Abstract
Wnt/β-catenin signaling is highly conserved throughout metazoans, is required for numerous essential events in development, and serves as a stem cell niche signal in many contexts. Misregulation of the pathway is linked to several human pathologies, most notably cancer. Wnt stimulation results in stabilization and nuclear import of β-catenin, which then acts as a transcriptional co-activator. Transcription factors of the T-cell family (TCF) are the best-characterized nuclear binding partners of β-catenin and mediators of Wnt gene regulation. This review provides an update on what is known about the transcriptional activation of Wnt target genes, highlighting recent work that modifies the conventional model. Wnt/β-catenin signaling regulates genes in a highly context-dependent manner, and the role of other signaling pathways and TCF co-factors in this process will be discussed. Understanding Wnt gene regulation has served to elucidate many biological roles of the pathway, and we will use examples from stem cell biology, metabolism, and evolution to illustrate some of the rich Wnt biology that has been uncovered.
Keywords: Wnt, beta-catenin, gene regulation, target location, T-cell factor
Introduction
The Wnt/β-catenin (Wnt/β-cat) pathway is conserved throughout metazoans and is essential for development and tissue homeostasis in adult organisms (reviewed in 1– 3). Aberrant Wnt/β-cat signaling is linked to several diseases, e.g. many cancers 4, 5 as well as bone and metabolic disorders 6. Intense investigation into the mechanisms of this pathway has uncovered some of the basics of how Wnts influence gene expression. A better understanding of how this signaling cascade operates has also provided genetic tools to explore various aspects of Wnt biology (reviewed in 7, 8). In addition, the identification of Wnt transcriptional targets has enhanced our knowledge of the biological importance of Wnt/β-cat signaling. In this short review, we will summarize recent findings on how the Wnt/β-cat pathway regulates transcription and provide examples of how identifying Wnt targets has broadened our knowledge of stem cell biology, the regulation of metabolism, and the evolution of physical traits.
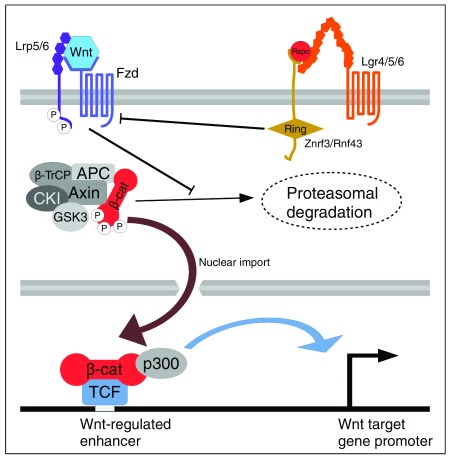
The Wnt/β-cat pathway regulates the levels and subcellular localization of β-cat ( Figure 1). In unstimulated cells, β-cat is constantly degraded by a “destruction complex” containing the molecular scaffolds Axin and adenomatous polyposis coli (APC), the protein kinases glycogen synthase kinase 3 (GSK3)α/β and casein kinase I (CKI), and the ubiquitin E3 ligase β-transducin repeat-containing E3 ubiquitin protein ligase (β-TrCP) (reviewed in 9). Upon Wnt binding to a receptor complex containing Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), the destruction complex is inactivated, allowing the stabilization and nuclear import of β-cat (reviewed in 10). Nuclear β-cat is then recruited to chromatin by transcription factors (TFs), with members of the T-cell factor (TCF)/lymphoid enhancer-binding factor 1 (LEF1) family being the best characterized 11– 15 (reviewed in 16 and 17). In many cases, TCFs act as transcriptional switches, repressing Wnt targets in the absence of signaling, in part by the recruitment of transducin-like enhancer of split (TLE)/Groucho (Gro) co-repressors, which are displaced by β-cat, which then recruits co-activators such as the histone acetyltransferases CREB-binding protein (CBP) and p300 (reviewed in 18).
Figure 1. Overview of vertebrate Wnt/β-catenin (Wnt/β-cat) signaling.
Wnt binding to Frizzled (Fzd) and low-density lipoprotein receptor-related protein 5/6 (Lrp5/6) co-receptors promotes the phosphorylation of Lrp5/6’s cytoplasmic tail. These interactions block the ability of the destruction complex to phosphorylate and ubiquitinate β-cat, preventing its degradation by the proteasome. Stabilized β-cat enters the nucleus, where it is recruited to Wnt-regulated enhancers by transcription factors (TFs) of the T-cell factor (TCF) family. R-spondin (Rspo) potentiates Wnt/β-cat signaling by increasing the number of Fzd receptors. Rspo forms a complex with Lgr4/5/6 and zinc and ring finger 3 (Znrf3)/ring finger protein 43 (Rnf43), preventing the latter from ubiquitinating Fzd receptors. APC, adenomatous polyposis coli; CKI, casein kinase I; GSK3, glycogen synthase kinase 3; β-TrCP, β-transducin repeat-containing E3 ubiquitin protein ligase.
The pathway described above is highly conserved from sponges to humans 1, 3, but vertebrates have evolved a mechanism to regulate the sensitivity of cells to Wnt signaling. R-spondins (Rspos) are secreted proteins that potentiate Wnt/β-cat signaling (reviewed in 19). Rspos bind to two cell surface receptors, leucine-rich repeat-containing G-protein-coupled receptor (Lgr)4/5/6 and the E3 ubiquitin ligases zinc and ring finger 3 (Znrf3)/ring finger protein 43 (Rnf43) 20, 21. In the absence of Rspo, Znrf3/Rnf43 ubiquitinate Fzd receptors, targeting them for degradation 20. In this manner, Rspo signaling through Lgr and Znrf3/Rnf43 sensitizes the ability of cells to respond to Wnt signals by increasing the number of Fzd receptors ( Figure 1).
In the nucleus, TCF/β-cat can act through enhancers that can be hundreds of kilobases away from the proximal promoters of Wnt targets (e.g 22– 26). Enhancer-promoter communication can be explained by chromatin looping, and there was some prior evidence for this in Wnt gene regulation 26– 28. Jones and colleagues significantly extend these findings, demonstrating that Wnt-dependent looping occurs at multiple targets 22. Cohesin complexes are strongly associated with chromatin loops (reviewed in 29). Consistent with this, chromatin immunoprecipitation sequencing (ChIP-seq) was used to show a signal-dependent recruitment of cohesin subunits to Wnt-regulated enhancers 22. They also found that pathway activation does not greatly increase RNA polymerase II (Pol II) occupancy at promoters of Wnt targets, but it does increase phosphorylation of the C-terminal domain of Pol II, indicating that Wnt/β-cat signaling stimulates transcriptional elongation 22. This study provides the clearest description to date of some of the chromatin events that tie the binding of TCF and β-cat to enhancers with the initiation of transcription at Wnt target loci, and it will be interesting to see if they are typical for Wnt gene activation beyond the human embryonic stem cells used in this report.
Updates to the standard model of Wnt gene regulation
The traditional assumption is that the recruitment of β-cat to chromatin results in transcriptional activation of nearby promoters (reviewed in 10, 18). However, a recent study systematically addressing this point found that the vast majority of β-cat binding sites in the chromatin of Xenopus gastrulating embryos had no detectable effect on gene expression 30. There was a strong overlap of the >10,000 β-cat ChiP-seq peaks identified in this report with TLE and p300 peaks from prior studies 31, 32, suggesting that many of these regions are functioning according to the standard model of Wnt-regulated enhancers. The authors propose a model of β-cat recruitment to regulatory DNA acting as a primer, with inputs from other signaling pathways required for activating transcription 30, 33. Interestingly, a priming role for β-cat has previously been proposed to occur at Wnt targets at an earlier developmental stage in Xenopus, prior to the onset of zygotic transcription at midblastula transition 34.
The work of Hoppler and colleagues highlights the challenges of using ChIP-seq to identify Wnt transcriptional targets. Another recent ChIP-seq/transcriptome analysis also found that only a small fraction of β-cat peaks were functional 35. The same is true when TCF peaks are matched to Wnt-regulated genes 23, 36– 39. But the study by Nakamura et al. 30 is interesting because it also considers p300 occupancy, which has a better track record of predicting functional enhancers 40, 41. That being said, even the most sophisticated models using multiple chromatin markers are still not perfect in locating functional enhancers 42. These studies highlight the complex nature of gene regulation and that clearly the recruitment of β-cat to chromatin is not sufficient for the activation of transcription.
Input from multiple signaling pathways on Wnt-regulated enhancers is one way to integrate information to precisely control gene expression, but cross-talk with other pathways can also occur outside the nucleus. Hippo signaling is a prominent example of cross-regulation with the Wnt/β-cat pathway that has received recent attention. Hippo signaling is an important regulator of cell proliferation and survival in animals (reviewed in 43, 44). A kinase cascade results in activation of the protein kinase large tumor suppressor kinase (LATS)1/2, which phosphorylates and inhibits the cytosolic proteins yes-associated protein (YAP) and tafazzin (TAZ). In the absence of LATS1/2 activity, YAP and TAZ translocate to the nucleus and serve as co-regulators for TEAD family TFs 43, 44. Initial reports found that YAP/TAZ inhibited Wnt/β-cat signaling 45– 47. In contrast to these reports, Piccolo and co-workers found that TAZ was targeted for degradation by the β-cat destruction complex 48. Wnt stimulation resulted in nuclear accumulation of both β-cat and TAZ, and a significant portion of the Wnt-induced transcriptional regulation was TAZ dependent in mammalian cell culture 48. Additional characterization revealed that YAP and TAZ were components of the destruction complex, which are dislodged upon Wnt stimulation 49. These authors provided evidence that Wnt-dependent YAP/TAZ release prevents β-TrCP association with the destruction complex, thus preventing β-cat degradation. Thus, YAP and TAZ can be viewed as integral components of the Wnt/β-cat signaling pathway in addition to their role in Hippo signaling 49.
Subsequent reports on the intersections between Hippo and Wnt/β-cat signaling support a complex and context-dependent relationship between the pathways. For example, LATS2 has been shown to directly inhibit β-cat’s interaction with other co-activators 50. YAP-dependent inhibition of Wnt/β-cat signaling has been reported in Lgr5 + intestinal stem cells 51 and an antagonistic relationship between the Hippo and Wnt pathways was also observed in hepatocellular carcinomas 52. However, cooperation between the pathways consistent with the Piccolo model has been described during chronic inflammation-induced metaplasia in corneal epithelium 53. In addition, Wnt3a activates both TCF and TEAD reporters in skeletal muscle cells 54. Adding to the mechanistic insight linking YAP and β-cat, SET domain-containing lysine methyltransferase 7 (SETD7) is present in the destruction complex and methylates YAP, which is required for its ability to promote the nuclear accumulation of β-cat 55. Hippo and Wnt/β-cat signaling are connected through multiple mechanisms, and understanding the cell-specific cues that favor one interaction over another will be an important goal for future studies.
The TCF transcriptional switch in vertebrates
Invertebrates such as Drosophila and Caenorhabditis elegans have one TCF gene, which plays a dual role on Wnt targets, inhibiting expression in the absence of signaling and mediating transcriptional activation when bound by β-cat (reviewed in 18). Vertebrates possess four or five TCF genes, with individual TCFs being more specialized, e.g. TCF3/TCF7L1 functions exclusively as a repressor 56– 59. In zebrafish, TCF3a and TCF3b repress Sry-related HMG box ( Sox) 4a expression to inhibit spinal cord neurogenesis in a Wnt/β-cat signaling-independent manner 60. Recently, Merrill and co-workers reported a dramatic genetic interaction between TCF3 alleles in mice that also supports a major role for β-cat-independent repression 61. TCF3 null mutants die during gastrulation 59, while TCF3 mutants lacking the β-cat binding domain ( ∆NTCF3) die during late embryogenesis 62. Surprisingly, TCF3 null/ ∆NTCF3 heterozygotes survive into adulthood with no obvious defects 61. This result demonstrates that TCF3 has an essential role in development that is independent of binding to β-cat.
How do some Wnt target genes undergo a transcriptional switch from repression by TCF3 to β-cat-dependent transcriptional activation by other TCFs? One model is that Wnt/β-cat signaling activates homeodomain-interacting protein kinase 2 (HIPK2), a kinase which phosphorylates TCF3, removing it from chromatin 63, 64. In mouse embryonic stem cells, several papers have reported a downregulation of TCF3 in backgrounds where Wnt/β-cat is elevated 61, 65, 66. Interestingly, the three reports differ on whether this effect acts at the level of transcription and/or post-transcriptionally. Under conditions in which mouse embryonic stem cells differentiate into endoderm, TCF3 downregulation coincided with elevated expression of the endodermal marker Forkhead Box A2 (FoxA2) and a loss of TCF3 on FoxA2 regulatory chromatin 66. Indeed, mouse embryonic stem cells lacking TCF3 have elevated FoxA2 expression and can differentiate into endoderm (albeit more slowly than normal) in the absence of Wnt stimulation 66. While this indicates that derepression of Wnt targets is a major driver for endoderm differentiation, another endoderm marker, Sox17, is directly activated by TCF4/TCF7L2 and β-cat 67. In sum, it appears as if derepression as well as β-cat activation of Wnt targets contribute to endoderm differentiation in vertebrates.
TCFs and Wnt target location
All TCFs contain a HMG domain that can bind DNA in a sequence-specific manner (reviewed in 16). However, there is considerable degeneracy in the consensus binding site 68, to the degree that HMG-DNA recognition cannot be sufficient to drive TCF distribution on chromatin (reviewed in 1). This makes identifying Wnt targets solely through computational searches problematic ( Table 1). One way that some TCFs increase their DNA binding specificity is via a second domain, termed the C-clamp, located adjacent to the HMG domain and which binds GC-rich motifs called helper sites 69. The C-clamp is a novel Zn-binding domain 70 and C-clamp-helper site recognition is widely employed in Wnt target gene regulation in Drosophila and C. elegans 70– 73. In vertebrates, C-clamps are found in some isoforms of TCF1/TCF7L and TCF4/TCF7L2, where their presence extends the target selection of these TCF isoforms 74, 75.
Table 1. Approaches to identify Wnt target genes directly activated by the pathway.
We define “direct Wnt targets” as genes whose regulatory DNA can be physically associated with T-cell factors (TCFs) or other transcription factors (TFs) and whose expression is modulated by the recruitment of β-catenin to regulatory chromatin by these TFs. The approaches outlined below each have their advantages and disadvantages, and a combination of them is required to establish with confidence that a gene is a Wnt target gene in a particular context.
| Approach | Advantages | Disadvantages |
|---|---|---|
|
Computational searches for TCF binding sites
Position-weight matrices constructed based on validated lists of TCF binding sites can be used to screen cis-regulatory DNA for additional sites (e.g. 109). The efficiency of this approach can be improved by adding multiple sequences bound by TFs (e.g. helper sites in invertebrates; see 69). The functional relevance of binding sites can be verified with reporter assays. |
• Quickly identifies potentially
regulated genes • The identification of binding sites also establishes candidates for mutagenesis to rigorously test their functionality |
• Most effective when the search
space is restricted to short stretches of DNA (<20 kb) rather than the whole genome • Not all consensus TCF sites will be functional • TCFs and other TFs have degenerate binding sites that could be functional, which could be missed if the calling criteria are too stringent |
|
Transcriptome analyses of Wnt-regulated genes
Microarrays or RNA sequencing can be used to identify genes whose expression changes in Wnt-on and Wnt-off conditions in cell culture (e.g. 74) or embryos (e.g. 30) |
• Identifies the full array of
genes regulated by Wnt pathway activation • Many genetic and biochemical reagents are available to manipulate the Wnt pathway |
• Does not distinguish between
direct and indirect targets of Wnt signaling • In vivo analyses in animal tissues are limited by the specificity of the genetic drivers used for the manipulations |
|
Chromatin immunoprecipitation sequencing
(ChIP-seq) analyses of TCF or β-catenin genomic occupancy ChIP-seq with TCFs and β-catenin with or without Wnt activation can identify candidate Wnt-regulated enhancers. This approach can be combined with ChIP-seq for other TFs (e.g. 76) or with transcriptome analyses to assign genes to regulatory DNA sequences (e.g. 30). |
• Biochemically establishes
the presence of Wnt effectors at cis-regulatory elements • Provides evidence of direct regulation by the Wnt pathway |
• Many TCF/β-catenin binding sites
have no detectable function • Quality of the antibody used plays a major role • While this approach can identify putative Wnt-dependent cis-regulatory elements, identifying which gene the element regulates can be difficult, especially for long-range enhancers |
Even in Drosophila, where there is one TCF gene containing a C-clamp, there is evidence that it associates with chromatin in conjunction with other TFs 76. Consistent with this, genome-wide surveys of TCF or β-cat binding in vertebrates reveal the presence of several TF binding site motifs besides the TCF site consensus 1, 30, 39, 77, 78. Other TFs have been reported to co-localize with TCFs 36, 38, 39, 79, 80, and in the cases of Cdx2 79, Sp5/8 81, and TEAD TFs 82, a co-dependency with TCFs or β-cat for chromatin association has been reported. In addition, TCF binding to chromatin is highly cell type specific 36 and is dynamic over time in the same cell type 38, 39. The data support a picture where different Wnt-regulated enhancers have different binding site grammars, which likely is a major mechanism by which Wnt/β-cat signaling regulates transcription programs in a cell-specific manner.
Are TCFs the major transcriptional mediators of Wnt/β-cat signaling in vertebrate systems? There are several TFs besides TCFs that can bind β-cat and regulate reporters in a β-cat-dependent manner (reviewed in 1, 10, 16), but information on their physiological relevance is limited. Identifying the β-cat binding domains on these TFs would provide valuable tools for investigating these interactions. For example, it is well known that deletion of the N-terminus of TCFs (∆NTCFs) results in potent dominant negatives 16. Expression of a ∆NTCF4 in colorectal carcinoma cells resulted in a reduction of >95% of the β-cat ChIP-seq peaks 83. One interpretation of these dramatic results is that TCFs are the predominant β-cat recruiters in these cells, at least under the experimental conditions used. These data do not rule out cooperation between TCFs and other TFs in β-cat recruitment and highlight the importance of generating TF mutants with specific defects in β-cat binding.
Wnt target genes inform about stem cell biology
Wnt signaling is considered crucial for tissue maintenance by regulating stem cells in many tissues, and examining the expression of Wnt targets has been a successful strategy for identifying Wnt-regulated stem cell populations 2. Lineage-tracing approaches using knock-in alleles of Cre recombinase into the genomic loci of Wnt targets allows fate mapping of the progeny of Wnt-active stem cells. The first major success of this approach was the identification of stem cells in the small intestinal crypts. Lgr5 was initially identified as a Wnt target in colon cancer cell lines 84. Subsequent in vivo analysis showed that its expression in the intestinal epithelium was limited to crypt base stem cells, and the ability of Lgr5 + cells to give rise to epithelial cell types was confirmed through lineage tracing 85. A gradient of Wnt signaling has been demonstrated to be essential for the maintenance of Lgr5 + intestinal stem cells, bolstering the idea of Lgr5 as a Wnt target 86. Lgr5 has since been shown to mark stem cell populations in the hair follicle 87, ovarian epithelium 88, and numerous other tissues 89. It is unclear whether it is a Wnt target in all cases.
A more widely expressed Wnt target is Axin2, whose expression domains resemble Wnt expression patterns 90. Axin2 was first used for fate mapping in the mammary gland 91 and has recently been used to investigate the origin of liver cells. The polyploid nature of hepatocytes has long raised the question of whether they arise from cell division or by differentiation from a stem cell progenitor. The liver is divided into hexagonal lobules, each containing a central vein in the middle. A population of mostly diploid Axin2-expressing cells surrounds the central vein 92. Lineage tracing by fluorescently labeling Axin2 + cells showed that they give rise to progeny that can be found throughout the lobule. Centrally located cells remain labeled, suggesting self-renewal 93. These results establish Axin2 + cells as progenitors of polyploid hepatocytes.
In contrast to the stem cells of the intestine and liver, stem cells of the nail epithelium are seemingly agnostic to Wnt/β-cat signaling but require the pathway for differentiation. Keratin-14 ( K14)-expressing cells located in the nail matrix were identified as nail stem cells (NSCs) through lineage tracing 94. A conditional knockout of β-cat in K14 + cells impaired nail growth, with the entire nail epithelium showing elevated levels of NSC markers. Surprisingly, overexpressing a stabilized β-cat in K14 + cells did not impact nail growth 94. In addition to its being a continuously growing tissue in adults, the nail epithelium has been studied for its role in digit tip regeneration. A population of Wntless ( Wls)-expressing cells—Wls is an acyltransferase required for the secretion of Wnt proteins 95—flanks the NSCs and is essential for digit tip regeneration. Digit tip regeneration does not happen after amputations that remove this population, but this defect can be rescued by the expression of β-cat in K14 + cells. In this context, the Wnt/β-cat pathway appears to be a permissive signal that is essential for differentiation but has no influence on NSCs. Consistent with this, NSCs do not express high levels of Axin2 94.
Wnt target genes in metabolic regulation
The Warburg effect or aerobic glycolysis is seen in cancer cells, which preferentially metabolize glucose through lactic acid fermentation instead of the TCA cycle, even in the presence of oxygen 96. A colon cancer cell line with elevated Wnt/β-cat signaling expressing a ∆NTCF4 isoform containing a C-clamp showed reduced proliferation and a metabolic shift towards oxidative respiration. Consistent with this, genes controlling the cell cycle and metabolism were downregulated by this dominant negative TCF4 74, 97. Interestingly, ∆NTCFs lacking a C-clamp did not affect proliferation but still caused the metabolic shift 97. Pyruvate dehydrogenase kinase 1 (PDK1), which blocks oxidative respiration, was found to be the key Wnt target promoting aerobic glycolysis in these cancer cells 97. PDK1 and the lactate transporter monocarboxylate transport protein (MCT)-1 are direct targets of the Wnt pathway in this context 97, 98 and lactate dehydrogenase, another enzyme driving the Warburg effect, was indirectly activated by the Wnt target c-Myc 97, 99. Interestingly, fluorescence lifetime microscopy (FLIM), which can provide an indicator of the relative rates of glycolysis and oxidative phosphorylation in live tissue, found that aerobic glycolysis also occurs in Wnt-dependent Lgr5 + intestinal stem cells 97, 100.
Wnt target genes and animal evolution
In contrast to transcriptional profiling, Wnt targets that are important in animal evolution have been identified through linkage studies. The three-spined stickleback has become a premier system for studying the evolution of physical traits, since the marine species has repeatedly lost its body armor and ventral spines after colonizing freshwater lakes 101– 103. Characterization of marine/freshwater hybrids identified the Ectodysplasin ( Eda) locus as a major gene responsible for the loss of lateral armor plates in the freshwater species 104. Further refinement identified a single point mutation in an enhancer just downstream of Eda 105. This enhancer is a target of Wnt/β-cat signaling, and the freshwater allele has reduced activation 105. While this 3.2 kb enhancer contains several putative TCF binding sites, they are not close to the polymorphism (K. M. Cadigan, unpublished data), and it is not clear whether Eda is a direct target of the pathway. A similar story exists for the evolution of wing spots in Drosophila guttifera, where the yellow gene is activated by Wnt/β-cat signaling in the pupal wing, though the activation appears to be indirect 106.
Another link between Wnt/β-cat and evolution comes from a genome-wide association study (GWAS) which identified a polymorphism near the KITLG locus that is responsible for blond hair in humans 107. KITLG encodes a ligand for the KIT receptor, known to control pigmentation in mammals 108. Interestingly, the polymorphism resides in a predicted TCF binding site, with the blond allele showing reduced activation by Wnt/β-cat signaling 109. This study directly links the regulation of a direct Wnt target to an important physical trait. This is reminiscent of a polymorphism 335 kb upstream of the c-myc locus, also in a functional TCF site, where the higher-affinity allele is linked to increased risk in colorectal and other cancers 26, 110. Both examples illustrate how a detailed understanding of Wnt gene regulation can facilitate the molecular understanding of polymorphisms in the human population.
Future directions
An increasing number of molecular approaches can now be employed to identify Wnt transcriptional targets in cells or tissues. Continued definition of Wnt transcriptional programs will further the understanding of how the Wnt/β-cat signaling pathway achieves its varied roles in development, stem cell maintenance, and metabolic regulation as well as in disease states and molecular evolution. It is clear that the activation of Wnt targets is highly context dependent, and the emerging picture is that a combination of TCFs and a diverse assortment of other TFs work together in different cells at different times. Unraveling the molecular mechanisms behind context specificity in Wnt responses will not only address a central question of gene regulation but also enhance our knowledge of the diversity of Wnt biology.
Abbreviations
ChIP-seq, chromatin immunoprecipitation sequencing; Eda, Ectodysplasin; FoxA2, Forkhead Box A2; Fzd, Frizzled; K14, keratin-14; LATS, large tumor suppressor kinase; Lgr, leucine-rich repeat-containing G-protein-coupled receptor; NSC, nail stem cell; PDK1, pyruvate dehydrogenase kinase 1; Rnf43, ring finger protein 43; Rspo, R-spondin; Sox, Sry-related HMG box; TAZ, Tafazzin; TCF, T-cell factor; TF, transcription factor; TLE, transducin-like enhancer of split; β-TrCP, β-transducin repeat-containing E3 ubiquitin protein ligase; Wls, Wntless; Wnt/β-cat, Wnt/β-catenin; YAP, yes-associated protein; Znrf3, zinc and ring finger 3.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Randall Moon, Institute for Stem Cell and Regenerative Medicine, University of Washington/HHMI, Seattle, WA, USA
Stefan Hoppler, University of Aberdeen, Aberdeen, UK
Hans Clevers, Department of Developmental Biology and Stem Cell Research, Hubrecht Institute and University Medical Center, Utrecht, Netherlands
Funding Statement
Funding was received from the National Institutes of Health (grant number R01 GM108468).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Archbold HC, Yang YX, Chen L, et al. : How do they do Wnt they do?: regulation of transcription by the Wnt/β-catenin pathway. Acta Physiol (Oxf). 2012;204(1):74–109. 10.1111/j.1748-1716.2011.02293.x [DOI] [PubMed] [Google Scholar]
- 2. Clevers H, Loh KM, Nusse R: Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346(6205):1248012. 10.1126/science.1248012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Loh KM, van Amerongen R, Nusse R: Generating Cellular Diversity and Spatial Form: Wnt Signaling and the Evolution of Multicellular Animals. Dev Cell. 2016;38(6):643–55. 10.1016/j.devcel.2016.08.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Holland JD, Klaus A, Garratt AN, et al. : Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25(2):254–64. 10.1016/j.ceb.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 5. Polakis P: Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5): pii: a008052. 10.1101/cshperspect.a008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karner CM, Long F: Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci. 2017;74(9):1649–57. 10.1007/s00018-016-2425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Birchmeier W: Orchestrating Wnt signalling for metabolic liver zonation. Nat Cell Biol. 2016;18(5):463–5. 10.1038/ncb3349 [DOI] [PubMed] [Google Scholar]
- 8. Grigoryan T, Wend P, Klaus A, et al. : Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22(17):2308–41. 10.1101/gad.1686208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamos JL, Weis WI: The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(1):a007898. 10.1101/cshperspect.a007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valenta T, Hausmann G, Basler K: The many faces and functions of β-catenin. EMBO J. 2012;31(12):2714–36. 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behrens J, von Kries JP, Kuhl M, et al. : Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382(6592):638–42. 10.1038/382638a0 [DOI] [PubMed] [Google Scholar]
- 12. Brunner E, Peter O, Schweizer L, et al. : Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385(6619):829–33. 10.1038/385829a0 [DOI] [PubMed] [Google Scholar]
- 13. Huber O, Korn R, McLaughlin J, et al. : Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59(1):3–10. 10.1016/0925-4773(96)00597-7 [DOI] [PubMed] [Google Scholar]
- 14. Molenaar M, van de Wetering M, Oosterwegel M, et al. : XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–9. 10.1016/S0092-8674(00)80112-9 [DOI] [PubMed] [Google Scholar]
- 15. van de Wetering M, Cavallo R, Dooijes D, et al. : Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88(6):789–99. 10.1016/S0092-8674(00)81925-X [DOI] [PubMed] [Google Scholar]
- 16. Cadigan KM, Waterman ML: TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012;4(11): pii: a007906. 10.1101/cshperspect.a007906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hrckulak D, Kolar M, Strnad H, et al. : TCF/LEF Transcription Factors: An Update from the Internet Resources. Cancers (Basel). 2016;8(7): pii: E70. 10.3390/cancers8070070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadigan KM: TCFs and Wnt/β-catenin Signaling: More than One Way to Throw the Switch. Curr Top Dev Biol.S.P. and F. Payre, ed. (Academic Press),2012;98:1–34. 10.1016/B978-0-12-386499-4.00001-X [DOI] [PubMed] [Google Scholar]
- 19. Schuijers J, Clevers H: Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 2012;31(12):2685–96. 10.1038/emboj.2012.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Lau W, Peng WC, Gros P, et al. : The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–16. 10.1101/gad.235473.113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Zebisch M, Xu Y, Krastev C, et al. : Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4: 2787. 10.1038/ncomms3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Estarás C, Benner C, Jones KA: SMADs and YAP compete to control elongation of β-catenin:LEF-1-recruited RNAPII during hESC differentiation. Mol Cell. 2015;58(5):780–93. 10.1016/j.molcel.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Hatzis P, van der Flier LG, van Driel MA, et al. : Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Mol Cell Biol. 2008;28(8):2732–44. 10.1128/MCB.02175-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park J, Ma W, O'Brien LL, et al. : Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23(3):637–51. 10.1016/j.devcel.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Sur IK, Hallikas O, Vähärautio A, et al. : Mice lacking a Myc enhancer that includes human SNP rs6983267 are resistant to intestinal tumors. Science. 2012;338(6112):1360–3. 10.1126/science.1228606 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Wright JB, Brown SJ, Cole MD: Upregulation of c- MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol. 2010;30(6):1411–20. 10.1128/MCB.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pomerantz MM, Ahmadiyeh N, Jia L, et al. : The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet. 2009;41(8):882–4. 10.1038/ng.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yochum GS, Sherrick CM, Macpartlin M, et al. : A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5' and 3' Wnt responsive enhancers. Proc Natl Acad Sci U S A. 2010;107(1):145–50. 10.1073/pnas.0912294107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Remeseiro S, Cuadrado A, Losada A: Cohesin in development and disease. Development. 2013;140(18):3715–8. 10.1242/dev.090605 [DOI] [PubMed] [Google Scholar]
- 30. Nakamura Y, de Paiva Alves E, Veenstra GJ, et al. : Tissue- and stage-specific Wnt target gene expression is controlled subsequent to β-catenin recruitment to cis-regulatory modules. Development. 2016;143(11):1914–25. 10.1242/dev.131664 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Akkers RC, van Heeringen SJ, Jacobi UG, et al. : A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev Cell. 2009;17(3):425–34. 10.1016/j.devcel.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yasuoka Y, Suzuki Y, Takahashi S, et al. : Occupancy of tissue-specific cis-regulatory modules by Otx2 and TLE/Groucho for embryonic head specification. Nat Commun. 2014;5: 4322. 10.1038/ncomms5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura Y, Hoppler S: Genome-wide analysis of canonical Wnt target gene regulation in Xenopus tropicalis challenges β-catenin paradigm. Genesis. 2017;55(1–2): e22991. 10.1002/dvg.22991 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Blythe SA, Cha SW, Tadjuidje E, et al. : beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell. 2010;19(2):220–31. 10.1016/j.devcel.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe K, Biesinger J, Salmans ML, et al. : Integrative ChIP-seq/microarray analysis identifies a CTNNB1 target signature enriched in intestinal stem cells and colon cancer. PLoS One. 2014;9(3):e92317. 10.1371/journal.pone.0092317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frietze S, Wang R, Yao L, et al. : Cell type-specific binding patterns reveal that TCF7L2 can be tethered to the genome by association with GATA3. Genome Biol. 2012;13(9):R52. 10.1186/gb-2012-13-9-r52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norton L, Chen X, Fourcaudot M, et al. : The mechanisms of genome-wide target gene regulation by TCF7L2 in liver cells. Nucleic Acids Res. 2014;42(22):13646–61. 10.1093/nar/gku1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trompouki E, Bowman TV, Lawton LN, et al. : Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147(3):577–89. 10.1016/j.cell.2011.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao C, Deng Y, Liu L, et al. : Dual regulatory switch through interactions of Tcf7l2/Tcf4 with stage-specific partners propels oligodendroglial maturation. Nat Commun. 2016;7: 10883. 10.1038/ncomms10883 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Dogan N, Wu W, Morrissey CS, et al. : Occupancy by key transcription factors is a more accurate predictor of enhancer activity than histone modifications or chromatin accessibility. Epigenetics Chromatin. 2015;8:16. 10.1186/s13072-015-0009-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Visel A, Blow MJ, Li Z, et al. : ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457(7231):854–8. 10.1038/nature07730 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. He Y, Gorkin DU, Dickel DE, et al. : Improved regulatory element prediction based on tissue-specific local epigenomic signatures. Proc Natl Acad Sci U S A. 2017;114(9):E1633–E1640. 10.1073/pnas.1618353114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meng Z, Moroishi T, Guan KL: Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. 10.1101/gad.274027.115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Moya IM, Halder G: The Hippo pathway in cellular reprogramming and regeneration of different organs. Curr Opin Cell Biol. 2016;43:62–8. 10.1016/j.ceb.2016.08.004 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Attisano L, Wrana JL: Signal integration in TGF-β, WNT, and Hippo pathways. F1000Prime Rep. 2013;5:17. 10.12703/P5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Imajo M, Miyatake K, Iimura A, et al. : A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31(5):1109–22. 10.1038/emboj.2011.487 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Varelas X, Miller BW, Sopko R, et al. : The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18(4):579–91. 10.1016/j.devcel.2010.03.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Azzolin L, Zanconato F, Bresolin S, et al. : Role of TAZ as mediator of Wnt signaling. Cell. 2012;151(7):1443–56. 10.1016/j.cell.2012.11.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Azzolin L, Panciera T, Soligo S, et al. : YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158(1):157–70. 10.1016/j.cell.2014.06.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Li J, Chen X, Ding X, et al. : LATS2 suppresses oncogenic Wnt signaling by disrupting β-catenin/BCL9 interaction. Cell Rep. 2013;5(6):1650–63. 10.1016/j.celrep.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gregorieff A, Liu Y, Inanlou MR, et al. : Yap-dependent reprogramming of Lgr5 + stem cells drives intestinal regeneration and cancer. Nature. 2015;526(7575):715–8. 10.1038/nature15382 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Kim W, Khan SK, Gvozdenovic-Jeremic J, et al. : Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J Clin Invest. 2017;127(1):137–52. 10.1172/JCI88486 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Nowell CS, Odermatt PD, Azzolin L, et al. : Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol. 2016;18(2):168–80. 10.1038/ncb3290 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Huraskin D, Eiber N, Reichel M, et al. : Wnt/β-catenin signaling via Axin2 is required for myogenesis and, together with YAP/Taz and Tead1, active in IIa/IIx muscle fibers. Development. 2016;143(17):3128–42. 10.1242/dev.139907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Oudhoff MJ, Antignano F, Chenery AL, et al. : Intestinal Epithelial Cell-Intrinsic Deletion of Setd7 Identifies Role for Developmental Pathways in Immunity to Helminth Infection. PLoS Pathog. 2016;12(9):e1005876. 10.1371/journal.ppat.1005876 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Dorsky RI, Itoh M, Moon RT, et al. : Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130(9):1937–47. 10.1242/dev.00402 [DOI] [PubMed] [Google Scholar]
- 57. Kim CH, Oda T, Itoh M, et al. : Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407(6806):913–6. 10.1038/35038097 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Liu F, van den Broek O, Destrée O, et al. : Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132(24):5375–85. 10.1242/dev.02152 [DOI] [PubMed] [Google Scholar]
- 59. Merrill BJ, Pasolli HA, Polak L, et al. : Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131(2):263–74. 10.1242/dev.00935 [DOI] [PubMed] [Google Scholar]
- 60. Gribble SL, Kim H, Bonner J, et al. : Tcf3 inhibits spinal cord neurogenesis by regulating sox4a expression. Development. 2009;136(5):781–9. 10.1242/dev.027995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shy BR, Wu CI, Khramtsova GF, et al. : Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/β-catenin signaling. Cell Rep. 2013;4(1):1–9. 10.1016/j.celrep.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu CI, Hoffman JA, Shy BR, et al. : Function of Wnt/β-catenin in counteracting Tcf3 repression through the Tcf3-β-catenin interaction. Development. 2012;139(2):2118–29. 10.1242/dev.076067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hikasa H, Sokol SY: Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem. 2011;286(14):12093–100. 10.1074/jbc.M110.185280 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Hikasa H, Ezan J, Itoh K, et al. : Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell. 2010;19(4):521–32. 10.1016/j.devcel.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Atlasi Y, Noori R, Gaspar C, et al. : Wnt signaling regulates the lineage differentiation potential of mouse embryonic stem cells through Tcf3 down-regulation. PLoS Genet. 2013;9(5):e1003424. 10.1371/journal.pgen.1003424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morrison G, Scognamiglio R, Trumpp A, et al. : Convergence of cMyc and β-catenin on Tcf7l1 enables endoderm specification. EMBO J. 2016;35(3):356–68. 10.15252/embj.201592116 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Engert S, Burtscher I, Liao WP, et al. : Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development. 2013;140(15):3128–38. 10.1242/dev.088765 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Badis G, Berger MF, Philippakis AA, et al. : Diversity and complexity in DNA recognition by transcription factors. Science. 2009;324(5935):1720–3. 10.1126/science.1162327 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Ravindranath AJ, Cadigan KM: The Role of the C-Clamp in Wnt-Related Colorectal Cancers. Cancers (Basel). 2016;8(8): pii: E74. 10.3390/cancers8080074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ravindranath AJ, Cadigan KM: Structure-function analysis of the C-clamp of TCF/Pangolin in Wnt/ß-catenin signaling. PLoS One. 2014;9(1):e86180. 10.1371/journal.pone.0086180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Archbold HC, Broussard C, Chang MV, et al. : Bipartite recognition of DNA by TCF/Pangolin is remarkably flexible and contributes to transcriptional responsiveness and tissue specificity of wingless signaling. PLoS Genet. 2014;10(9):e1004591. 10.1371/journal.pgen.1004591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhambhani C, Ravindranath AJ, Mentink RA, et al. : Distinct DNA binding sites contribute to the TCF transcriptional switch in C. elegans and Drosophila. PLoS Genet. 2014;10(2):e1004133. 10.1371/journal.pgen.1004133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang CU, Blauwkamp TA, Burby PE, et al. : Wnt-mediated repression via bipartite DNA recognition by TCF in the Drosophila hematopoietic system. PLoS Genet. 2014;10(8):e1004509. 10.1371/journal.pgen.1004509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoverter NP, Ting JH, Sundaresh S, et al. : A WNT/p21 circuit directed by the C-clamp, a sequence-specific DNA binding domain in TCFs. Mol Cell Biol. 2012;32(18):3648–62. 10.1128/MCB.06769-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hoverter NP, Zeller MD, McQuade MM, et al. : The TCF C-clamp DNA binding domain expands the Wnt transcriptome via alternative target recognition. Nucleic Acids Res. 2014;42(22):13615–32. 10.1093/nar/gku1186 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Junion G, Spivakov M, Girardot C, et al. : A transcription factor collective defines cardiac cell fate and reflects lineage history. Cell. 2012;148(3):473–86. 10.1016/j.cell.2012.01.030 [DOI] [PubMed] [Google Scholar]
- 77. Bhambhani C, Cadigan KM: Finding a Needle in a Genomic Haystack: Genome-Wide to Wnt/TCF Transcriptional Targets. In Wnt Signaling in Development and Disease: Molecular Mechanisms and Biological Functions Hoppler S, and Moon RT, eds. (John Wiley & Sons, Inc).2014;73–87. 10.1002/9781118444122.ch5 [DOI] [Google Scholar]
- 78. Wang Y, Wang R, Jin VX: Inference of hierarchical regulatory network of TCF7L2 binding sites in MCF7 cell line. Int J Comput Biol Drug Des. 2016;9(1–2):25–53. 10.1504/IJCBDD.2016.074990 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Verzi MP, Hatzis P, Sulahian R, et al. : TCF4 and CDX2, major transcription factors for intestinal function, converge on the same cis-regulatory regions. Proc Natl Acad Sci U S A. 2010;107(34):15157–62. 10.1073/pnas.1003822107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang X, Peterson KA, Liu XS, et al. : Gene regulatory networks mediating canonical Wnt signal-directed control of pluripotency and differentiation in embryo stem cells. Stem Cells. 2013;31(12):2667–79. 10.1002/stem.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kennedy MW, Chalamalasetty RB, Thomas S, et al. : Sp5 and Sp8 recruit β-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription. Proc Natl Acad Sci U S A. 2016;113(13):3545–50. 10.1073/pnas.1519994113 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Jiao S, Li C, Hao Q, et al. : VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. 2017;8: 14058. 10.1038/ncomms14058 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Schuijers J, Mokry M, Hatzis P, et al. : Wnt-induced transcriptional activation is exclusively mediated by TCF/LEF. EMBO J. 2014;33(2):146–56. 10.1002/embj.201385358 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. van de Wetering M, Sancho E, Verweij C, et al. : The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111(2):241–50. 10.1016/S0092-8674(02)01014-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Barker N, van Es JH, Kuipers J, et al. : Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. van Es JH, Haegebarth A, Kujala P, et al. : A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32(10):1918–27. 10.1128/MCB.06288-11 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Jaks V, Barker N, Kasper M, et al. : Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–9. 10.1038/ng.239 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Flesken-Nikitin A, Hwang CI, Cheng CY, et al. : Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495(7440):241–5. 10.1038/nature11979 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Barker N, Tan S, Clevers H: Lgr proteins in epithelial stem cell biology. Development. 2013;140(12):2484–94. 10.1242/dev.083113 [DOI] [PubMed] [Google Scholar]
- 90. Jho EH, Zhang T, Domon C, et al. : Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–83. 10.1128/MCB.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van Amerongen R, Bowman AN, Nusse R: Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11(3):387–400. 10.1016/j.stem.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 92. Benhamouche S, Decaens T, Godard C, et al. : Apc tumor suppressor gene is the "zonation-keeper" of mouse liver. Dev Cell. 2006;10(6):759–70. 10.1016/j.devcel.2006.03.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Wang B, Zhao L, Fish M, et al. : Self-renewing diploid Axin2 + cells fuel homeostatic renewal of the liver. Nature. 2015;524(7564):180–5. 10.1038/nature14863 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Takeo M, Chou WC, Sun Q, et al. : Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature. 2013;499(7457):228–32. 10.1038/nature12214 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Port F, Basler K: Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic. 2010;11(10):1265–71. 10.1111/j.1600-0854.2010.01076.x [DOI] [PubMed] [Google Scholar]
- 96. Warburg O: On the origin of cancer cells. Science. 1956;123(3191):309–14. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 97. Pate KT, Stringari C, Sprowl-Tanio S, et al. : Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33(13):1454–73. 10.15252/embj.201488598 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Sprowl-Tanio S, Habowski AN, Pate KT, et al. : Lactate/pyruvate transporter MCT-1 is a direct Wnt target that confers sensitivity to 3-bromopyruvate in colon cancer. Cancer Metab. 2016;4:20. 10.1186/s40170-016-0159-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thompson CB: Wnt meets Warburg: another piece in the puzzle? EMBO J. 2014;33(13):1420–2. 10.15252/embj.201488785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Stringari C, Edwards RA, Pate KT, et al. : Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci Rep. 2012;2: 568. 10.1038/srep00568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bell MA, Foster SA: The Evolutionary Biology of the Threespine Stickleback. (Oxford University Press).1994. Reference Source [Google Scholar]
- 102. Chan YF, Marks ME, Jones FC, et al. : Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327(5963):302–5. 10.1126/science.1182213 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Peichel CL, Nereng KS, Ohgi KA, et al. : The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414(6866):901–5. 10.1038/414901a [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Colosimo PF, Hosemann KE, Balabhadra S, et al. : Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 2005;307(5717):1928–33. 10.1126/science.1107239 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. O'Brown NM, Summers BR, Jones FC, et al. : A recurrent regulatory change underlying altered expression and Wnt response of the stickleback armor plates gene EDA. eLife. 2015;4:e05290. 10.7554/eLife.05290 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Werner T, Koshikawa S, Williams TM, et al. : Generation of a novel wing colour pattern by the Wingless morphogen. Nature. 2010;464(7292):1143–8. 10.1038/nature08896 [DOI] [PubMed] [Google Scholar]
- 107. Sulem P, Gudbjartsson DF, Stacey SN, et al. : Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39(12):1443–52. 10.1038/ng.2007.13 [DOI] [PubMed] [Google Scholar]
- 108. Reissmann M, Ludwig A: Pleiotropic effects of coat colour-associated mutations in humans, mice and other mammals. Semin Cell Dev Biol. 2013;24(6–7):576–86. 10.1016/j.semcdb.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 109. Guenther CA, Tasic B, Luo L, et al. : A molecular basis for classic blond hair color in Europeans. Nat Genet. 2014;46(7):748–52. 10.1038/ng.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 110. Tuupanen S, Turunen M, Lehtonen R, et al. : The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet. 2009;41(8):885–90. 10.1038/ng.406 [DOI] [PubMed] [Google Scholar]