Abstract
Background.
Optimal adjuvant management of adult low-grade gliomas is controversial. Recently described tumor classification based on molecular subtype has the potential to individualize adjuvant therapy but has not yet been evaluated as part of a prospective trial.
Methods.
Patients aged 18 or older with newly diagnosed World Health Organization grade II low-grade gliomas and gross residual disease after surgical resection were enrolled in the study. Patients received monthly cycles of temozolomide for up to 1 year or until disease progression. For patients with available tissue, molecular subtype was assessed based upon 1p/19q codeletion and isocitrate dehydrogenase-1 R132H mutation status. The primary outcome was radiographic response rate; secondary outcomes included progression-free survival (PFS) and overall survival (OS).
Results.
One hundred twenty patients were enrolled with median follow-up of 7.5 years. Overall response rate was 6%, with median PFS and OS of 4.2 and 9.7 years, respectively. Molecular subtype was associated with rate of disease progression during treatment (P<.001), PFS (P=.007), and OS (P<.001). Patients with 1p/19q codeletion demonstrated a 0% risk of progression during treatment. In an exploratory analysis, pretreatment lesion volume was associated with both PFS (P<.001) and OS (P<.001).
Conclusions.
While our study failed to meet the primary endpoint for objective radiographic response, patients with high-risk low-grade glioma receiving adjuvant temozolomide demonstrated a high rate of radiographic stability and favorable survival outcomes while meaningfully delaying radiotherapy. Patients with 1p/19q codeletion are potential candidates for omission of adjuvant radiotherapy, but further work is needed to directly compare chemotherapy with combined modality therapy.
Keywords: clinical trials, low-grade glioma, molecular markers
Importance of the study
We report the results of a phase II clinical trial evaluating the utility of temozolomide as a means of delaying adjuvant radiotherapy in patients with lowgrade gliomas. Patients demonstrated a high rate of radiographic stability on treatment, and the regimen meaningfully delayed the receipt of radiotherapy. In the first prospective study to report results based on molecular subtype, we find that subtype based on 1p19q codeletion and IDH1-R132H mutation was prognostic of progression-free and overall survival. 1p19q codeleted patients had favorable survival outcomes, and a 0% risk of progression on treatment. While RTOG 9802 recently demonstrated a survival benefit for combined chemotherapy and radiation in high-risk low grade gliomas, our results raise the possibility that omission of radiotherapy may be considered in a select subgroup of patients with favorable molecular and clinical features. Further work is needed to directly compare chemotherapy to combined modality therapy in appropriately selected patients.
Management of adult low-grade gliomas (LGGs) remains controversial. Adjuvant radiotherapy has been shown to improve progression-free survival (PFS) compared with observation,1 and may be employed as initial adjuvant treatment. However, radiation fields for LGGs generally encompass a large volume of normal brain tissue and can cause long-term sequelae, including cognitive changes.2–5 While neurocognitive testing of long-term LGG survivors receiving radiotherapy has demonstrated progressive cognitive decline,5 tumor progression itself is associated with cognitive decline and compromised quality of life (QoL),4 so the benefit from adjuvant radiotherapy depends on the balance between improved tumor control and potential late toxicity.
Chemotherapy has recently been shown to have activity in adult LGGs, with the Radiation Therapy Oncology Group (RTOG) 9802 study demonstrating improved PFS with the combination of radiotherapy followed by procarbazine, lomustine, and vincristine (PCV) chemotherapy compared with radiotherapy alone, with updated results demonstrating improved overall survival (OS).6,7 This study clearly established the efficacy of chemotherapy in LGGs, and for many provided convincing evidence that combined modality therapy should be standard of care for all patients requiring adjuvant treatment.8
By demonstrating the efficacy of chemotherapy in this population, RTOG 9802 also highlighted the possibility that chemotherapy alone may be used as an alternative to radiation in a select group of patients, thus sparing the known late toxicity of radiation. Multiple single-arm studies in patients with newly diagnosed and recurrent LGGs have shown objective responses in patients treated with PCV alone, further supporting this possibility.9–12
Temozolomide (TMZ) was not an established chemotherapy agent at the time the RTOG 9802 trial was designed but has since been shown to improve OS in combination with radiation for high-grade gliomas.13 TMZ has a favorable toxicity profile in comparison with PCV and is a promising alternative agent in LGGs. Multiple phase II trials have demonstrated the activity of TMZ in patients with newly diagnosed and recurrent LGGs,14–19 though these studies are limited by small patient numbers and limited follow-up.
While the efficacy of chemotherapy has been demonstrated in this population, relatively little work has been performed to risk stratifying patients in order to determine optimal candidates for omission of adjuvant radiotherapy. Recent comprehensive genomic analysis of LGG tumors demonstrated distinct molecular subgroups based on 1p/19q codeletion and isocitrate dehydrogenase (IDH) mutation status, which carry more prognostic value than traditional World Health Organization (WHO) classification.20,21 Molecular classification thus has enormous potential to enable tailored adjuvant therapy, but the utility of such classification has not been evaluated in a prospective trial.
Here we present the long-term results of a phase II clinical trial investigating the efficacy of TMZ as primary adjuvant therapy in patients with newly diagnosed LGG and report results based on molecular subtype in an effort to determine optimal candidates for this regimen.
Patients and Methods
Patient Characteristics
Eligibility criteria included patients over age 18 with histologically proven supratentorial WHO grade II oligodendroglioma, astrocytoma, and oligoastrocytoma, reviewed by a neuropathologist at our institution. All patients were required to have undergone either subtotal surgical resection or biopsy within 4 months prior to enrollment, with evaluable residual disease on postoperative MRI. Patients were required to have a KPS ≥60 and no evidence of compromised hematologic, renal, or hepatic function on laboratory testing. Exclusion criteria included history of malignancy in remission for less than 5 years, known HIV-positive status or AIDS-related illness, and women who were pregnant, breastfeeding, or of childbearing potential not using contraception. The protocol was approved by our institutional review board, and all patients provided informed consent.
Treatment
TMZ was administered orally, once per day for 5 consecutive days at a starting dose of 200mg/m2/day and repeated every 28 days for up to 12 cycles. Further treatment was allowed at the discretion of the investigator for an additional 12 cycles. After the first cycle, dose modifications were required for subsequent cycles for nadir absolute neutrophil count (ANC) <1000/mm3, nadir platelet count <50000/mm3, or any grade 3 or 4 nonhematologic toxicity. Delay of TMZ administration was required for ANC <1500/mm3, platelets <1000/mm3, or any nonhematologic grade 2, 3, or 4 toxicity on the intended administration date.
Patient Evaluations
Within 14 days prior to initiating therapy, all patients underwent a baseline evaluation, including medical history, physical examination, neurologic examination, vital signs, KPS, and laboratory testing. Patients also underwent baseline brain MRI with and without gadolinium contrast. Patients underwent repeat laboratory testing 28 days following the start of each cycle. On the first day of every odd cycle, patients underwent repeat physical and neurologic examination and MRI. MRIs were required prior to the scheduled bimonthly examination if there was clinical evidence of progression.
Treatment Response Evaluation
Assessment of treatment response was determined by MRI, in conjunction with neurologic examination and steroid requirement assessment, derived from Macdonald’s criteria.22 Complete response was defined as disappearance of the fluid attenuated inversion recovery (FLAIR) lesion on consecutive MRI scans, with stable or improved neurologic exam and a stable dose of steroids for 5 days prior to the MRI at a dose equal to or less than that at the time of the previous scan. Partial response was defined as either a 50% reduction in lesion size or a determination that tumor burden was “definitely better” than prior scan as determined by the reading neuroradiologist, with stable or improved neurologic examination and steroid dose as described above. Progressive disease was defined as definite enlargement of any existing lesion, or any new lesion. All other situations were considered stable disease.
Molecular Subtype Analysis
Molecular subtype was determined for 97 patients through either clinical testing at the time of treatment or through a post-hoc analysis performed for patients without available clinical testing. For patients undergoing clinical testing, 1p/19q codeletion testing was performed via fluoresence in situ hybridization (FISH) analysis; and, when available, IDH1-R132H mutation testing was performed via immunohistochemial (IHC) staining. Based on evidence that all patients with 1p/19q codeletion also contain an IDH mutation,23 patients with 1p/19q codeletion were imputed to have an IDH mutation even if IDH1-R132H testing was not performed. For patients without clinical data undergoing post-hoc analysis, subtyping was performed using an algorithm incorporating 1p/19q codeletion, IDH1 mutation, and loss of alpha thalassemia/mental retardation syndrome X-linked (ATRX).23 First, IHC was performed for loss of ATRX and for presence of the IDH1-R132H mutations. For patients with ATRX loss, 1p/19q codeletion was imputed to be absent, since 1p/19q codeletion has been shown to be mutually exclusive with ATRX loss.23 For patients with retained ATRX expression, 1p/19q codeletion testing was performed using FISH. All patients were then classified as 1p/19q codeleted (1p/19q codel, regardless of IDH1 status), 1p/19q intact/IDH1-R132H mutated (IDH1mut), or 1p/19q intact/IDH1-R132H wild-type (IDH1wt).
Volumetric Analysis
In a post-hoc analysis, volumetric assessment was performed on 71 patients with available postoperative, pretreatment brain MRI. The lesion volume was defined as the hyperintense region on FLAIR imaging and was delineated using a computer-assisted tumor volume definition tool (Smartbrush, Brainlab). The relationship between tumor volume and survival outcomes was analyzed with tumor volume as both a continuous variable and as a binary variable with a volume threshold determined through classification and regression tree (CART) analysis.24 CART recursively partitions subjects into mutually exclusive groups as defined by predictor cutpoints, with participants in each group having similar outcome probabilities. The primary cutpoint derived from CART analysis with OS as the outcome was used to place patients into 2 groups based on a threshold tumor volume.
Statistical Design
The primary endpoint of the study was objective response rate, as defined above. The null hypothesis for response rate was 2% for patients with oligoastrocytomas; the study was powered to detect a difference in response rate from 2% if the true response rate was 15% with 90% power, using a one-tailed binomial exact test with alpha of 0.1. Based on this sample size calculation, 40 patients were planned to be enrolled for each histologic group (oligodendroglioma, oligoastrocytoma, and astrocytoma), with separate response assessments for each group. Secondary endpoints included PFS, OS, and toxicity rate. Assessment of endpoints was performed separately for each histologic and molecular group, with no adjustment for multiple comparisons. Comparison of response and progression rates across groups was performed using Fisher’s exact test, and comparison of continuous variables across groups was performed using the Wilcoxon rank-sum test. The proportional hazards assumption was found to be violated for multiple variables in the study, so comparison of survival curves was performed using the Tarone–Ware test, a nonparametric test not reliant on a proportional hazards assumption.
Results
Patient Characteristics
From 2000 to 2013, 120 patients were enrolled on the trial (Table 1). Of 97 patients with available tissue, 44 (45%) were classified as 1p/19q codel, 37 (38%) as 1p/19q intact/IDH1mut, and 16 (16%) as 1p/19q intact/IDH1wt. Median follow-up for all patients was 7.5 years; there were 86 progression events and 56 deaths during follow-up.
Table 1.
Patient characteristics
| Characteristics | Patients, n=120 (%) |
|---|---|
| Age, y | |
| Median | 39 |
| Range | 19–71 |
| Sex | |
| Male | 67 (56) |
| Female | 53 (44) |
| KPS | |
| 90–100 | 106 (88) |
| <90 | 12 (10) |
| Unknown | 2 (2) |
| Midline crossing | |
| No | 104 (87) |
| Yes | 16 (13) |
| Extent of resection | |
| GTR | 0 |
| STR | 92 (77) |
| Biopsy only | 28 (23) |
| Histology | |
| Oligodendroglioma | 57 (48) |
| Oligoastrocytoma | 20 (17) |
| Astrocytoma | 43 (36) |
| 1p/19q status | |
| Codeleted | 44 (37) |
| Noncodeleted | 55 (46) |
| Unknown | 21 (18) |
| IDH1-R132H status* | |
| Mutated | 83 (70) |
| Intact | 18 (15) |
| Unknown | 19 (16) |
| Molecular subtype | |
| 1p/19q codel | 44 (37) |
| IDH1mut | 37 (31) |
| IDH1wt | 16 (13) |
| Unknown | 23 (19) |
*IDH1 status was either directly measured or imputed based on 1p/19q status. GTR, gross total resection; STR, subtotal resection.
Treatment Response
A partial radiographic response was observed in 7 patients (6%); there were no complete responses. A 5% response rate was observed in the oligoastrocytoma subtype (90% lower CI: 0.5%, P=.33 for difference from 2%), failing to meet the primary study endpoint. Response rates for oligodendrogliomas and astrocytomas were 7% (90% lower CI: 3.1%) and 4% (90% lower CI: 1.2%), respectively. An additional 97 patients (81%) demonstrated stable disease, yielding an 87% rate of stable or improved disease during treatment. Sixteen patients progressed during treatment (2 oligodendrogliomas, 5 oligoastrocytomas, 9 astrocytomas).
Classified by molecular subtype, 5 responders were 1p/19q codel (11% response rate), compared with 1 IDH1mut (3% response rate), and zero IDH1wt (P=.17 for difference in response rates). Conversely, no 1p/19q codel patients progressed during treatment, compared with 3 IDH1 mut (8%) and 9 IDH1wt (56%). Progression rates during treatment were significantly different by molecular subtype (P<.001).
Survival Endpoints
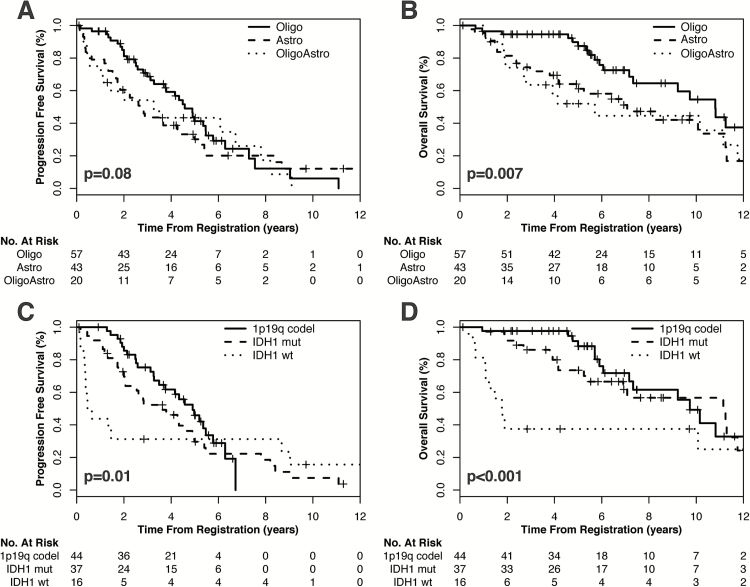
Median PFS was 3.8 years (95% CI: 3.0–5.0 y); PFS did not differ significantly by histology (P=.08) but differed by molecular subtype (P=.007). Median OS was 9.7 years (95% CI: 7.2–11.3 y); both histology and molecular subtype were associated with OS (P=.01 and P<.001, respectively). Median PFS and OS based on clinical and pathologic characteristics are shown in Table 2. Kaplan–Meier curves of PFS and OS by both histology and molecular subtype are shown in Fig. 1.
Table 2.
Median PFS and OS by clinical, histopathologic, molecular and radiographic characteristics
| Characteristic (n) | Median PFS, y (95% CI) |
P | Median OS, y (95% CI) |
P |
|---|---|---|---|---|
| Age | .87 | .69 | ||
| <40 (61) | 3.8 (2.6–5.8) | 10.2 (7.3–11.8) | ||
| ≥40 (59) | 3.8 (2.7–5.3) | 9.2 (6.4–NA) | ||
| Performance status | .72 | .51 | ||
| KPS 90–100 (106) | 3.7 (2.8–4.9) | 10.8 (7.1–NA) | ||
| KPS < 90 (12) | 4.3 (2.7–6.3) | 9.2 (5.7–11.1) | ||
| Extent of resection | .003 | .01 | ||
| STR (92) | 4.3 (3.5–5.4) | 10.1 (7.1–NA) | ||
| Biopsy only (28) | 2.0 (1.3–5.2) | 7.2 (2.4–NA) | ||
| Histology | .08 | .007 | ||
| Oligodendroglioma (57) | 4.6 (3.8–5.7) | 10.8 (7.3–NA) | ||
| Astrocytoma (43) | 3.3 (1.1–NA) | 7.1 (5.0–NA) | ||
| Oligoastrocytoma (20) | 2.7 (1.8–5.0) | 5.7 (2.8–NA) | ||
| Molecular subgroup | .01 | <.001 | ||
| 1p/19q codel (44) | 4.9 (3.8–NA) | 9.7 (7.3–NA) | ||
| IDH1 mut (31) | 3.6 (2.1–5.4) | 11.2 (6.9–NA) | ||
| Wild type (13) | 0.6 (0.4–NA) | 1.8 (1.1–NA) | ||
| Pretreatment tumor volume | <.001 | <.001 | ||
| Volume ≤ 68cm3 (54) | 4.9 (4.2–7.6) | 11.2 (10.8–NA) | ||
| Volume > 68cm3 (17) | 1.7 (1.3–3.7) | 4.2 (2.4–NA) |
P-values are calculated using the Tarone–Ware test.
Fig. 1.
Kaplan–Meier curves demonstrating PFS and OS by (A, B) WHO histologic classification and by (C, D) molecular subgroup. Oligo, oligodendroglioma; astro, astrocytoma; oligoastro, oligoastrocytoma.
Functional and Neurologic Status
At baseline, patients enrolled in the study in general had excellent functional status, with 88% of patients having a baseline KPS of 90%–100%. Clinical information after completing treatment with TMZ was available for 93 patients; 72 patients (77%) demonstrated stable KPS, while 13 patients (14%) experienced improved KPS, and 8 (9%) experienced a decline in KPS. Eighty-nine patients (96%) experienced no change in neurologic symptoms during treatment, while 2 experienced symptom improvement and 2 experienced symptom worsening.
Volumetric Analysis
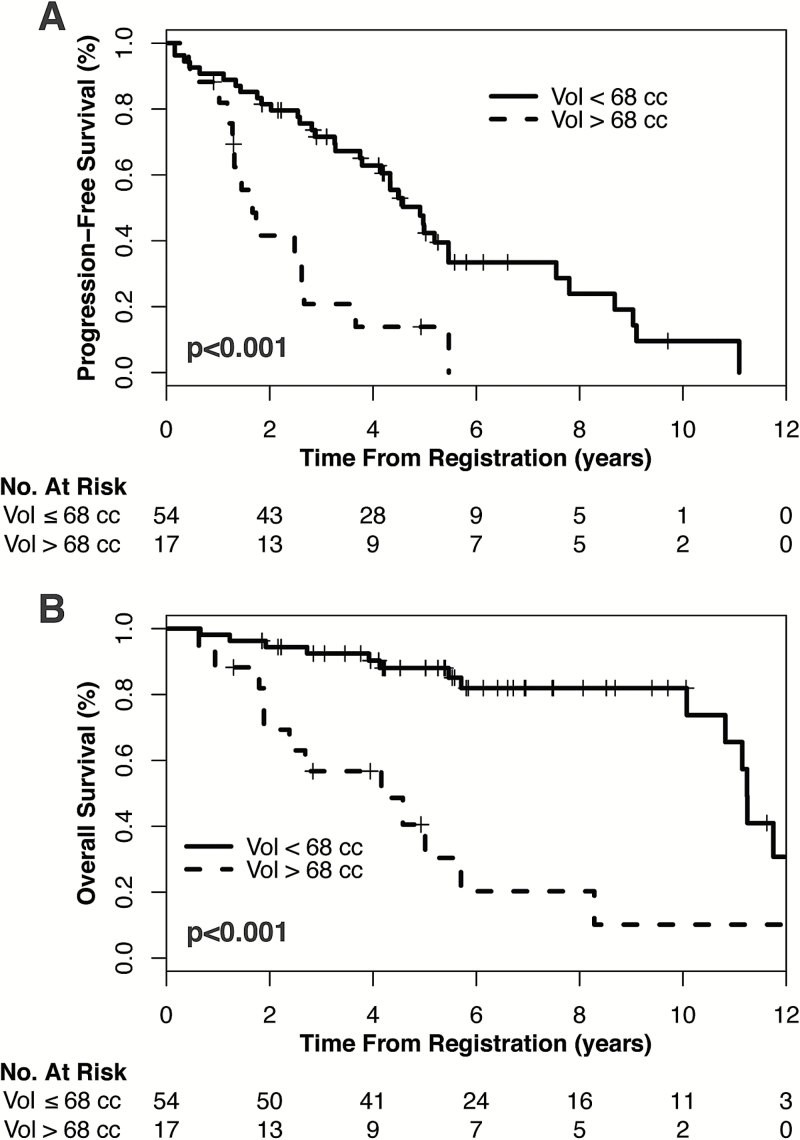
In an exploratory, post-hoc analysis of 71 patients with available imaging, median postoperative, pretreatment tumor volume was 26.5cm3 (interquartile range, 9.8cm3–64.7cm3). On univariate Cox analysis, pretreatment volume was associated with both PFS (hazard ratio [HR] 1.07 for each 10cm3 volume increase, 95% CI: 1.03–1.11, P<.001) and OS (HR 1.09 for each 10cm3 volume increase, 95% CI: 1.04–1.13, P<.001). Tumor volume remained prognostic of both PFS and OS after stratification by molecular status (PFS: HR 1.06 for each 10cm3 volume increase, 95% CI: 1.02–1.10, P=.007; OS: HR 1.06 for each 10cm3 volume increase, 95% CI: 1.02–1.12, P=.006) and by extent of resection (PFS HR 1.06 for each 10cm3 volume increase, 95% CI: 1.02–1.11, P=.004; OS HR 1.07 for each 10cm3 volume increase, 95% CI: 1.02–1.12, P=.005). Using a threshold determined by CART analysis, pretreatment tumor volume of ≤68cm3 conferred significantly improved PFS (P<.001) and OS (P<.001, Fig. 2). This threshold remained prognostic in the 1p/19q codel subgroup (PFS: P=.01, OS: P=.001) and IDH1mut subgroup (PFS: P=.02; OS: P=.001); there were insufficient events to evaluate the prognostic value of this threshold in the IDH1wt subgroup.
Fig. 2.
Kaplan–Meier curves demonstrating (A) PFS and (B) OS based on tumor volume as measured on postsurgical, pretreatment MRI.
Subsequent Therapy
Disease progression was observed in 86 patients (72%), with follow-up after progression available for 80 patients. At the time of first progression, 59% underwent salvage surgery, 45% received further chemotherapy, and 54% underwent salvage radiotherapy. Including follow-up after first progression, 56 patients (70% of progressors) eventually underwent salvage radiotherapy. Thus 64 patients (53%) had not received radiation at last follow-up, with a median follow-up of 5.8 years. Malignant transformation, defined as pathologically proven WHO grade III or IV glioma, was observed in 33 of 55 patients undergoing further surgery after disease progression. The rate of malignant transformation did not vary significantly by molecular subtype (P=.26), and pretreatment tumor volume was not significantly higher in patients who underwent malignant transformation (P=.23).
Treatment Compliance and Toxicity
The mean number of courses of TMZ completed was 10 (range 1–12); 86 patients (72%) completed the entire course of 12 cycles. The rate of grades 2–4 toxicity was 10%, with two grade 4 events (both thrombocytopenia requiring transfusion).
Discussion
After recent long-term results from RTOG 9802 demonstrated a survival benefit with the addition of PCV chemotherapy to adjuvant radiation, the treatment paradigm for LGGs has rapidly evolved to incorporate chemotherapy into the adjuvant setting.7 Based on these results, many have advocated that combined chemoradiotherapy should be the standard of care for all LGGs requiring adjuvant treatment.8 However, given the known long-term toxicity of large-field cranial irradiation, adjuvant chemotherapy without radiation may be a viable treatment option for selected patients as a means of delaying progression and potentially prolonging survival while simultaneously delaying or eliminating late radiation toxicity.
Several prospective studies have examined the efficacy of TMZ alone in patients with LGG (Table 3), but these studies are limited by their inclusion of both newly diagnosed and progressive disease, relatively small cohorts, and limited follow-up precluding evaluation of long-term clinical outcomes.14–19 A subset of these studies demonstrated a relationship between treatment response and 1p/19q codeletion16,18 but did not perform comprehensive molecular classification, including IDH1 status, in their analysis. More generally, no prior prospective study has examined the influence of recently established LGG molecular groups20,21 on clinical outcomes.
Table 3.
Comparison to prior studies investigating use of TMZ in LGG
| Study | # Patients | Median F/U | Response Rate (CR + PR) | 3y PFS | 3y OS |
|---|---|---|---|---|---|
| Brada et al14 | 30 | 3 y | 10% | 66% | 82% |
| Quinn et al17 | 46 | <1 y | 61% | NR | NR |
| Hoang-Xuan et al16 | 60 | 1.2 y | 17% | NR | NR |
| Kesari et al15 | 44 | 3 y | 20% | 57% | 81% |
| UCSF TMZ | 120 | 7.5 y | 6% | 58% | 81% |
F/U, follow-up; CR, complete response; PR, partial response. NR, not reported; UCSF, University of California, San Francisco.
In this study, we report outcomes of 120 patients with newly diagnosed LGG treated with adjuvant TMZ after subtotal resection or biopsy. Our primary outcome was radiographic response to treatment; the observed response rate failed to meet the prespecified criteria for a positive study. However, we report a high rate (86%) of stable or improved disease during treatment with TMZ.
We also report long-term clinical outcomes, with median PFS and OS of 3.8 and 9.7 years, respectively. Although our study was not powered to directly compare our survival results with historical controls, our results are similar to the radiation-only arm of RTOG 9802, which reported median PFS and OS of 4.0 and 7.8 years, respectively (Table 4).7 Of note, all patients in our study would meet criteria for RTOG 9802 and the histologic makeup of the studies are similar, while 10% of patients on RTOG 9802 had undergone gross total resection and would not be eligible for our study. Therefore, with similar histologic characteristics and a higher burden of residual disease, our study demonstrates similar PFS and OS as the radiation-alone arm of RTOG 9802, suggesting that TMZ may provide clinical efficacy comparable to adjuvant radiation. RTOG 9802 also reported survival outcomes specifically for patients with IDH1-R132H mutations. While authors do not report median results, based on available information the median PFS for patients with IDH1-R132H mutation undergoing radiotherapy alone was roughly 4.5 years, while median OS was roughly 10 years, similar to our outcomes of 4.3 and 10.2 years, respectively. Our results are also similar to those of RTOG 0424, a phase II study evaluating the use of concurrent TMZ and radiation in patients with high-risk LGG25 (Table 4). Furthermore, a majority of patients on our study (53%) did not receive salvage radiation at the time of last follow-up, with a median follow-up of 5.8 years. Thus, the use of TMZ appears to meaningfully delay the need for adjuvant radiotherapy and associated long-term sequelae. Given that patients on our study received heterogeneous salvage treatment, it remains to be seen whether combined chemoradiotherapy given as salvage at the time of progression yields equivalent survival to upfront adjuvant chemoradiotherapy as established by RTOG 9802.
Table 4.
Comparison to recent cooperative group studies utilizing adjuvant radiation
| RTOG 9802 RT7 | RTOG 9802 RT+PCV7 | RTOG 0424 RT+TMZ23 |
UCSF TMZ |
|
|---|---|---|---|---|
| Median age, y | 40 | 41 | 49 | 39 |
| Histology | ||||
| Oligodendroglioma | 45% | 40% | 23% | 48% |
| Oligoastrocytoma | 31% | 31% | 22% | 17% |
| Astrocytoma | 23% | 29% | 55% | 36% |
| Extent of resection | ||||
| GTR | 9% | 11% | 19% | 0% |
| STR | 45% | 41% | 61% | 77% |
| Biopsy only | 47% | 48% | 16% | 23% |
| Median PFS (y) | 4.0 | 10.4 | 4.5 | 3.8 |
| Median OS (y) | 7.8 | 13.3 | NR (>5 y) | 9.7 |
RT, radiotherapy; UCSF, University of California, San Francisco; GTR, gross total resection; STR, subtotal resection; NR, not reached.
In the first prospective study incorporating the newly established LGG molecular classification, we present clinical results based on molecular subtypes for 97 patients on the study. We show that molecular subtype is significantly associated with risk of progression during treatment with TMZ, and with both PFS and OS. In particular, patients with 1p/19q codeletion demonstrated a 0% rate of progression during treatment with TMZ, with median PFS and OS of 4.9 and 9.7 years, respectively. With minimal risk of progression during treatment and favorable long-term survival, adjuvant chemotherapy could be considered in this population as a means of delaying radiotherapy. However, in the absence of a comparison group undergoing no adjuvant therapy, it remains unclear whether these patients would benefit from adjuvant chemotherapy over observation alone, followed by definitive therapy at the time of progression. Conversely, over half of patients with intact 1p/19q and wild-type IDH1-R132H progressed during treatment with TMZ, demonstrating median PFS and OS of 0.6 and 1.8 years, respectively, suggesting that chemotherapy alone may not be sufficient in this group.
In a post-hoc, exploratory analysis, we also demonstrate that postoperative pretreatment tumor volume is highly prognostic of clinical outcomes, highlighting the importance of maximal safe tumor resection, as has been reported in other studies.26–29 Maximal safe resection may be of particular importance in patients for whom adjuvant radiation is not planned for additional local control. However, given the exploratory nature of this analysis, these results should be considered hypothesis generating and interpreted accordingly. Furthermore, the clinical utility of our volume threshold based on CART analysis has yet to be validated in an independent dataset. While we show that pretreatment volume remained prognostic after stratification by molecular status and extent of resection, our study lacked sufficient power to perform multivariate analysis to determine whether pretreatment tumor volume was an independent prognostic factor after controlling for molecular status and other clinical variables.
While the predominant concern for adverse long-term treatment effects is related to cognitive decline from radiotherapy, the recent observation of the apparent induction of malignant transformation and development of a hypermutated state through the use of adjuvant TMZ has raised concerns that TMZ may itself compromise outcomes for patients prone to this transformation.30,31 However, it is unclear whether the use of TMZ alters the natural history of the disease for such patients. In this study, 33 patients underwent malignant transformation after therapy, though it is unclear what fraction of these patients developed a hypermutated state attributable to TMZ use. The rate of malignant transformation observed was comparable to those previously published,32,33 and there was not a higher rate of malignant transformation based on molecular subtype or pretreatment tumor volume. Work is ongoing to determine which patients are most at risk for TMZ-induced mutagenesis, the frequency of this phenomenon, and whether this event adversely affects patient outcomes. Furthermore, while TMZ has not been previously linked to long-term cognitive decline,34 the current study lacked prospective assessments of long-term cognitive function. Previous published work demonstrated stable or improved QoL during treatment on this study.35
A phase III trial currently in follow-up phase, European Organisation for Research and Treatment of Cancer (EORTC) 22033–26033, may provide further insight into the appropriate use of adjuvant chemotherapy in LGGs. The study randomized patients with high-risk or progressive LGGs to TMZ alone or radiotherapy alone. Preliminary results were reported in abstract form, demonstrating equivalent PFS and OS between the 2 arms; but with median follow-up of under 4 years, long-term clinical outcomes have not been assessed.36 The study also features assessment of long-term QoL and neurocognitive function and may better evaluate the tradeoff between optimizing survival endpoints and the potentially detrimental effect of radiation or chemotherapy on the quality of survival.
A second ongoing phase III trial, EORTC 26081–22086 (the “CODEL” study), may provide additional insight. The study initially enrolled only patients with anaplastic (WHO grade III) 1p/19q codeleted gliomas and randomized patients to adjuvant radiotherapy in combination with either PCV or TMZ chemotherapy, or to adjuvant TMZ alone, but was amended in 2015 to include patients with low-grade (WHO grade II) 1p/19q codeleted gliomas. The study also recently closed the TMZ-only arm based on preliminary results demonstrating inferior PFS compared with patients receiving radiotherapy.37 However, it is important to note that these results were seen exclusively in patients with anaplastic gliomas, and it is not clear if they can be generalized to grade II tumors. Indeed, patients on the CODEL trial under treatment with TMZ alone demonstrated a 42% rate of progression during treatment and a median PFS of 2.5 years, in stark contrast to our reported results of 0% and 4.9 years, respectively. This discrepancy suggests a differential response to TMZ between grade II and grade III tumors and argues that tumor grade may continue to play an important role in selecting patients for adjuvant therapy even after accounting for molecular status.
Several limitations to our study should be mentioned. First, our analysis of molecular phenotype relied on assessment of the canonical IDH1-R132H mutation; patients who did not demonstrate this mutation did not undergo IDH1 and IDH2 sequencing to evaluate for alternate mutations, so it is possible that some patients classified as IDH1wt harbor undetected IDH mutations. Nonetheless, our proportion of IDH1wt patients did not differ substantially from previously published results,20,21,23 and the rate of noncanonical IDH mutations has been reported as under 10%,38 suggesting that detecting these noncanonical IDH mutations would not significantly alter our results. Second, assessment of O6-DNA methylguanine-methyltransferase (MGMT) methylation status, which has been previously associated with benefit to therapy with TMZ,13 was not evaluated in this study as a potential prognostic factor. However, the presence of MGMT methylation has been found to be highly correlated with IDH mutational status39 (84% of patients with IDH mutation were MGMT methylated), so MGMT status would likely provide minimal additional prognostic information. Third, the design of this study predated the development of the RANO (Response Assessment in Neuro-Oncology) criteria,40,41 so the criteria for treatment response are based on older metrics and may not be directly comparable to measurements of response rates in more recent studies. The optimal criteria for response in LGG continues to evolve; work is ongoing incorporating volumetric analysis of serial imaging of patients on our study to determine optimal metrics to measure treatment response. Finally, our single-arm study precludes direct comparison to alternative treatments; comparison to historical controls is limited by both potential differences in patient characteristics between studies and by the fact that our study was not powered to compare survival outcomes to historical controls. More studies are needed directly comparing adjuvant chemotherapy with combined modality therapy in appropriately selected patients.
In conclusion, in this study we find that adjuvant TMZ for newly diagnosed LGG achieved a low radiographic response rate, failing to meet the primary endpoint of the study. However, treatment with TMZ yielded a high rate of radiographic and clinical stability while meaningfully delaying the receipt of radiotherapy. We report the first results from a prospective study stratified by LGG molecular subtype and show that patients with 1p/19q codeletion have minimal risk of progression during treatment. Conversely, patients who are 1p/19q intact and IDH1wt demonstrated rapid progression on TMZ and may benefit from combined modality adjuvant therapy.
Funding
This study was funded by Roche, with support from the Dabierre family.
Conflict of interest statement. M.W. is employed at Illumina, Inc (family member) and has stock ownership (family member). D.A.H.-K. is part of leadership at Cellworks and has stock ownership in Pfizer, Accuracy. J.C. has stock ownership, Telo Therapeutics. N.B. has honoraria from Genentech, VBL Therapeutics, and Omniox; consulting or an advisory role with Genentech; research funding from VBL Therapeutics, Bristol-Myers Squibb, Stemline Therapeutics, Merrimack, and Celldex. M.B. has honoraria from Actelion and is a consultant for Actelion. S.C. is a consultant for Neonc Technologies.
Acknowledgments
The authors would like to acknowledge the Dabierre family for their support of this study. Portions of this work were presented at the annual meeting of the American Society of Clinical Oncology in Chicago, IL, in May 2015, and at the annual meeting of the American Society for Radiation Oncology in San Antonio, TX, in October 2015.
References
- 1. van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 2. Donahue B. Short- and Long-Term Complications of Radiation Therapy for Pediatric Brain Tumors. Pediatr Neurosurg. 1992;18(4):207–217. [DOI] [PubMed] [Google Scholar]
- 3. Olson JD, Riedel E, DeAngelis LM. Long-term outcome of low-grade oligodendroglioma and mixed glioma. Neurology. 2000;54(7):1442–1448. [DOI] [PubMed] [Google Scholar]
- 4. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 5. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 6. Shaw EG, Wang M, Coons SW, et al. Randomized Trial of Radiation Therapy Plus Procarbazine, Lomustine, and Vincristine Chemotherapy for Supratentorial Adult Low-Grade Glioma: Initial Results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med. 2016;374(14):1344–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Bent MJ. Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol. 2014;16(12):1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckner JC, Gesme D, O’Fallon JR, et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003;21(2):251–255. [DOI] [PubMed] [Google Scholar]
- 10. Stege EMB-T, Kros JM, de Bruin HG, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103(4):802–809. [DOI] [PubMed] [Google Scholar]
- 11. Soffietti R, Rudà R, Bradac GB, et al. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43(5):1066–1073. [DOI] [PubMed] [Google Scholar]
- 12. Mason WP, Krol GS, DeAngelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46(1):203–207. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCI:C trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 14. Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14(12):1715–1721. [DOI] [PubMed] [Google Scholar]
- 15. Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res. 2009;15(1):330–337. [DOI] [PubMed] [Google Scholar]
- 16. Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–3138. [DOI] [PubMed] [Google Scholar]
- 17. Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21(4):646–651. [DOI] [PubMed] [Google Scholar]
- 18. Levin N, Lavon I, Zelikovitsh B, et al. Progressive low-grade oligodendrogliomas: response to temozolomide and correlation between genetic profile and O6-methylguanine DNA methyltransferase protein expression. Cancer. 2006;106(8):1759–1765. [DOI] [PubMed] [Google Scholar]
- 19. van den Bent MJ, Taphoorn MJB, Brandes AA, et al. Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol. 2003;21(13):2525–2528. [DOI] [PubMed] [Google Scholar]
- 20. The Cancer Genome Atlas Research Network. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERTPromoter Mutations in Tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 23. Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015;129(1):133–146. [DOI] [PubMed] [Google Scholar]
- 24. Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and Regression Trees.Monterey, CA: Wadsworth and Brooks/Cole;1984. [Google Scholar]
- 25. Fisher BJ, Hu C, Macdonald DR, et al. Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of radiation therapy oncology group 0424. Int J Radiat Oncol Biol Phys. 2015;91(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–708. [DOI] [PubMed] [Google Scholar]
- 27. Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. [DOI] [PubMed] [Google Scholar]
- 28. Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95(5):735–745. [DOI] [PubMed] [Google Scholar]
- 29. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. [DOI] [PubMed] [Google Scholar]
- 30. Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Thuijl HF, Mazor T, Johnson BE, et al. Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol. 2015;129(4):597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rees J, Watt H, Jäger HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72(1):54–64. [DOI] [PubMed] [Google Scholar]
- 33. Leu S, Felten von S, Frank S, et al. IDH mutation is associated with higher risk of malignant transformation in low-grade glioma. J Neurooncol. January 2016. [DOI] [PubMed] [Google Scholar]
- 34. McAleer MF, Brown PD. Neurocognitive Function Following Therapy for Low-Grade Gliomas. Semin Radiat Oncol. 2015;25(3):210–218. [DOI] [PubMed] [Google Scholar]
- 35. Liu R, Solheim K, Polley M-Y, et al. Quality of life in low-grade glioma patients receiving temozolomide. Neuro Oncol. 2009;11(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baumert BG, Mason WP, Ryan G, et al. Temozolomide chemotherapy versus radiotherapy in molecularly characterized (1p loss) low-grade glioma: A randomized phase III intergroup study by the EORTC/NCI:C-CTG/TROG/MRC-CTU (EORTC 22033–26033). J Clin Oncol. 2013;31(15). [Google Scholar]
- 37. Jaeckle K, Vogelbaum M, Ballman K, et al. ATCT-16CODEL (ALLIANCE-N0577; EORTC-26081/2208; NRG-1071; NCI:C-CEC-2): Phase III randomized study of RT VS. RT + TMZ vs. TMZ for newly diagnosed 1p/19q-codeleted anaplastic glioma. Analysis of patients treated on the original protocol design. Neuro Oncol. 2015;17(suppl 5):v4.4–v5. [Google Scholar]
- 38. Hartman C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009;118(4):469–474 [DOI] [PubMed] [Google Scholar]
- 39. Leu S, Felten von S, Frank S, et al. IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro Oncol. 2013;15(4):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 41. van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]