ABSTRACT
Activity-dependent modifications in the strength of excitatory synapses are considered to be major cellular mechanisms that contribute to the plasticity of neuronal networks underlying learning and memory. Key mechanisms for the regulation of synaptic efficacy involve the dynamic changes in size and number of dendritic spines, as well as the synaptic incorporation and removal of AMPA-type glutamate receptors (AMPAr). As key regulators of the actin cytoskeleton, the Rho subfamily of GTP-binding proteins play a critical role in synaptic development and plasticity. They shuttle between the active GTP-bound form and the inactive GDP-bound form under the regulation of dedicated guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). More than 80 human GEFs and 70 GAPs have been identified, most of which are expressed in the brain with a specific spatial and temporal expression pattern. However, the function of most GEFs and GAPs in the brain has not been elucidated. In this review, we highlight the novel neuronal function of the synaptic RhoGAP ARHGAP12 and the ID-associated RhoGEF TRIO and further propose 3 possible approaches of neurons utilizing Rho GTPase regulatory proteins to accurately modulate synaptic function.
KEYWORDS: ARHGAP12, excitatory synapse, hippocampal development, Rho GTPases, TRIO
Introduction
Chemical signals are transmitted between neurons via specialized structures named synapses. The vast majority of excitatory synapses in which glutamate is the predominant neurotransmitter are located on spines, actin cytoskeleton enriched protrusions on the dendrite. The adaptive plastic property of neurons, namely the ability of rapidly changing the morphology and the molecular composition at excitatory synapses in response to experience, is widely believed to be the cellular basis of learning and memory. The long-lasting increase and decrease in synaptic strength, known as long-term potentiation (LTP) and long-term depression (LTD), respectively, are 2 most well-studied forms of Hebbian synaptic plasticity. Considerable experimental evidence has demonstrated that alterations in the size and the amount of dendritic spines, which largely rely on remodeling of actin cytoskeleton, are correlated with the efficacy of excitatory synaptic transmission (reviewed in ref. 1). Interestingly, intimate association between LTP/LTD and enlargement/shrinkage of dendritic spines have been observed,2,3 which further supports the positive correlation between spine structure and synaptic strength, and leads to the concept of structural plasticity and functional plasticity.
Due to the capability of directly controlling actin dynamics and organization, Rho GTPase family members have been pointed as key contributors for orchestrating changes in synaptic structure and function. Belonging to the Ras superfamily of small GTPases, Rho GTPases function as molecular switches cycling between an active GTP-bound form and an inactive GDP-bound form. Among 22 members of Rho family identified so far, RhoA has been demonstrated to inhibit spine formation and maintenance while Rac1 and Cdc42 display opposing effect in both cellular and animal models.4-6 The activity of Rho GTPase is mainly regulated by guanine nucleotide exchange factors (GEFs), which are positive regulators, by GTPases activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs), which are negative regulators. Several critical GEFs and GAPs have been found to be localized at synapses and play multifaceted roles in regulating synaptic function.7-11 For instance, the extensively studied RhoGEF Kalirin7 (Kal7) has been found to exclusively localize to the postsynaptic compartment of excitatory synapses in hippocampus. Elevated Kal7 levels increase spine density and size, whereas downregulation of endogenous Kal7 leads to a decrease in spine and synapse density both in vitro and in vivo.12,13 Also, loss of Kal7 results in a decrease of NR2B containing N-methyl-D-aspartate receptors (NMDArs) in PSDs and specifically impairs NMDAr-dependent LTP and LTD.14 In addition, another GEF Tiam1 has been shown to regulate axon extension and neuronal migration both in vitro and in vivo.15-17 Knocking down Tiam1 significantly reduces dendritic arborization and spine density, as well as the frequency of miniature excitatory postsynaptic current (mEPSC) in cultured hippocampal neurons.18-20 More recently, Um et al. have demonstrated that Tiam1 forms a GEF/GAP complex with RacGAP Bcr at synapses, which is essential for rapidly regulating Rac1 signaling and maintaining synaptogenesis in an optimal range.21
Growing evidence highlights the association between synaptic dysfunction and neurodevelopment disorders including intellectual disability (ID) and autism spectrum disorders (ASDs). A series of mutations in genes encoding Rho GTPases signaling have been identified in patients with ID and/or ASDs.22 There is no doubt that revealing the precise function of individual Rho proteins will shed light on the mechanisms underlying ID and ASDs. Including ID associated proteins OPHN1, ARHGEF6 and Kalirin, more than 80 GEFs and more than 70 GAPs exist in the human genome,23-25 providing a ratio between the number of Rho GTPases and GEFs/GAPs about 4 to 1. Although lots of efforts have been made to unmask the detailed regulatory role of Rho proteins in different developmental stages of the central nervous system (CNS), the field still lacks a complete picture of how the brain utilizes “overabundant” molecules involved in Rho GTPase signaling to achieve a precise regulation of synaptic strength during development and cognition. It is hypothesized that excess GEFs and GAPs enable neurons to temporally and spatially restrict their responses to local extracellular cues and further ensure proper connectivity for learning and memory (reviewed in refs. 26, 27). Here, based on the previous findings and the recent functional characterizations of the novel synaptic RhoGAP ARHGAP12 28 and the ID associated RhoGEF TRIO,29 we further propose 3 possible approaches, in addition to previously summarized regulatory manners (reviewed in ref. 27), for neurons to accurately control synaptic strength via Rho GTPase signaling pathways during development.
Diverse approaches accomplishing precise regulation of synaptic efficacy via Rho GTPase signaling
Using individual multifunctional GEFs/GAPs at synapses
We recently uncovered a dual function for the previously uncharacterized RhoGAP, ARHGAP12, specifically at hippocampal excitatory synapses during development28 (Fig. 1A). ARHGAP12 is able to orchestrate synaptic efficacy by modulating both spine morphology and surface α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAr) levels at the post-synaptic compartment. Overexpression of ARHGAP12 reduced both spine density and volume while knocking down ARHGAP12 resulted in increased spine volume without affecting spine density. Functionally, elevated levels of ARHGAP12 significantly depressed CA3-CA1 synapses, and on the contrary, potentiated excitatory synaptic transmission was observed in ARHGAP12 downregulated neurons. Our data further showed that ARHGAP12 is able to actively regulate excitatory AMPAr endocytosis. More importantly, we demonstrated that 2 distinct pathways are engaged to mediate structural and functional alterations respectively. On one hand, the GAP activity of ARHGAP12 allows it to regulate the activity of its target Rac1 GTPase and subsequently modulate the morphology of dendritic spines. On the other hand, by interacting with the F-BAR protein CIP4, ARHGAP12 is involved in the endocytic machinery and further regulates AMPAr endocytosis. A similar mechanism has been observed in studies of OPHN1, whose mutations have been associated with ID.30 OPHN1 regulates spine structure through its RhoGAP activity and maintains normal AMPAr recycling via interacting with Homer1b/c.8,31 Moreover, via interactions with endophilin A2/3, OPHN1 also mediates persistent decreases in surface AMPArs in mGluR-LTD.32 Together, these findings strongly indicate that neurons can optimize synaptic efficacy by making use of individual multifunctional proteins involved in Rho GTPase signaling at synapses (Fig. 3A). It is not hard to imagine that several advantages may come from this approach. Firstly, using one protein to regulate cellular events that alter synaptic structure and function in the same direction would greatly reinforce and ensure desired synaptic modifications in response to experience and/or development to occur correctly. Secondly, it enables neurons to respond to external cues more rapidly since one signal might already be sufficient to trigger structural and functional modifications simultaneously. Lastly, it provides an optimal energetic setting at synapses in the CNS. It has been shown that the largest component of brain energy is used at synapses and disruptions thereof have been found to present pathological effects (reviewed in ref. 33). Using multifunctional Rho GTPases and their regulators may contribute to turning on an energy-saving mode at synapses and maximize energy supply for normal cognitive processes.
Figure 1.
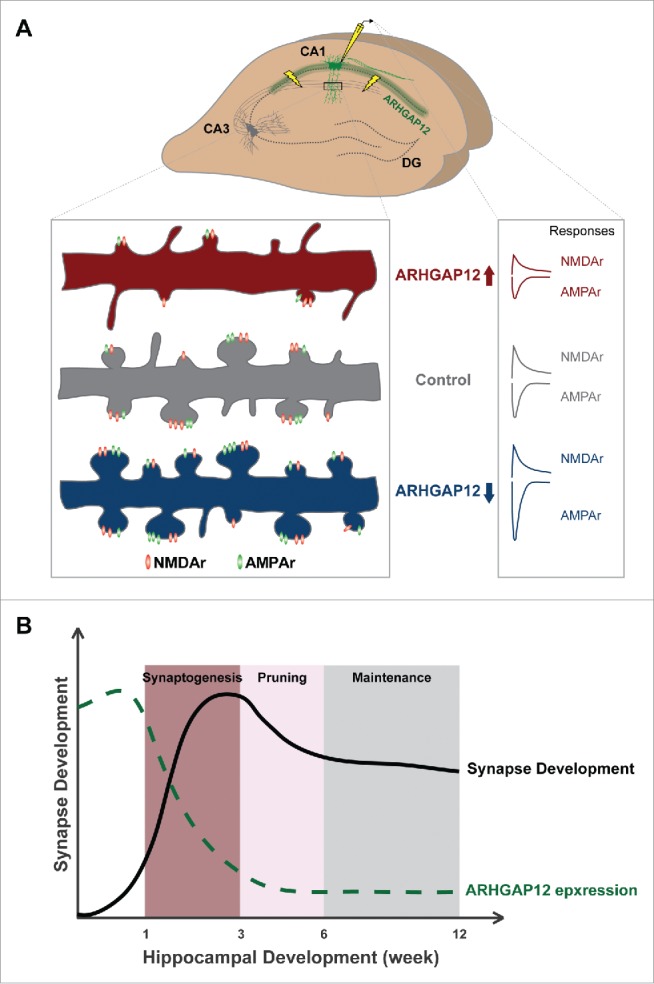
Synaptic function of ARHGAP12 during hippocampal development. (A) ARHGAP12 is almost exclusively expressed in CA1 excitatory neurons. Overexpression of ARHGAP12 significantly reduces both spine density and volume while downregulation leads to increased spine size without affecting the density. Electrophysiologically, elevated ARHGAP12 levels depress both AMPAr- and NMDAr-mediated synaptic transmission whereas reduced ARHGAP12 levels specifically enhance AMPAr-mediated transmission. In addition, knocking down ARHGAP12 promotes silent synapses converting to functional synapses. (B) ARHGAP12 acts as a synaptic “brake” during hippocampal development. In rodent, rapid synaptogenesis in the hippocampus occurs between the second and the third postnatal week followed by a selective pruning in adolescence and maintenance in adulthood. The expression of ARHGAP12 protein in CA1 subregion gradually declines in the first 3 postnatal weeks and sustains low levels throughout hippocampal development, which releases the “brake” and allows for synaptogenesis.
Figure 3.
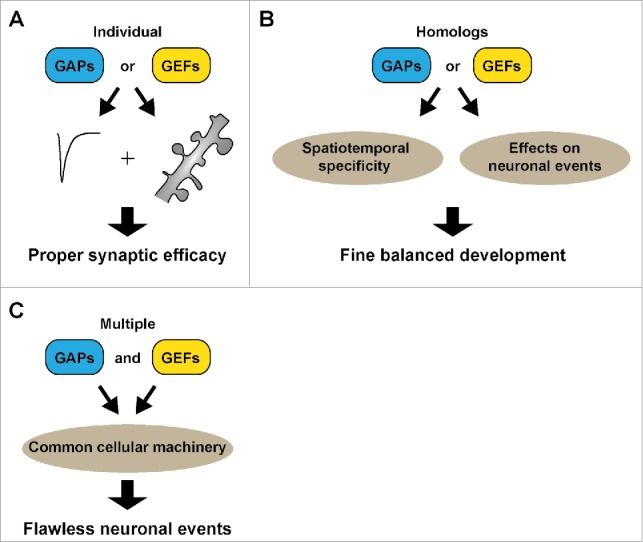
Three approaches utilized by neurons to precisely regulate synaptic efficacy. (A) Using individual multifunctional Rho GEFs/GAPs to control synaptic structure and function. (B) Using homologs displaying distinct effects on neuronal and synaptic development. (C) Recruiting multiple GEFs/GAPs to target a common cellular event.
In line with the spatiotemporal specificity of ARHGAP12, namely its unique expression pattern in excitatory neurons of CA1 subregion at early developmental stages, our results suggest that ARHGAP12 functions as a synaptic “brake” during hippocampal development by limiting silent synapses converting to active synapses (Fig. 1B). Additionally, we also observed a positive feedback loop between synaptic activity and ARHGAP12 mediated signaling pathway, in the sense that synaptic activity is required for ARHGAP12 repression, and in turn, ARHGAP12 downregulation enhances synaptic efficacy. Overall, these results indicate that by regulating the levels of individual multifunctional molecules involved in Rho GTPase singnaling, neurons may adjust to environmental stimuli and developmental changes, and consequently maintain synaptic strength and connectivity in an optimal range.
Notably, Arhgap12 mRNA was recently identified as a potential target of FMRP, an RNA binding protein that represses translation34 and loss of function mutations in FMRP gene result in Fragile X Syndrome (FXS). A form of protein synthesis-dependent synaptic plasticity, mGluR-LTD, has been demonstrated to be exaggerated in a mouse model of FXS (Fmr1 KO mice), due to the absence of FMRP mediated repression of “LTD” proteins. Several mechanisms including PICK1-GluA2 interaction,35,36 OPHN1-endophilin interaction,32 PKC-dependent phosphorylation of GluA2, and activation of the Rac1-LimK-cofilin signaling pathway35-38 have been implied in the regulation of mGluR-LTD. Based on these reports and our observation of ARHGAP12 being a repressor of spine morphology and synaptic strength, we speculate that ARHGAP12 could be perfectly situated to act as a coordinator to structurally and functionally weaken synapses during plasticity. Moreover, it could also serve as a potential target to reverse the exaggerated mGluR-LTD phenotype observed in FXS. One of the current focuses in our group is to seek direct experimental evidence to evaluate this hypothesis. Experiments combining electrophysiological, molecular biological and behavioral approaches in Arhgap12 knockout animals will provide novel insight of how ARHGAP12 is involved in mGluR-LTD and how neurons command multifunctional GEFs and GAPs to precisely regulate cognitive processes.
Using homologs displaying opposing effect
Many of the GAPs and GEFs are highly identical in terms of their structure. As it has been proposed, the spatiotemporal expression pattern could explain the functional divergence. A typical example is α-chimaerin, a RhoGAP possessing GAP activity toward Rac1 and to a lesser extend to Cdc42. There are 2 isoforms of α-chimaerin, α1- and α2- chimaerin, which both contain C1 and RhoGAP domains but differ in that the α2-chimaerin contains an N-terminal SH2 domain that is absent from α1.39 Interestingly, both isoforms strongly differ in their temporal expression. Whereas α2-chimaerin is strongly expressed early in development, the expression of α1-chimaerin coincides with synaptogenesis, resulting in a high expression in mature neurons. In concordance, both isoforms have been found to have very distinct functions. Αlpha2-chimaerin has been found important for axon guidance and neuron migration,9,40,41 whereas α1-chimaerin has a specific role in spine pruning in the hippocampus and cerebellum.42,43
In addition to the discoveries above, homologs involved in Rho GTPase signaling may also target common cellular processes during development in a counterproductive manner. A striking example occurs along the functional characterization of Trio and its ortholog Kalirin. Initial studies indicated that Trio full-knockout mice display embryonic lethality.44 Moreover, disrupted cerebellum development, such as abnormal neurite growth and granule cell migration, has been observed in conditional knockout mice of Trio.45 Recently, we identified TRIO as a responsible gene for mild to borderline ID and further revealed its contribution during neurite outgrowth and basal synaptic transmission.29 Our results showed that reduced Trio levels promote neurite outgrowth and specifically enhance AMPAr-mediated transmission, indicating that the endogenous TRIO restricts these 2 critical cellular events (Fig. 2). Surprisingly, although sharing more than 80% of the sequence, the effect exhibited by TRIO is opposing to that displayed by Kalirin, which stimulates neurites outgrowth and is required for activity-dependent spine enlargement and enhancement of AMPAr-mediated transmission. This scenario is of particular interest since it represents another approach of neurons accurately regulating multiple critical events of neuronal development. By choosing structural identical homologs displaying opposite synaptic function, neurons may achieve a fine balance that is required for all neuronal events including migration and synaptogenesis and accurately steer brain development in the proper direction (Fig. 3B). Of note, a recent study reported that neurons with elevated levels of a specific isoform of TRIO, TRIO-9, displayed increased AMAPr-mediated synaptic transmission.46 Also, knocking down endogenous TRIO at 1 day in vitro (1 DIV) when Trio is the most profoundly expressed led to a deficit in synaptic transmission,46 an opposite effect of reducing TRIO levels at a later time point, 4 DIV. These findings imply that variants of GEFs and GAPs might exhibit distinct function and aberrations in their levels occurring at different stages of development may result in diverse, even completely opposing impact at synapses. Future experiments are needed to test this idea.
Figure 2.
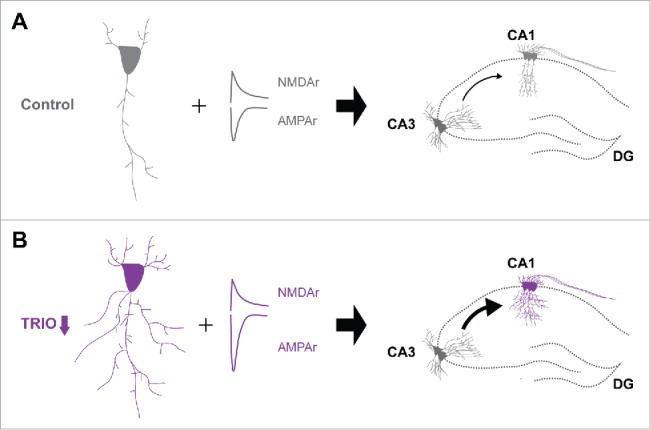
Synaptic function of TRIO in the hippocampus. Reduced expression of TRIO increases dendritic complexity and enhances synaptic transmission at CA3-CA1 synapses.
Recruiting multiple GEFs/GAPs to target common cellular events
A recent study reported the contribution of ARHGAP12 in regulating phagocytosis,47 an important event responsible for eliminating particles in diverse cell types. A common feature of the phagocytic process, regardless of the type of receptor bound or the size of the target particles, is the involvement of actin cytoskeleton remodeling.48 Interestingly, other studies showed that both phagocytosis and excitatory synaptogenesis require Rac1 activation downstream of BAI1.20,49 Schlam et al. have demonstrated that ARHGAP12 acts synergistically with other 2 GAPs, SH3BP1 and ARHGAP25, to disassemble actin, a critical step underlying phagosome maturation for completing the internalization of large targets. Sustained Rac or Cdc42 activities resulted from silencing one of the 3 Rho GAPs can compromise phagocytic efficiency.47 These findings, in RAW 264.7 cell lines, may provide a hint of why and how multiple Rho regulators are required in CNS. Instead of being functionally redundant, multiple Rho regulators, with overlapping functions, might be engaged in the same neuronal event to ensure it takes place flawlessly (Fig. 3C). Given that both endogenous ARHGAP12 and TRIO are the most abundant at early developmental stages in the hippocampus and that both are present at synapses and limit synaptic transmission, it stands to reason that ARHGAP12 and TRIO may serve as GEF-GAP partners and restrict synaptic strength collaboratively in order to keep neuronal connectivity optimal. Future experiments are required to evaluate this possibility, clarify respective contributions of ARHGAP12 and TRIO and further identify more pivotal GEFs and/or GAPs partners in controlling normal synaptic function.
Conclusion
Detailed functional analysis of Rho GTPases and their regulatory proteins will provide mechanistic insight of how the normal brain regulates cognition and how neurological disorders occur. In addition to the continuous investigation of unraveling neuronal function of individual GEFs and GAPs, future experiments might also aim for generating an expression profile of Rho regulators at the single cell, perhaps even at single synapse level. Indeed, novel imaging techniques combined with reporter molecules allow to investigate Rho GTPase signaling at single synapse level.50 Different cell types at different brain regions may possess their unique identities reflected by distinct compositions of GEFs and GAPs, which might ultimately determine their responses and contributions in cognitive processes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to colleagues whose work could not be cited due to space limitations.
Funding
The research of the authors is supported by grants from the “Hypatia fellowship award of the Radboudumc” [to N.N.K.]; the “FP7-Marie Curie International Reintegration Grant” [to N.N.K. grant number 277091]; the Jerome Lejeune Foundation [to N.N.K]; the Netherlands Organization for Scientific Research (open ALW ALW2PJ/13082 to HvB/NNK).
References
- [1].Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol 2010; 189:619-29; PMID:20457765; https://doi.org/ 10.1083/jcb.201003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 1999; 399:66-70; PMID:10331391; https://doi.org/ 10.1038/19978 [DOI] [PubMed] [Google Scholar]
- [3].Zhou Q, Homma KJ, Poo M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004; 44:749-57; PMID:15572107; https://doi.org/ 10.1016/j.neuron.2004.11.011 [DOI] [PubMed] [Google Scholar]
- [4].Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci 2004; 19:3151-64; PMID:15217371; https://doi.org/ 10.1111/j.0953-816X.2004.03380.x [DOI] [PubMed] [Google Scholar]
- [5].Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 2000; 20:5329-38; PMID:10884317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tashiro A, Minden A, Yuste R. Regulation of dendritic spine morphology by the Rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 2000; 10:927-38; PMID:11007543; https://doi.org/ 10.1093/cercor/10.10.927 [DOI] [PubMed] [Google Scholar]
- [7].Nakano-Kobayashi A, Nadif Kasri N, Newey SE, Van Aelst L. The Rho-linked mental retardation protein OPHN1 controls synaptic vesicle endocytosis via endophilin A1. Curr Biol 2009; 19:1133-9; PMID:19481455; https://doi.org/ 10.1016/j.cub.2009.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev 2009; 23:1289-302; PMID:19487570; https://doi.org/ 10.1101/gad.1783809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iwata R, Ohi K, Kobayashi Y, Masuda A, Iwama M, Yasuda Y, Yamamori H, Tanaka M, Hashimoto R, Itohara S, et al.. RacGAP α2-Chimaerin Function in Development Adjusts Cognitive Ability in Adulthood. Cell Rep 2014; 8:1257-64; PMID:25159148; https://doi.org/ 10.1016/j.celrep.2014.07.047 [DOI] [PubMed] [Google Scholar]
- [10].Jaudon F, Raynaud F, Wehrle R, Bellanger J-M, Doulazmi M, Vodjdani G, Gasman S, Fagni L, Dusart I, Debant A, et al.. The RhoGEF DOCK10 is essential for dendritic spine morphogenesis. Mol Biol Cell 2015; 26:2112-27; PMID:25851601; https://doi.org/ 10.1091/mbc.E14-08-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim J-Y, Oh MH, Bernard LP, Macara IG, Zhang H. The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons. J Biol Chem 2011; 286:37615-24; PMID:21900250; https://doi.org/ 10.1074/jbc.M111.268029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ma X-M, Kiraly DD, Gaier ED, Wang Y, Kim E-J, Levine ES, Eipper B a, Mains RE. Kalirin-7 is required for synaptic structure and function. J Neurosci 2008; 28:12368-82; PMID:19020030; https://doi.org/ 10.1523/JNEUROSCI.4269-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ma XM, Huang JP, Kim EJ, Zhu Q, Kuchel GA. Mains RE, Eipper B a. Kalirin-7, an important component of excitatory synapses, is regulated by estradiol in hippocampal neurons. Hippocampus 2011; 21:661-77; PMID:20333733; https://doi.org/ 10.1002/hipo.20780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lemtiri-Chlieh F, Zhao L, Kiraly DD, Eipper B a, Mains RE, Levine ES. Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity. BMC Neurosci 2011; 12:126; PMID:22182308; https://doi.org/ 10.1186/1471-2202-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. The in vivo roles of STEF / Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J 2003; 22:4190-4201; PMID:12912917; https://doi.org/ 10.1093/emboj/cdg413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matsuo N, Terao M, Nabeshima YI, Hoshino M. Roles of STEF/Tiam1, guanine nucleotide exchange factors for Rac1, in regulation of growth cone morphology. Mol Cell Neurosci 2003; 24:69-81; PMID:14550769; https://doi.org/ 10.1016/S1044-7431(03)00122-2 [DOI] [PubMed] [Google Scholar]
- [17].Patricia K, Gabriela P, Caceres A. Evidence for the Involvement of Tiam1 in Axon Formation. Mol Biol Cell 2001; 21:2261-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol 2006; 8:227-37; PMID:16474385; https://doi.org/ 10.1038/ncb1368 [DOI] [PubMed] [Google Scholar]
- [19].Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, Weinberg RJ, Greenberg ME. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 2005; 45:525-38; PMID:15721239; https://doi.org/ 10.1016/j.neuron.2005.01.024 [DOI] [PubMed] [Google Scholar]
- [20].Duman JG, Tzeng CP, Tu Y-K, Munjal T, Schwechter B, Ho TS-Y, Tolias KF. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci 2013; 33:6964-78; PMID:23595754; https://doi.org/ 10.1523/JNEUROSCI.3978-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Um K, Niu S, Duman JG, Cheng JX, Tu YK, Schwechter B, Liu F, Hiles L, Narayanan AS, Ash RT, et al.. Dynamic Control of Excitatory Synapse Development by a Rac1 GEF/GAP Regulatory Complex. Dev Cell 2014; 29:701-15; PMID:24960694; https://doi.org/ 10.1016/j.devcel.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ba W, van der Raadt J, Nadif Kasri N. Rho GTPase signaling at the synapse: Implications for intellectual disability. Exp Cell Res 2013; 319:2368-74; PMID:23769912; https://doi.org/ 10.1016/j.yexcr.2013.05.033 [DOI] [PubMed] [Google Scholar]
- [23].Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167-80; PMID:15688002; https://doi.org/ 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- [24].Moon SY, Zheng Y. Rho GTPase- activating proteins in cell regulation. Trends Cell Biol 2003; 13:13-22; PMID:12480336; https://doi.org/ 10.1016/S0962-8924(02)00004-1 [DOI] [PubMed] [Google Scholar]
- [25].Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007; 129:865-77; PMID:17540168; https://doi.org/ 10.1016/j.cell.2007.05.018 [DOI] [PubMed] [Google Scholar]
- [26].Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol 2011; 94:133-48; PMID:21530608; https://doi.org/ 10.1016/j.pneurobio.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Duman JG, Mulherkar S, Tu Y-K, Cheng J, Tolias KF. Mechanisms for spatiotemporal regulation of Rho-GTPase signaling at synapses. Neurosci Lett 2015; 601:4-10; PMID:26003445; https://doi.org/ 10.1016/j.neulet.2015.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ba W, Selten MM, van der Raadt J, van Veen H, Li L-L, Benevento M, Oudakker AR, Lasabuda RSE, Letteboer SJ, Roepman R, et al.. ARHGAP12 Functions as a Developmental Brake on Excitatory Synapse Function. Cell Rep 2016; 14:1-14; PMID:26725109; https://doi.org/ 10.1016/j.celrep.2016.01.037 [DOI] [PubMed] [Google Scholar]
- [29].Ba W, Yan Y, Reijnders MRF, Schuurs-Hoeijmakers JHM, Feenstra I, Bongers EMHF, Bosch DGM, de Leeuw N, Pfundt R, Gilissen C, et al.. TRIO loss of function is associated with mild intellectual disability and affects dendritic branching and synapse function. Hum Mol Genet 2016; 25:892-902; PMID:26721934; https://doi.org/ 10.1093/hmg/ddv618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Roest Crollius H, Carrié A, Fauchereau F, Cherry M, et al.. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature 1998; 392:923-6; PMID:9582072; https://doi.org/ 10.1038/31940 [DOI] [PubMed] [Google Scholar]
- [31].Nakano-Kobayashi A, Tai Y, Nadif Kasri N, Van Aelst L. The X-linked Mental Retardation Protein OPHN1 Interacts with Homer1b/c to Control Spine Endocytic Zone Positioning and Expression of Synaptic Potentiation. J Neurosci 2014; 34:8665-71; PMID:24966368; https://doi.org/ 10.1523/JNEUROSCI.0894-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nadif Kasri N, Nakano-Kobayashi A, Van Aelst L. Rapid synthesis of the X-linked mental retardation protein OPHN1 mediates mGluR-dependent LTD through interaction with the endocytic machinery. Neuron 2011; 72:300-15; PMID:22017989; https://doi.org/ 10.1016/j.neuron.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Harris JJ, Jolivet R, Attwell D. Synaptic Energy Use and Supply. Neuron 2012; 75:762-77; PMID:22958818; https://doi.org/ 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- [34].Ascano M, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, et al.. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 2012; 492:382-6; PMID:23235829; https://doi.org/ 10.1038/nature11737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rocca D, Amici M, Antoniou A, Suarez E, Halemani N, Murk K, McGarvey J, Jaafari N, Mellor J, Collingridge G, et al.. The Small GTPase Arf1 Modulates Arp2/3-mediated actin polymerization via PICK1 to regulate synaptic plasticity. Neuron 2013; 79:293-307; PMID:23889934; https://doi.org/ 10.1016/j.neuron.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JTR. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 2008; 57:872-82; PMID:18367088; https://doi.org/ 10.1016/j.neuron.2008.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 2010; 65:445-59; PMID:20188650; https://doi.org/ 10.1016/j.neuron.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou Z, Hu J, Passafaro M, Xie W, Jia Z. GluA2 (GluR2) regulates metabotropic glutamate receptor-dependent long-term depression through N-cadherin-dependent and cofilin-mediated actin reorganization. J Neurosci 2011; 31:819-33; PMID:21248105; https://doi.org/ 10.1523/JNEUROSCI.3869-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hall C, Sin WC, Teo M, Michael GJ, Smith P, Dong JM, Lim HH, Manser E, Spurr NK, Jones TA, et al.. Alpha 2-chimerin, an SH2-containing GTPase-activating protein for the ras-related protein p21rac derived by alternate splicing of the human n-chimerin gene, is selectively expressed in brain regions and testes. Mol Cell Biol 1993; 13:4986-98; PMID:8336731; https://doi.org/ 10.1128/MCB.13.8.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Beg A a., Sommer JE, Martin JH, Scheiffele P. α2-Chimaerin Is an Essential EphA4 Effector in the Assembly of Neuronal Locomotor Circuits. Neuron 2007; 55:768-78; PMID:17785183; https://doi.org/ 10.1016/j.neuron.2007.07.036 [DOI] [PubMed] [Google Scholar]
- [41].Ip JPK, Shi L, Chen Y, Itoh Y, Fu W-Y, Betz A, Yung W-H, Gotoh Y, Fu AKY, Ip NY. α2-chimaerin controls neuronal migration and functioning of the cerebral cortex through CRMP-2. Nat Neurosci 2011; 15:39-47; PMID:22138645; https://doi.org/ 10.1038/nn.2972 [DOI] [PubMed] [Google Scholar]
- [42].Buttery P, Beg A a, Chih B, Broder A, Mason C a, Scheiffele P. The diacylglycerol-binding protein alpha1-chimaerin regulates dendritic morphology. Proc Natl Acad Sci U S A 2006; 103:1924-9; PMID:16446429; https://doi.org/ 10.1073/pnas.0510655103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Van de Ven TJ, VanDongen HMA, VanDongen AMJ. The nonkinase phorbol ester receptor alpha 1-chimerin binds the NMDA receptor NR2A subunit and regulates dendritic spine density. J Neurosci 2005; 25:9488-96; PMID:16221859; https://doi.org/ 10.1523/JNEUROSCI.2450-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].O'Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci U S A 2000; 97:12074-8; https://doi.org/ 10.1073/pnas.97.22.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peng YJ, He WQ, Tang J, Tao T, Chen C, Gao YQ, Zhang WC, He XY, Dai YY, Zhu NC, et al.. Trio is a key guanine nucleotide exchange factor coordinating regulation of the migration and morphogenesis of granule cells in the developing cerebellum. J Biol Chem 2010; 285:24834-44; PMID:20516067; https://doi.org/ 10.1074/jbc.M109.096537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herring BE, Nicoll RA. Kalirin and Trio proteins serve critical roles in excitatory synaptic transmission and LTP. Proc Natl Acad Sci 2016; 113:2264-9; PMID:26858404; https://doi.org/ 10.1073/pnas.1600179113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schlam D, Bagshaw RD, Freeman SA, Collins RF, Pawson T, Fairn GD, Grinstein S. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun 2015; 6:8623; PMID:26465210; https://doi.org/ 10.1038/ncomms9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Freeman SA, Grinstein S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol Rev 2014; 262:193-215; PMID:25319336; https://doi.org/ 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- [49].Park D, Tosello-Trampont A-C, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007; 450:430-4; PMID:17960134; https://doi.org/ 10.1038/nature06329 [DOI] [PubMed] [Google Scholar]
- [50].Yasuda R. Studying signal transduction in single dendritic spines. Cold Spring Harb Perspect Biol 2012; 4:1-16; https://doi.org/ 10.1101/cshperspect.a005611 [DOI] [PMC free article] [PubMed] [Google Scholar]


