ABSTRACT
The plasma membrane is generally associated with underling actin cytoskeleton. When the plasma membrane detaches from actin filaments, it is expanded by the intracellular pressure and the spherical membrane protrusion which lacks underlying actin cortex, termed bleb, is formed. Bleb is widely used for migration across species; however, the molecular mechanism underlying membrane blebbing remains largely unknown. Our recent study revealed that 2 small GTPases, Rnd3 and RhoA, are important regulators of membrane blebbing. In the expanding blebs, Rnd3 is recruited to the plasma membrane and inhibits RhoA activity by activating RhoGAP. On the other hand, RhoA is activated at the retracting membrane and removes Rnd3 from plasma membrane by the activity of ROCK (Rho-associated protein kinase). ROCK is also important for the rapid reassembly of actin cortex and retraction of membrane blebs by activating Ezrin. We propose that a Rnd3 and RhoA cycle underlies the core machinery of continuous membrane blebbing.
KEYWORDS: actin, blebbing, Rhoa, Rnd3, ROCK
The plasma membrane is generally associated with underling actin cytoskeleton. The interaction between plasma membrane and actin cytoskeleton is mediated by some anchor proteins such as ezrin/radixin/moesin (ERM) family proteins. When the plasma membrane detaches from actin filaments, spherical membrane protrusion is formed by the intracellular pressure.1-3 This membrane protrusion termed bleb is observed during cytokinesis4 and cell migration.5
Some cancer cells use membrane blebbing as a mode of motility during metastasis.6 When cancer cells are cultured on rigid substrates, cells move with forming actin-rich plasma membrane protrusions, such as lammellipodia and filopodia. On the other hand, cells migrate by forming membrane blebs when embedded in the 3D extracellular matrix such as collagen gels.7-9 Recently, several groups reported that cancer cells start to use membrane blebbing-associated cell migration in response to the physical changes of extracellular conditions. Down-regulation of cell adhesion to the extracellular matrix and up-regulation of physical confinement induce the formation of large polarized blebs in cancer cells.10-12 This bleb-based migration is the faster mode of migration as compared to the migration using lammellipodia and filopodia
The blebbing-associated cell migration is not only observed in cancer cells, but also under physiological conditions. For example, primordial germ cells (PGCs) migrate with forming membrane blebs in zebrafish13 and Drosophila melanogaster embryos.14 Dictyostelium also use membrane blebbing for migration during chemotaxis.15 Thus, membrane blebbing is widely used for migration across species.
The regulation of actin cytoskeleton during membrane blebbing
The process of expansion and retraction of blebs is largely depends on actin cytoskeleton organized underneath plasma membrane. Bleb forms when plasma membrane is detached from actin cytoskeleton. This is caused by the local increase of intracellular pressure or by the local rupture of actin cytoskeletons. After formation, bleb continues to expand; however, bleb stops expanding when the rapid reassembly of actin filaments is initiated underneath blebbing membrane. Then, actin filaments are polymerized and cover the protruded blebbing membrane. Finally, myosins are accumulated to the actin filaments, and actomyosin contraction drives the retraction of bleb16 (Fig. 1).
Figure 1.

The life cycle of bleb. Membrane blebs are formed when when plasma membrane is detached from underlying actin cytoskeleton. The detachment is caused by the increase of intracellular pressure or by the local rupture of actin filaments. After expansion of membrane protrusion, ERM family proteins and Eps8 are recruited to the protruded bleb membrane. Then, reassembly of actin filaments occurs rapidly and covers the protruded membrane. Finally, myosin is recruited to actin cortex and the acto-myosin cortex retracts the protruded membranes.
As just described, the processes of a bleb-cycle seem to be simple; however, it is unknown how the assembly of actin cortex is regulated in these processes.17 To elucidate how actin cortex is rebuilt during retraction of blebs, Charras et al. observed changes of localization of GFP-tagged regulatory proteins of actin filaments during a bleb cycle.16 This seminal paper showed that regulatory proteins of actin filaments are recruited to the protruded membrane in a staged manner. Among tested proteins, Ezrin/Radixin/Moesin (ERM) family proteins rapidly come to the plasma membrane prior to the regrowth of actin filaments. On the other hand, the recruitment of myosin II and myosin light chain to the membrane blebs occurs after actin filaments covers the protruded membrane and coincided with the onset of bleb retraction. Subsequently, Stastna et al. and Bovellan et al. demonstrated that formin family proteins, key regulators of actin nucleation, are recruited to the membrane blebs and essential for the reassembly of actin filaments.18,19 In addition, Logue et al. showed that Eps8, a plus end capping protein, is recruited to the plasma membrane on a similar timescale as ezrin and essential for the formation of membrane blebs.20 Thus, important regulators of actin cortex during membrane blebs were identified; however how these proteins are coordinated during reassembly of actin filaments in membrane blebs has remained unclear.
RhoA-ROCK-Rnd3 feedback loop regulates the expansion and retraction of blebs
In our recent study, we revealed that the actin-cortex reassembly during membrane blebbing is regulated by the activities of 2 different small GTPases, Rnd3 and RhoA.21 In the retracting phase of membrane blebs, we found that RhoA is activated at the plasma membrane. RhoA GTP-form and ROCK are recruited to the plasma membrane at the onset of bleb retraction. As already reported,16 GFP-ezrin did not show a restricted distribution pattern but localized uniformly at the blebbing membrane. However, we found that ezrin is phosphorylated at Thr-567 and adopts an active open conformation at retracting membrane. Ezrin is reported to be phosphorylated and activated by ROCK.22 We showed that activation of ezrin is required for the recruitment of Eps8 to the plasma membrane. The activation of ezrin and recruitment of Eps8 at the plasma membrane induces the rapid retraction of the protruded membrane by promoting the reassembly of the actin cortex. In addition to the activation of ezrin, active RhoA also activates another RhoA effector protein, formins, which are essential for the regrowth of actin filaments at the retracting membrane.18,19
In the expanding phase of membrane blebs, we found that Rnd3 is recruited to the expanding plasma membrane. The membrane localization of Rnd3 was gradually lost when reassembly of actin filaments started (Fig. 2). Rnd3 was reported to bind p190RhoGAP and enhance its activity.23 Rnd3 is a small GTPase which takes a constitutively GTP bound active form. Rnd3 antagonizes RhoA activity by activating p190RhoGAP.24
Figure 2.

Rnd3 gradually disappears from blebbing membrane at the onset of the retraction phase. Membrane blebbing of a DLD1 cell, a human colon cancer cell, transfected with Lifeact–RFP and GFP-tagged Rnd3. Rnd3 was recruited to expanding membrane blebs; however, the membrane localization of Rnd3 is gradually lost during the retraction of membrane blebs. (Scale bar, 2 μm.)
On the contrary, it was reported that Rnd3 binds to ROCK25 and is a substrate of ROCK.26 ROCK phosphorylates Rnd3 at serine 240 which leads to the sequestration of Rnd3 in the cytoplasm via its binding to 14-3-3 protein.3 Therefore, RhoA-ROCK inhibits Rnd3 activity. When Rnd3 S240A mutant, which is resistant to inhibition by ROCK, is overexpressed, Eps8 was no longer recruited to the plasma membrane and the reassembly of actin cortex was significantly delayed, resulting in the reduction of retraction speed of protruded membrane and the formation of abnormally large blebs.21
Taken together, we propose that the interlocked feedback system between Rnd3 and RhoA rapidly switches during membrane expansion to retraction in blebbing membrane. At the expanding plasma membrane, Rnd3 and RhoGAP are present at the plasma membrane and inhibit RhoA-ROCK activity. During this phase, reassembly of actin cortex was largely inhibited and bleb continues to expand passively by the intracellular pressure. However, when the protruded membrane area is increased, the concentration of Rnd3 at the plasma membrane gradually decreases. And sporadic activation of RhoA may be stabilized locally by the phosphorylation of Rnd3 by RhoA-ROCK pathway and the removal of Rnd3 from the plasma membrane. The local reduction of Rnd3 at the plasma membrane leads to the down regulation of RhoGAP activity, which amplifies the RhoA activation, and RhoA-ROCK pathway becomes dominant at the plasma membrane. ROCK phosphorylates ezrin and promotes recruitment of Eps8 to the plasma membrane. Finally, actin filaments are reassembled locally, and the retraction of membrane blebs starts (Fig. 3).21
Figure 3.
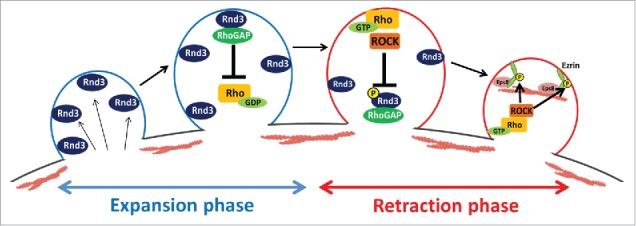
A model of the regulation of actin cortex by Rnd3 and RhoA during membrane blebbing. In the expansion phase of membrane blebs, Rnd3 and p190RhoGAP inhibit the activation of RhoA and regrowth of actin filaments. As the surface area of the protruded membrane increases during expansion, the concentration of Rnd3 at the plasma membrane decreases and sporadic activation of RhoA may occur. Locally activated RhoA activates ROCK. ROCK phosphorylates Rnd3, which leads to the removal of Rnd3 and p190RhoGAP from the plasma membrane. Thus, RhoA activation is amplified and sustained by the positive-feedback loop. ROCK also phosphorylates ezrin, which leads to the recruitment of Eps8 to the plasma membrane. Activated ezrin, Eps8 and activated formins promote the reassembly of actin cortex and retraction of membrane blebs.
Future directions
Finally, we would like to discuss some unsolved important issues briefly. As shown in Figure 2, Rnd3 accumulates at expanding bleb membrane, but the molecular mechanism remains elusive. We found that when cells were treated with LatrunculinB to disrupt actin cortex, Rnd3 persisted at the protruded membrane.21 This observation indicates that Rnd3 preferentially localizes to the cortex-free plasma membrane. Furthermore, it is unclear what is a trigger which switches from the Rnd3-dominant expansion phase to the RhoA-dominant retraction phase during bleb cycle. We showed that interlocked feedback loops between Rnd3 and RhoA is a core machinery of bleb cycle. However, some as yet un-identified cue may promote the transition from the expanding phase to the retraction phase. One of such candidates is a change of physical properties of bleb membrane such as membrane tension or membrane curvature. Membrane tension reportedly changes during bleb expansion.1 And it was recently reported that the plasma membrane tension modulates the activity of small GTPases.8 Therefore, it will be interesting to consider about the relationships between the Rnd3 activity and the changes of physiological condition of blebbing membrane such as plasma membrane tension and intracellular pressure.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Tinevez JY, Schulze U, Salbreux G, Roensch J, Joanny JF, Paluch E. Role of cortical tension in bleb growth. Proc Natl Acad Sci U S A 2009; 106:18581-6; PMID:19846787; https://doi.org/ 10.1073/pnas.0903353106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albrecht-Buehler G. Does blebbing reveal the convulsive flow of liquid and solutes through the cytoplasmic meshwork? Cold Spring Harb Symp Quant Biol 1982; 46 Pt 1:45-9; PMID:6955087; https://doi.org/ 10.1101/SQB.1982.046.01.008 [DOI] [PubMed] [Google Scholar]
- [3].Cunningham CC. Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol 1995; 129:1589-99; PMID:7790356; https://doi.org/ 10.1083/jcb.129.6.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Conrad GW, Davis SE. Polar lobe formation and cytokinesis in fertilized eggs of Ilyanassa obsoleta. III. Large bleb formation caused by Sr2+, ionophores X537A and A23187, and compound 48/80. Dev Biol 1980; 74:152-72; PMID:6765932; https://doi.org/ 10.1016/0012-1606(80)90058-5 [DOI] [PubMed] [Google Scholar]
- [5].Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol 2008; 181:879-84; PMID:18541702; https://doi.org/ 10.1083/jcb.200802081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Madsen CD, Hooper S, Tozluoglu M, Bruckbauer A, Fletcher G, Erler JT, Bates PA, Thompson B, Sahai E. STRIPAK components determine mode of cancer cell migration and metastasis. Nat Cell Biol 2015; 17:68-80; PMID:25531779; https://doi.org/ 10.1038/ncb3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol 2008; 9:730-6; PMID:18628785; https://doi.org/ 10.1038/nrm2453 [DOI] [PubMed] [Google Scholar]
- [8].Trinkaus JP, Trinkaus M, Fink RD. On the convergent cell movements of gastrulation in Fundulus. J Exp Zool 1992; 261:40-61; PMID:1729385; https://doi.org/ 10.1002/jez.1402610107 [DOI] [PubMed] [Google Scholar]
- [9].Bergert M, Chandradoss SD, Desai RA, Paluch E. Cell mechanics control rapid transitions between blebs and lamellipodia during migration. Proc Natl Acad Sci U S A 2012; 109:14434-9; PMID:22786929; https://doi.org/ 10.1073/pnas.1207968109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu YJ, Le Berre M, Lautenschlaeger F, Maiuri P, Callan-Jones A, Heuze M, Takaki T, Voituriez R, Piel M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015; 160:659-72; PMID:25679760; https://doi.org/ 10.1016/j.cell.2015.01.007 [DOI] [PubMed] [Google Scholar]
- [11].Ruprecht V, Wieser S, Callan-Jones A, Smutny M, Morita H, Sako K, Barone V, Ritsch-Marte M, Sixt M, Voituriez R, et al.. Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell 2015; 160:673-85; PMID:25679761; https://doi.org/ 10.1016/j.cell.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bergert M, Erzberger A, Desai RA, Aspalter IM, Oates AC, Charras G, Salbreux G, Paluch EK. Force transmission during adhesion-independent migration. Nat Cell Biol 2015; 17:524-9; PMID:25774834; https://doi.org/ 10.1038/ncb3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell 2006; 11:613-27; PMID:17084355; https://doi.org/ 10.1016/j.devcel.2006.09.023 [DOI] [PubMed] [Google Scholar]
- [14].Jaglarz MK, Howard KR. The active migration of Drosophila primordial germ cells. Development 1995; 121:3495-503; PMID:8582264 [DOI] [PubMed] [Google Scholar]
- [15].Zatulovskiy E, Tyson R, Bretschneider T, Kay RR. Bleb-driven chemotaxis of Dictyostelium cells. J Cell Biol 2014; 204:1027-44; PMID:24616222; https://doi.org/ 10.1083/jcb.201306147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol 2006; 175:477-90; PMID:17088428; https://doi.org/ 10.1083/jcb.200602085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Charras GT. A short history of blebbing. J Microsc 2008; 231:466-78; PMID:18755002; https://doi.org/ 10.1111/j.1365-2818.2008.02059.x [DOI] [PubMed] [Google Scholar]
- [18].Stastna J, Pan X, Wang H, Kollmannsperger A, Kutscheidt S, Lohmann V, Grosse R, Fackler OT. Differing and isoform-specific roles for the formin DIAPH3 in plasma membrane blebbing and filopodia formation. Cell Res 2012; 22:728-45; PMID:22184005; https://doi.org/ 10.1038/cr.2011.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bovellan M, Romeo Y, Biro M, Boden A, Chugh P, Yonis A, Vaghela M, Fritzsche M, Moulding D, Thorogate R, et al.. Cellular control of cortical actin nucleation. Curr Biol 2014; 24:1628-35; PMID:25017211; https://doi.org/ 10.1016/j.cub.2014.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Logue JS, Cartagena-Rivera AX, Baird MA, Davidson MW, Chadwick RS, Waterman CM. Erk regulation of actin capping and bundling by Eps8 promotes cortex tension and leader bleb-based migration. Elife 2015; 4:e08314; PMID:26163656; https://doi.org/ 10.7554/eLife.08314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Aoki K, Maeda F, Nagasako T, Mochizuki Y, Uchida S, Ikenouchi J. A RhoA and Rnd3 cycle regulates actin reassembly during membrane blebbing. Proc Natl Acad Sci U S A 2016; 113(13):E1863-71; https://doi.org10.1073/pnas.1600968113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 1998; 140:647-57; PMID:9456324; https://doi.org/ 10.1083/jcb.140.3.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol 2006; 7:54-62; PMID:16493413; https://doi.org/ 10.1038/nrm1788 [DOI] [PubMed] [Google Scholar]
- [24].Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr Biol 2003; 13:1106-15; PMID:12842009; https://doi.org/ 10.1016/S0960-9822(03)00418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Villalonga P, Guasch RM, Riento K, Ridley AJ. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol Cell Biol 2004; 24:7829-40; PMID:15340047; https://doi.org/ 10.1128/MCB.24.18.7829-7840.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J 2005; 24:1170-80; PMID:15775972; https://doi.org/ 10.1038/sj.emboj.7600612 [DOI] [PMC free article] [PubMed] [Google Scholar]


