Abstract
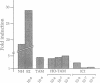
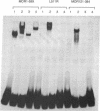
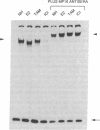
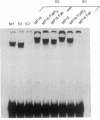
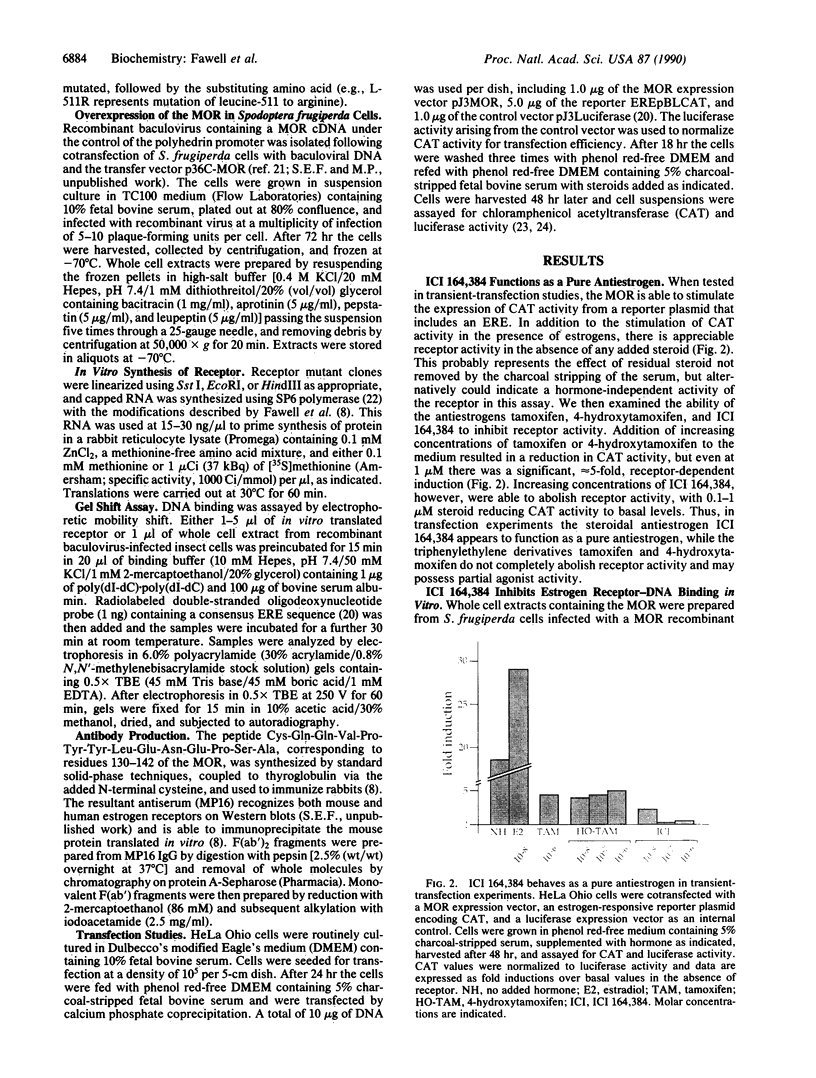
Many estrogen-antagonist and -agonist ligands have been synthesized, some of which have proved clinically important in the treatment of hormone-dependent breast tumors and endocrine disorders. Here we show that the "pure" antiestrogen ICI 164,384 inhibits mouse estrogen receptor-DNA binding in vitro. The effects of this steroid on DNA binding can be overcome by addition of anti-receptor antibody whose epitope lies N-terminal to the receptor DNA-binding domain. Since this antibody is also capable of restoring DNA-binding activity to receptor mutants that either lack the dimerization domain or bear deleterious mutations within it, we propose that ICI 164,384 reduces DNA binding by interfering with receptor dimerization. In contrast, when complexed with the antagonist/partial agonist tamoxifen, the estrogen receptor is capable of binding to DNA in vitro, but tamoxifen does not promote the agonist-induced conformational change obtained with estradiol. The implications of these data are discussed in relation to the in vivo properties of these drugs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowler J., Lilley T. J., Pittam J. D., Wakeling A. E. Novel steroidal pure antiestrogens. Steroids. 1989 Jul;54(1):71–99. doi: 10.1016/0039-128x(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., Parker M. G. A proposed consensus steroid-binding sequence--a reply. Mol Endocrinol. 1989 Jun;3(6):1002–1005. doi: 10.1210/mend-3-6-1002. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Giambiagi N., Pasqualini J. R. Interaction of three monoclonal antibodies with the nonactivated and activated forms of the estrogen receptor. Endocrinology. 1990 Mar;126(3):1403–1409. doi: 10.1210/endo-126-3-1403. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gordon M. S., Notides A. C. Computer modeling of estradiol interactions with the estrogen receptor. J Steroid Biochem. 1986 Aug;25(2):177–181. doi: 10.1016/0022-4731(86)90414-0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Oestradiol induction of a glucocorticoid-responsive gene by a chimaeric receptor. Nature. 1987 Jan 1;325(6099):75–78. doi: 10.1038/325075a0. [DOI] [PubMed] [Google Scholar]
- Ham J., Parker M. G. Regulation of gene expression by nuclear hormone receptors. Curr Opin Cell Biol. 1989 Jun;1(3):503–511. doi: 10.1016/0955-0674(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Harper M. J., Walpole A. L. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil. 1967 Feb;13(1):101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- Johansson H., Terenius L., Thorén L. The binding of estradiol-17beta to human breast cancers and other tissues in vitro. Cancer Res. 1970 Mar;30(3):692–698. [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Leclercq G., Heuson J. C., Schoenfeld R., Mattheiem W. H., Tagnon H. J. Estrogen receptors in human breast cancer. Eur J Cancer. 1973 Sep;9(9):665–673. doi: 10.1016/0014-2964(73)90009-1. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt A. D., West N. B., Brenner R. M. Analysis of monomeric-dimeric states of the estrogen receptor with monoclonal antiestrophilins. J Steroid Biochem. 1986 Mar;24(3):677–686. doi: 10.1016/0022-4731(86)90842-3. [DOI] [PubMed] [Google Scholar]
- Martinez E., Wahli W. Cooperative binding of estrogen receptor to imperfect estrogen-responsive DNA elements correlates with their synergistic hormone-dependent enhancer activity. EMBO J. 1989 Dec 1;8(12):3781–3791. doi: 10.1002/j.1460-2075.1989.tb08555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Gronemeyer H., Turcotte B., Bocquel M. T., Tasset D., Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989 May 5;57(3):433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Page M. J. p36C: an improved baculovirus expression vector for producing high levels of mature recombinant proteins. Nucleic Acids Res. 1989 Jan 11;17(1):454–454. doi: 10.1093/nar/17.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S., Yamamoto K. R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987 May;6(5):1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Tora L., White J., Brou C., Tasset D., Webster N., Scheer E., Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989 Nov 3;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Wilson A. P., Nicholson R. I., Davies P., Wakeling A. E. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30(1-6):263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Wilson A. P., Nicholson R. I., Davies P., Wakeling A. E. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30(1-6):263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- White R., Lees J. A., Needham M., Ham J., Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987 Oct;1(10):735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]