cytoskeleton. Indeed, interfering with either actin assembly or disassembly precludes the initial contact of macrophages with their targets,2 suggesting that phagocytes actively capture their prey by launching actin-driven membrane extensions. Such dynamic membrane extensions are expected to be especially important for securing highly motile or sparsely opsonized pathogens. Membrane ruffling may also facilitate the effective capture of apoptotic cells. In fact, efferocytosis is so efficient that apoptotic bodies are notoriously difficult to detect in vivo, despite their overwhelming frequency.7
Analogous to the ongoing and dynamic protrusion of the plasma membrane for the capture of particulate targets, professional phagocytes also continuously sample their fluid milieu by ruffling their membranes.1 As these actin-driven membrane ruffles converge and close, they give rise to large (>250 nm), phase-bright vacuoles known as macropinosomes. Astonishingly, macrophages internalize the equivalent of their entire surface every 30 minutes,8 while iDCs take up their whole volume approximately every 2.5 hours.9 Sustaining such striking rates of fluid uptake requires of the constant fueling of actin- and membrane-remodeling machineries, placing significant demands on the cellular energy budget. Nonetheless, professional phagocytes incur such expenses in order to continuously monitor for signs of danger.
In a wide range of cells, macropinocytosis can be induced by (and indeed, is often studied immediately after) the addition of growth factors. However, it is important to emphasize that professional phagocytes are unique in that they ruffle their membranes and macropinocytose even in the apparent absence of growth factors. This growth factor-independent macropinocytosis differs from its growth factor-induced mode in 2 important aspects. First, growth factor-independent macropinocytosis seems to primarily serve an immunological role, while growth factor-induced macropinocytosis is mainly intended for nutrient acquisition. Second, growth factor-independent-macropinocytosis appears to be restricted to professional phagocytes, while growth factor-induced macropinocytosis occurs in a large variety of cell types.
Despite these key disparities, it is frequently assumed that both forms of macropinocytosis are physiologically and mechanistically identical. This assumption is now challenged by recent studies delineating the molecular features that enable professional phagocytes to ruffle their membranes in a growth factor-independent manner. Specifically, we recently provided evidence that macrophages and iDCs carry significantly higher levels of phosphatidic acid (PtdOH) in their plasma membranes than their more sedentary, epithelial counterparts.1 As that report showed, constitutive biosynthesis of PtdOH at the plasma membrane is instrumental for the activation of the small GTPase RAC1/2 at sites of membrane protrusion, and therefore for the nucleation of branched actin networks in these regions. In agreement with this notion, inhibition of plasmalemmal PtdOH biosynthesis markedly prevents membrane ruffling and macropinocytosis in macrophages and iDCs.1
Mechanistically, PtdOH signals membrane rearrangements and macropinocytosis by 3 interdependent pathways, all of which converge on RAC1/2 activation and actin remodelling. First, PtdOH facilitates the dissociation of RAC1/2 from its Rho-specific guanine nucleotide dissociation inhibitor (RhoGDI),10 thereby promoting the association of RAC1/2 with the plasmalemma. Second, PtdOH serves as a landing and/or stabilizing platform for Rho family guanine nucleotide exchange factors (RhoGEFs) that activate RAC1/2, including DOCK2 and TIAM1.11,1 Both of these GEFs carry C-terminal polybasic clusters that facilitate their electrostatic interaction with PtdOH at the plasma membrane. The latter claim is partly borne out by observations wherein pharmacological inhibition of PtdOH biosynthesis leads to the dissociation of TIAM1 form the plasmalemma,1 and a DOCK2 mutant lacking its C-terminal polybasic stretch does not bind PtdOH effectively.11 Third, PtdOH promotes the recruitment and activation of type I phosphatidylinositol phosphate kinase (PIPKI),12,13 the primary enzyme responsible for phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] biosynthesis.14 Additionally, PIPKI has been reported to act as an adaptor in the integrin-dependent recruitment of RAC1/2 to the plasmalemma.15
Albeit transient and locally restricted, accumulation of PtdIns(4,5)P2 drives robust actin polymerization by promoting the activity of a plethora actin-binding proteins that catalyze filament assembly, while concomitantly inhibiting those responsible for disassembly.16 Specifically, PtdIns(4,5)P2 cooperates with active Rho GTPases in the release of WASP nucleation promoting factors from an auto-inhibited state, thereby triggering Arp2/3-mediated actin polymerization.17-19 In addition to promoting actin nucleation, PtdIns(4,5)P2 supports linkages between the plasma membrane and pre-existing actin filaments through proteins of the ezrin/moesin/radixin (ERM) family.20 Lastly, PtdIns(4,5)P2 inhibits proteins responsible for capping the plus ends of growing actin filaments,21,22 and also sequesters members of the ADF/cofilin family.23 The latter catalyze the disassembly of F-actin by introducing torsional stress in aged (ADP-bound) regions of the actin filament.24 Thus, due to its unique ability to simultaneously promote both RAC1/2 activity and PtdIns(4,5)P2 biosynthesis, plasmalemmal PtdOH represents a multilayered and versatile phospholipid employed by professional phagocytes to regulate the timing and location of actin polymerization.
When we first provided evidence that the constitutive biosynthesis of PtdOH is responsible for the ongoing ruffling and macropinocytosis of professional phagocytes,1 we were unable to identify the receptor(s) and ligand(s) responsible for eliciting these responses. Nonetheless, we had noted that ruffling abated, and PtdOH levels concomitantly decreased, in response to pertussis toxin. The latter is an ADP-ribosylating agent that uncouples Gα subunits from their cognate G protein-coupled receptors (GPCRs), thereby precluding their signaling capacity.25 This observation led us to attempt halting membrane ruffling by inhibiting a series of serpentine receptors known to couple to Gα subunits, including receptors for ATP26 and sphingosine-1-phosphate.27 However, we were incapable of precluding or even slowing down ruffling by these means. Inhibition of protein synthesis and, remarkably, serum starvation were also without effect. It was this last observation (that growth factors in serum are dispensable for the ongoing membrane ruffling of professional phagocytes) that led us to investigate with greater mechanistic detail the hallmarks, receptors and ligands that characterize growth factor-independent macropinocytosis.
Such studies recently led us to identifying extracellular calcium as an absolute requirement for the constitutive (but not growth factor-induced) macropinocytosis of professional phagocytes.28 That study also described that extracellular calcium is detected through the calcium-sensing receptor (CaSR), a GPCR expressed in a variety of cell types, including monocytes, macrophages and dendritic cells.28,29 Consistent with our earlier findings, ligation of CaSR by extracellular calcium triggers plasmalemmal PtdOH biosynthesis, TIAM1 recruitment and RAC1/2 activation at sites of membrane ruffling. As such, either removal of extracellular calcium or inhibition of CaSR dramatically reduced plasmalemmal PtdOH levels and concomitantly inhibited constitutive macropinocytosis.
Interestingly, cells that undergo macropinocytosis exclusively in response to growth factors can be rendered constitutively macropinocytic by forcing them to express CaSR. This is borne out by a previous study demonstrating that heterologous expression of CaSR in HEK293 cells–which normally lack CaSR expression–is sufficient to induce robust membrane ruffling.30 We confirmed and extended those observations by showing that heterologous expression of CaSR in HEK293 cells was sufficient for establishing a growth factor-independent macropinocytosis program.28
Mechanistically, CaSR elicits these responses in professional phagocytes by coupling the biosynthesis of PtdOH with the conversion of PtdIns(4,5)P2 into other bioactive lipids. Specifically, phosphatidylinositol 3-kinase (PI3K) is activated by ligated CaSR, in turn resulting in the phosphorylation of PtdIns(4,5)P2 into PtdIns(3,4,5)P3. In parallel, CaSR triggers the recruitment of phospholipase C (PLC) to the plasmalemma, where it further promotes PtdIns(4,5)P2 consumption by hydrolyzing the phosphoinositide into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). The formation of DAG is very quickly followed by its phosphorylation into PtdOH by diacylglycerol kinases, facilitating recruitment of RAC GEFs with polybasic stretches. The signaling pathways leading from CaSR ligation of extracellular calcium to RAC1/2 activation and constitutive membrane ruffling are illustrated in Figure 1.
Figure 1.
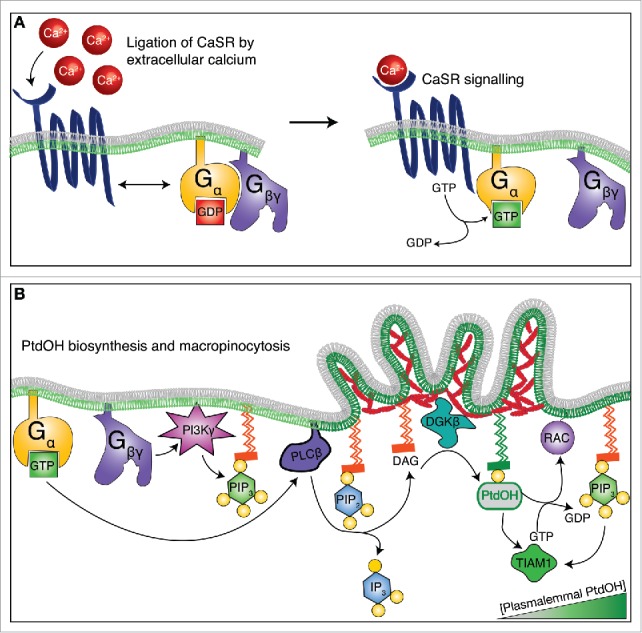
The growth factor-independent macropinocytosis and membrane ruffling of professional phagocytes is driven by extracellular calcium. (A) In response to extracellular calcium, the 7-transmembrane receptor CaSR changes conformation and catalyzes nucleotide exchange on its coupled Gα protein. The Gβγ subunit then dissociates from Gα-GTP, but each component of the complex gains signaling functions. (B) Active (GTP-bound) Gα activates PLC, which in turn mediates the breakdown of PtdIns(4,5)P2 into DAG and Ins(1,4,5)P3. In parallel, the Gβγ subunit promotes the formation of PtdIns(3,4,5)P3 by activating PI3K. The formation of DAG is rapidly followed by its phosphorylation by DGK into PtdOH, which serves multiple signaling roles in membrane ruffling and macropinocytosis. In particular, PtdOH recruits GEFs that carry polybasic amino acid clusters and activate the small GTPase RAC1/2. Following its activation, RAC1/2 catalyzes the nucleation of the branched actin networks that propel membrane ruffles and macropinosome formation.
Calcium-dependent macropinocytosis also differs from the growth factor-induced form in one remarkable aspect: extracellular danger signals sensed by cytoplasmic pattern recognition receptors can initially be taken up by CaSR-driven macropinosomes.28 Having entered the endosomal system, these conserved microbial components are transported to the cytosol by endolysosomal peptide carriers.31 A specific instance is muramyl dipeptide (MDP), the minimal bioactive motif that gives rise to peptidoglycan in bacterial cell walls. Human macrophages readily macropinocytose fluorescently labeled MDP, and subsequently transfer it across endolysosomal barriers into the cytosol.28 Cytosolic MDP is then recognized by pattern recognition receptors of the nucleotide-binding oligomerization domain (NOD) family, which initiate NF-κB-dependent inflammatory responses.28 Strikingly, the uptake of NOD ligands by macropinocytosis and the accompanying activation of NF-κB are precluded if extracellular calcium is removed or if CaSR is inhibited. In this manner, calcium-dependent macropinocytosis offers a platform for the uptake of microbial-derived components and their ensuing recognition by cytosolic pattern recognition receptors. It is noteworthy that this function is analogous to the role that membrane-bound TLRs play in pattern recognition during internalization of particulate targets by phagocytosis.
At first glance, extracellular calcium may seem like an odd ligand to dictate the behavior and inflammatory responses of professional phagocytes. After all, calcium is normally assumed to be ubiquitously and homogenously present in circulation, which would make regulation of inflammation by modulating calcium abundance cumbersome. Moreover, the concentration of extracellular calcium is approximately 4 orders of magnitude higher than that of intracellular calcium, presenting a conundrum as to how any given tissue would be capable of sufficiently elevating extracellular calcium in their microenvironment in order to elicit inflammation.
Despite these difficult questions, previous intriguing studies have indicated that the concentration of calcium does in fact fluctuate considerably in extracellular fluids.29 As such, it seems entirely possible that extracellular calcium can behave as a chemotactic agent that orchestrates the migration of professional phagocytes to sites of infection or tissue damage. In fact, the tissue microenvironment at sites of atherosclerosis and general inflammation has been shown to be associated with deposition of calcium salts.29
Thus, much like chemokines, extracellular calcium operates through GPCR signaling to modify the membrane composition of professional phagocytes, activate Rho GTPases and mobilize the actin cytoskeleton. Together, these calcium-driven responses serve to guide professional phagocytes to sites where their dual function as housekeepers and assassins is much needed by infected, dead or dying cells.
Abbreviations
- CaSR
calcium-sensing receptor
- DAG
diacylglycerol
- F-actin
filamentous actin
- GPCR
G protein-coupled receptor
- iDC
immature dendritic cell
- IP3
inositol 1,4,5-trisphosphate
- MDP
muramyl dipeptide
- NOD
nucleotide-binding oligomerization domain
- PI3K
phosphatidylinositol 3-kinase
- PIPKI
type I phosphatidylinositol phosphate kinase
- PLC
phospholipase C
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- PtdOH
phosphatidic acid
- RhoGDI
Rho-specific guanine nucleotide dissociation inhibitor
- RhoGEF
Rho family guanine nucleotide exchange factor
- TLR
Toll-like receptor
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
D.S. is supported by a Restracomp studentship from The Hospital for Sick Children; J.C. is a Cystic Fibrosis Canada postdoctoral fellow.
References
- [1].Bohdanowicz M, Schlam D, Hermansson M, Rizzuti D, Fairn GD, Ueyama T, Somerharju P, Du G, Grinstein S. Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol Biol Cell 2013; 24(11):1700-12; PMID:23576545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Flannagan RS, Harrison RE, Yip CM, Jaqaman K, Grinstein S. Dynamic macrophage “probing” is required for the efficient capture of phagocytic targets. J Cell Biol 2010; 191:1205-18; PMID:21135140; https://doi.org/ 10.1083/jcb.201007056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med 1990; 172:631-40; PMID:2373994; https://doi.org/ 10.1084/jem.172.2.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol 1984; 2:395-428; PMID:6242349; https://doi.org/ 10.1146/annurev.iy.02.040184.002143 [DOI] [PubMed] [Google Scholar]
- [5].Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999; 401:811-5; PMID:10548109; https://doi.org/ 10.1038/44605 [DOI] [PubMed] [Google Scholar]
- [6].Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med 2010; 207:1807-17; PMID:20805564; https://doi.org/ 10.1084/jem.20101157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 2006; 27:244-50; PMID:16584921; https://doi.org/ 10.1016/j.it.2006.03.005 [DOI] [PubMed] [Google Scholar]
- [8].Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci Off J Soc Neurosci 2003; 23:1329-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol 1976; 68:665-87; PMID:1030706; https://doi.org/ 10.1083/jcb.68.3.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abramovici H, Mojtabaie P, Parks RJ, Zhong XP, Koretzky GA, Topham MK, Gee SH. Diacylglycerol kinase zeta regulates actin cytoskeleton reorganization through dissociation of Rac1 from RhoGDI. Mol Biol Cell 2009; 20:2049-59; PMID:19211846; https://doi.org/ 10.1091/mbc.E07-12-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, et al.. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science 2009; 324:384-7; PMID:19325080; https://doi.org/ 10.1126/science.1170179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jenkins GH, Fisette PL, Anderson RA. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem 1994; 269:11547-54; PMID:8157686 [PubMed] [Google Scholar]
- [13].Roach AN, Wang Z, Wu P, Zhang F, Chan RB, Yonekubo Y, Di Paolo G, Gorfe AA, Du G. Phosphatidic acid regulation of PIPKI is critical for actin cytoskeletal reorganization. J Lipid Res 2012; 53:2598-609; PMID:22991193; https://doi.org/ 10.1194/jlr.M028597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol Phosphate Kinases, a Multifaceted Family of Signaling Enzymes. J Biol Chem 1999; 274:9907-10; PMID:10187762; https://doi.org/ 10.1074/jbc.274.15.9907 [DOI] [PubMed] [Google Scholar]
- [15].Chao WT, Daquinag AC, Ashcroft F, Kunz J. Type I PIPK-α regulates directed cell migration by modulating Rac1 plasma membrane targeting and activation. J Cell Biol 2010; 190:247-62; PMID:20660631; https://doi.org/ 10.1083/jcb.200911110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev 2010; 90:259-89; PMID:20086078; https://doi.org/ 10.1152/physrev.00036.2009 [DOI] [PubMed] [Google Scholar]
- [17].Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 2000; 150:1299-310; PMID:10995436; https://doi.org/ 10.1083/jcb.150.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PtdIns(4,5)P2-dependent manner downstream of tyrosine kinases. EMBO J 1996; 15:5326-35; PMID:8895577 [PMC free article] [PubMed] [Google Scholar]
- [19].Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem 2001; 276:26448-52; PMID:11340081; https://doi.org/ 10.1074/jbc.M103856200 [DOI] [PubMed] [Google Scholar]
- [20].Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 2002; 3:586-99; PMID:12154370; https://doi.org/ 10.1038/nrm882 [DOI] [PubMed] [Google Scholar]
- [21].Kim K, McCully ME, Bhattacharya N, Butler B, Sept D, Cooper JA. Structure/function analysis of the interaction of phosphatidylinositol 4,5-bisphosphate with actin-capping protein: implications for how capping protein binds the actin filament. J Biol Chem 2007; 282:5871-9; PMID:17182619; https://doi.org/ 10.1074/jbc.M609850200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kuhn JR, Pollard TD. Single molecule kinetic analysis of actin filament capping. Polyphosphoinositides do not dissociate capping proteins. J Biol Chem 2007; 282:28014-24; PMID:17656356; https://doi.org/ 10.1074/jbc.M705287200 [DOI] [PubMed] [Google Scholar]
- [23].van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, DesMarais V, Yip SC, Backer JM, Eddy RJ, Condeelis JS. EGF-induced PtdIns(4,5)P2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol 2007; 179:1247-59; PMID:18086920; https://doi.org/ 10.1083/jcb.200706206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 1999; 15:185-230; PMID:10611961; https://doi.org/ 10.1146/annurev.cellbio.15.1.185 [DOI] [PubMed] [Google Scholar]
- [25].Mangmool S, Kurose H. Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX). Toxins 2011; 3:884-99; PMID:22069745; https://doi.org/ 10.3390/toxins3070884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Singh A, Boyer JL, Der CJ, Zohn IE. Transformation by a nucleotide-activated P2Y receptor is mediated by activation of Gαi, Gαq and Rho-dependent signaling pathways. J Mol Signal 2010; 5:11; PMID:20653955; https://doi.org/ 10.1186/1750-2187-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, van der Pol SM, Geerts D, van Horssen J, van der Valk P, van der Kam E, et al.. Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J Neuro Inflammation 2012; 9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Canton J, Schlam D, Breuer C, Gütschow M, Glogauer M, Grinstein S. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat Commun 2016; 7:11284; PMID:27050483; https://doi.org/ 10.1038/ncomms11284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Olszak IT, Poznansky MC, Evans RH, Olson D, Kos C, Pollak MR, Brown EM, Scadden DT. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest 2000; 105:1299-305; PMID:10792005; https://doi.org/ 10.1172/JCI9799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bouschet T, Martin S, Kanamarlapudi V, Mundell S, Henley JM. The calcium-sensing receptor changes cell shape via a β-arrestin-1 ARNO ARF6 ELMO protein network. J Cell Sci 2007; 120:2489-97; PMID:17623778; https://doi.org/ 10.1242/jcs.03469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nakamura N, Lill JR, Phung Q, Jiang Z, Bakalarski C, de Mazière A, Klumperman J, Schlatter M, Delamarre L, Mellman I. Endosomes are specialized platforms for bacterial sensing and NOD2 signalling. Nature 2014; 509:240-4; PMID:24695226; https://doi.org/ 10.1038/nature13133 [DOI] [PubMed] [Google Scholar]


