Abstract
Background.
Cancers of the brain and CNS constitute a group of rare and heterogeneous tumors. Increasing incidence in Western populations has been linked to improvements in diagnostic technology, although interpretation is hampered by changes in diagnosis and reporting. The present study examines geographic and temporal variations in incidence rates of brain and CNS cancers worldwide.
Methods.
Data from successive volumes of Cancer Incidence in Five Continents were used, including 96 registries in 39 countries. We used Joinpoint regression to estimate the average annual percentage change and its 95% CI.
Results.
Globally, a large variability in the magnitude of the diagnosis of new cases of brain and CNS cancer was found, with a 5-fold difference between the highest rates (mainly in Europe) and the lowest (mainly in Asia). Increasing rates of brain and CNS cancer were found in South America, namely in Ecuador, Brazil, and Colombia; in eastern Europe (Czech Republic and Russia), in southern Europe (Slovenia), and in the 3 Baltic countries. Trends were similar between sexes, although decreasing trends in men and women were seen in Japan and New Zealand.
Conclusions.
Important regional variations in brain and CNS cancers exist, and given an increasing burden and risk worldwide, there is a need for further etiological research that focuses on the elucidation of environmental risk. The trends are sufficiently complex and diffuse, however, to warrant a cautious approach to interpretation.
Keywords: brain and CNS tumors, cancer registries, epidemiology, incidence, time trends
Cancers of the brain and CNS comprize a group of rare and heterogeneous tumors with respect to genetics and biology.1 They constitute approximately 3% of the cancer cases worldwide and are more frequent among men than women.2 The prognoses of brain and CNS cancers vary by age and histological type; generally 5-year survival is low, with prognosis particularly poor for glioblastomas and at older ages.3 Observed increases in survival in higher-income countries are largely attributed to improvements in medical care and the availability of new therapies.4,5
Studies, particularly in Western countries, have indicated an increasing occurrence of brain and CNS cancers, most notably among elderly populations.6–8 The main driver is considered to be general improvements in diagnosis following the introduction of CT and MRI in the 1980s.6,7 Little is known about the etiological factors of brain cancers. Other than genetic risk factors, ionizing radiation exposure is known to increase the risk, while allergic conditions appear to decrease its risk.9,10 Exposure to non-ionizing radiation, especially radiofrequency fields from mobile phones but also low frequency fields, infections with some viruses, use of hormonal contraceptives, hormone replacement therapy, statins, vitamin D level, alcohol, height, BMI, and occupational exposures have been investigated, but no firm conclusions can be drawn at present.11–13
Given the specific anatomical location of brain and CNS tumors and the high probability of developing a life-threatening condition, many cancer registries record (and include in reporting) nonmalignant tumors of the brain and other and unspecified parts of the CNS. These nonmalignant tumors are estimated to be around two-thirds of all brain and CNS tumors.14 This fact in combination with the underlying diagnostic difficulties of these tumors,15 the lack of consistency in definition of specific histological types, and the importance of diagnostic advances with time calls for a careful assessment of international comparisons of secular trends in incidence16; in some instances such comparisons may not be valid17
Perhaps for the above reasons, systematic descriptions of the patterns and trends of brain and CNS cancers across world regions are rarely undertaken. They can, however, contribute to a better understanding of the specific problems of interpretation and may provide some insight into potential risk factors and consequently the future burden of these tumors. The present study aims to provide such a global status report of the geographic and temporal variations in the incidence of brain and CNS cancers in different countries across continents worldwide.
Methods
Data Sources
Annual incidence data of cancers of the brain and CNS (including malignant meningioma, ICD-10: C70-72) were derived from International Agency for Research on Cancer’s serial publication Cancer Incidence in Five Continents (CI5) volumes VIII, IX, and X (1993 to 2007)18 and additionally extracted from the European Cancer Observatory for Denmark, Norway, and Sweden.19 Incidence data in the CI5 publications were obtained from high quality population-based registries covering either the total population (in 17 countries) or region(s) within a given country. We included 96 registries in 39 countries (see Supplementary Table 2) across 9 world regions: (1) Africa, (2) Central and South America, (3) Northern America, (4) Asia, (5) central and eastern Europe (6) northern Europe, (7) southern Europe, (8) western Europe, and (9) Oceania.
Statistical Analysis
Data were analyzed in adult populations (≥15 y, due to different patterns of brain and CNS cancer in children) by sex and country except in the US, where separate analysis by race/ethnicity (US whites and US blacks) were undertaken. Truncated age-standardized incidence rates (per 100,000, ages ≥15 y) used the world standard population20 and are presented for the latest available period, 2003–2007, with 95% CIs.
Trends are presented for the years 1993–2007, with the average annual percentage change (AAPC) calculated for the latest 10-year period (commonly 1998–2007) as described by Kim21 and presented with corresponding 95% CIs for each country, except Uganda, excluded from this analysis due to small numbers. An AAPC with an associated P value <.05 was considered statistically significant. The choice of model was determined using a permutation method.21 All analyses were performed using the statistical software Stata and Joinpoint (batch mode).22 Trends in incidence by sex are shown with smoothed lines on fitting locally weighted regression (lowess) curves, in which 30% of the data were used in the smoothing.23
Results
Geographic Variations in Incidence 2003–2007
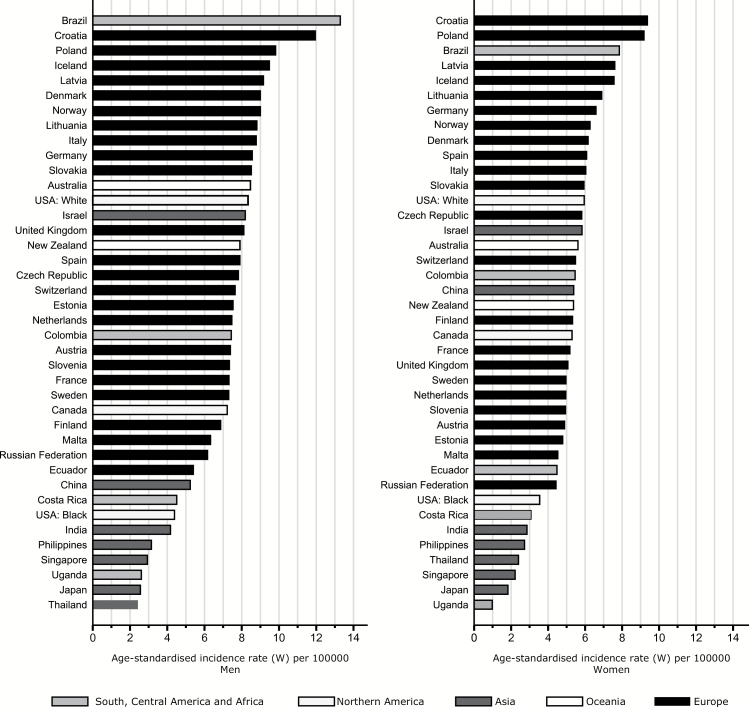
In the countries/registries considered, 78,034 new cases of brain and CNS cancers were diagnosed between 2003 and 2007; 54% in men and 46% in women (Table 1). Incidence was generally higher in men than in women, with a male to female ratio ranging from 2.7 to 1.0. Age-standardized incidence rates (per 100,000) in men were highest in Brazil (13.3), Croatia (12.0), and Poland (9.8), followed by the northern European countries of Iceland (9.5), Latvia (9.2), Denmark, and Norway (both 9.0). For women, rates in Croatia ranked first (9.4), followed by Poland (9.2), Brazil (7.9), Iceland (7.6), and Latvia (7.6). Rates were lowest among men in Thailand (2.4), Japan (2.6), Uganda (2.6), and Singapore (2.9), and in Uganda (1.0), Japan (1.8), Singapore (2.2), and Thailand (1.0) among women. Large differences were observed between the white and black populations of the US, with an almost 2-fold higher incidence among whites relative to blacks (8.3 vs 4.4 in white and black men and 6.0 vs 3.6 in women, respectively) (Fig. 1 and Supplementary Table 1).
Table 1.
Truncated age-standardized rates (ASR) and 95% CI of brain and CNS cancer incidence (per 100,000) by sex and country, ages ≥15 y, diagnoses 2003–2007.
| Country | Number of Registries | Total | Men | Women | % Women | M:F Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ASR(W) | 95% CI | Cases | ASR(W) | 95%CI | |||||
| Africa | ||||||||||
| Uganda | 1 | 39 | 31 | 2.6 | 1.4 – 3.7 | 8 | 1.0 | 0.2 – 1.7 | 20.5 | 2.7 |
| South and Central America | ||||||||||
| Brazil | 1 | 373 | 214 | 13.3 | 11.4 – 15.1 | 159 | 7.9 | 6.6 – 9.1 | 42.6 | 1.7 |
| Costa Rica* | 1 | 518 | 301 | 4.5 | 3.9 – 5.0 | 217 | 3.1 | 2.6 – 3.5 | 41.9 | 1.5 |
| Colombia | 1 | 428 | 220 | 7.4 | 6.4 – 8.4 | 208 | 5.5 | 4.7 – 6.2 | 48.6 | 1.4 |
| Ecuador | 1 | 235 | 121 | 5.4 | 4.4 – 6.3 | 114 | 4.5 | 3.6 – 5.3 | 48.5 | 1.2 |
| North America | ||||||||||
| Canada | 3 | 988 | 539 | 7.2 | 6.5 – 7.8 | 449 | 5.3 | 4.7 – 5.8 | 45.4 | 1.4 |
| USA: Black | 9 | 507 | 248 | 4.4 | 3.8 – 4.9 | 259 | 3.6 | 3.1 – 4.0 | 51.1 | 1.2 |
| USA: White | 9 | 7191 | 4012 | 8.3 | 8.0 – 8.5 | 3179 | 6.0 | 5.7 – 6.2 | 44.2 | 1.4 |
| Asia | ||||||||||
| China | 3 | 3901 | 1815 | 5.2 | 4.9 – 5.4 | 2086 | 5.4 | 5.1 – 5.6 | 53.5 | 1.0 |
| India | 3 | 2375 | 1464 | 4.2 | 3.9 – 4.4 | 911 | 2.9 | 2.7 – 3.0 | 38.4 | 1.5 |
| Israel | 1 | 1609 | 872 | 8.2 | 7.6 – 8.7 | 737 | 5.8 | 5.3 – 6.2 | 45.8 | 1.4 |
| Japan | 3 | 1700 | 911 | 2.6 | 2.2 – 2.7 | 789 | 1.8 | 1.6 – 1.9 | 46.4 | 1.4 |
| Philippines | 1 | 421 | 218 | 3.2 | 2.7 – 3.6 | 203 | 2.7 | 2.3 – 3.0 | 48.2 | 1.2 |
| Singapore* | 1 | 367 | 205 | 2.9 | 2.4 – 3.3 | 162 | 2.2 | 1.8 – 2.5 | 44.1 | 1.3 |
| Thailand | 3 | 351 | 170 | 2.4 | 2.0 – 2.7 | 181 | 2.4 | 2.1 – 2.6 | 51.6 | 1.0 |
| Oceania | ||||||||||
| Australia | 6 | 6888 | 3993 | 8.5 | 8.1 – 8.8 | 2895 | 5.6 | 5.4 – 5.7 | 42 | 1.5 |
| New Zealand* | 1 | 1269 | 729 | 7.9 | 7.3 – 8.4 | 540 | 5.4 | 4.9 – 5.8 | 42.6 | 1.5 |
| Europe | ||||||||||
| Eastern Europe | ||||||||||
| Czech Republic* | 1 | 3722 | 1953 | 7.8 | 7.4 – 8.1 | 1769 | 5.9 | 5.6 – 6.1 | 47.5 | 1.3 |
| Poland | 1 | 304 | 135 | 9.8 | 8.1 – 11.4 | 169 | 9.2 | 7.6 – 10.7 | 55.6 | 1.1 |
| Russian Federation | 1 | 1264 | 596 | 6.2 | 5.6 – 6.7 | 668 | 4.5 | 4.1 – 4.8 | 52.8 | 1.4 |
| Slovakia* | 1 | 1825 | 960 | 8.5 | 7.9 – 9.0 | 865 | 6.0 | 5.5 – 6.1 | 47.4 | 1.4 |
| Northern Europe | ||||||||||
| Denmark* | 1 | 2193 | 1251 | 9.0 | 8.4 – 9.5 | 933 | 6.2 | 5.7 – 6.6 | 42.9 | 1.4 |
| Estonia* | 1 | 401 | 215 | 7.5 | 6.4 – 8.5 | 186 | 4.8 | 4.0 – 5.5 | 46.4 | 1.6 |
| Finland* | 1 | 1738 | 908 | 6.9 | 6.4 – 7.3 | 830 | 5.4 | 4.9 – 5.8 | 47.8 | 1.3 |
| Iceland* | 1 | 117 | 62 | 9.5 | 7.1 – 11.8 | 55 | 7.6 | 5.5 – 9.7 | 47 | 1.2 |
| Latvia* | 1 | 972 | 456 | 9.2 | 8.3 – 10.0 | 516 | 7.6 | 6.8 – 8.3 | 53.1 | 1.2 |
| Lithuania* | 1 | 1351 | 635 | 8.8 | 8.0 – 9.5 | 716 | 6.9 | 6.3 – 7.4 | 53 | 1.3 |
| Norway* | 1 | 1752 | 984 | 9.0 | 8.3 – 9.6 | 768 | 6.3 | 5.6 – 6.9 | 43.8 | 1.4 |
| Sweden* | 1 | 2763 | 1609 | 7.3 | 6.7 – 7.8 | 1154 | 5.0 | 4.5 – 5.4 | 41.7 | 1.4 |
| United Kingdom | 9 | 14210 | 8313 | 8.1 | 7.9 – 8.2 | 5897 | 5.1 | 4.9 – 5.2 | 41.5 | 1.6 |
| Southern Europe | ||||||||||
| Croatia* | 1 | 2553 | 1298 | 12.0 | 11.3 – 12.6 | 1255 | 9.4 | 8.8 – 9.9 | 49.2 | 1.3 |
| Italy | 8 | 2491 | 1323 | 8.8 | 8.2 – 9.3 | 1168 | 6.1 | 5.6 – 6.5 | 46.9 | 1.4 |
| Malta* | 1 | 110 | 60 | 6.3 | 4.6 – 7.9 | 50 | 4.6 | 3.2 – 5.9 | 45.5 | 1.4 |
| Slovenia* | 1 | 660 | 356 | 7.3 | 6.5 – 8.0 | 304 | 5.0 | 4.3 – 5.6 | 46.1 | 1.5 |
| Spain | 7 | 1787 | 957 | 7.9 | 7.3 – 8.4 | 830 | 6.1 | 5.6 – 6.5 | 46.4 | 1.3 |
| Western Europe | ||||||||||
| Austria | 2 | 334 | 191 | 7.4 | 6.3 – 8.5 | 143 | 4.9 | 4.0 – 5.7 | 42.8 | 1.5 |
| France | 8 | 1972 | 1085 | 7.3 | 6.8 – 7.7 | 887 | 5.2 | 4.8 – 5.5 | 45 | 1.4 |
| Germany | 1 | 485 | 254 | 8.6 | 7.4 – 9.7 | 231 | 6.6 | 5.5 – 7.6 | 47.6 | 1.3 |
| Netherlands* | 1 | 5079 | 2945 | 7.5 | 7.2 – 7.7 | 2134 | 5.0 | 4.7 – 5.2 | 42 | 1.5 |
| Switzerland | 6 | 791 | 434 | 7.7 | 6.9 – 8.4 | 357 | 5.5 | 4.8 – 6.1 | 45.1 | 1.4 |
ASR(W) Age-standardized using the world population; % Women (proportion of cancers in women); M:F ratio (Male and Female Ratio); * identifies national registries.
Fig. 1.
Truncated age-standardized incidence rates of brain and CNS cancers (per 100,000, ages ≥15 y, world standard), 2003–2007 by sex.
Temporal Variations 1993–2007
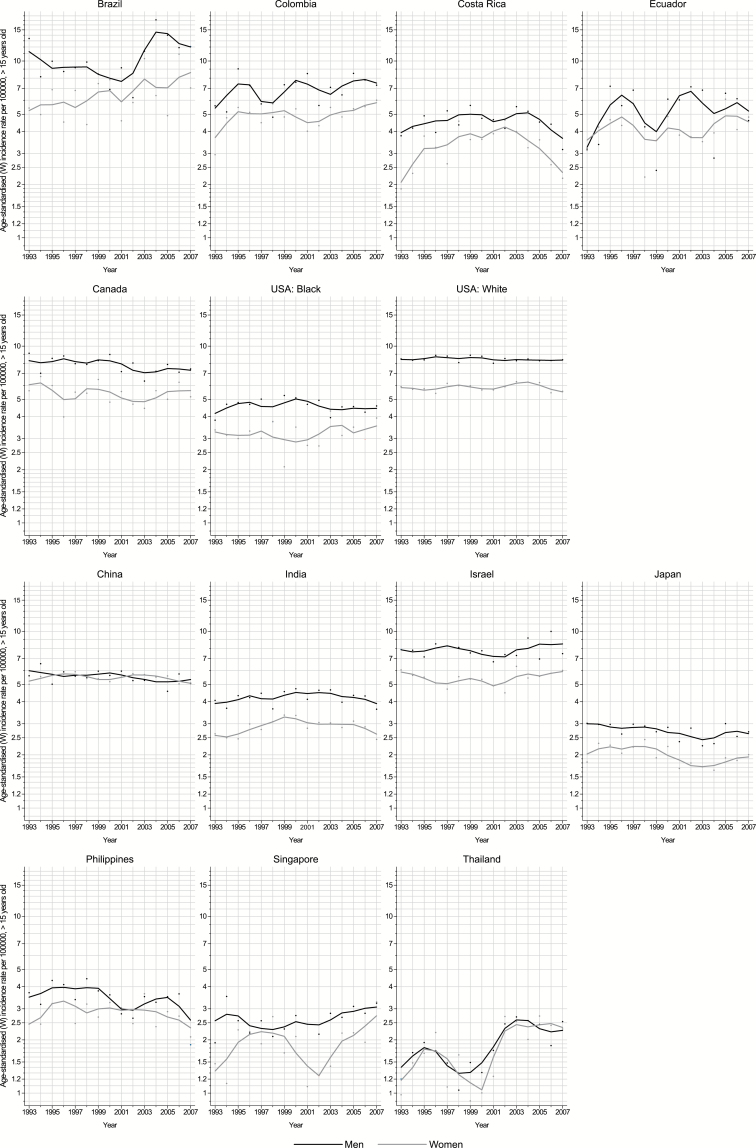
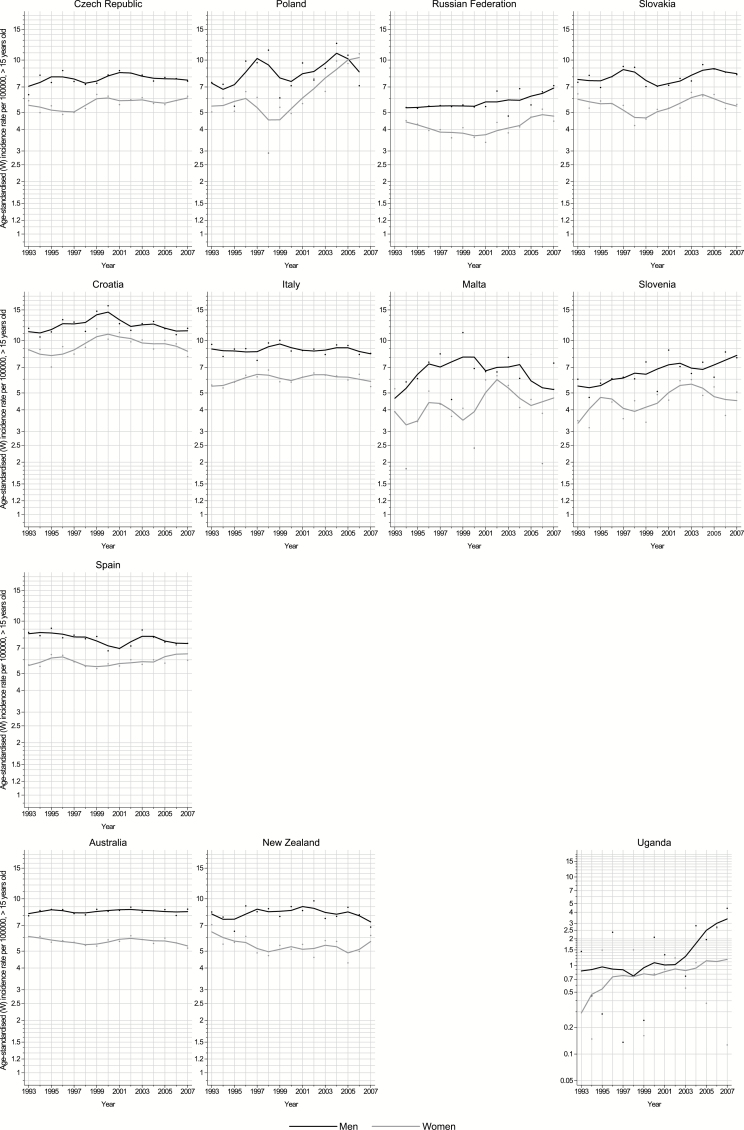
Figures 2, 3, and 4 show the annual incidence rate for each country included in this study by sex, between 1993 and 2007. Rising trends were observed in both sexes in Latvia, Lithuania, Norway, Slovenia, the Russian Federation, Ecuador, Colombia, Brazil, Singapore, and Thailand; in the remaining countries, rates tended to be relatively stable over the last decade.
Fig. 2.
Trends in truncated age-standardized incidence rates of brain and CNS cancers (per 100,000, ages ≥15 y, world standard) in selected populations in the Americas 1993–2007 by sex (black and gray lines: males and females, respectively).
Fig. 3.
Trends in truncated age-standardized incidence rates of brain and CNS cancers (per 100,000, ages ≥15 y, world standard) in selected populations in northern and western Europe 1993–2007 by sex (black and gray lines: males and females, respectively).
Fig. 4.
Trends in truncated age-standardized incidence rates of brain and CNS cancers (per 100,000, ages ≥15 y, world standard), 1993–2007 in in selected populations in southern and eastern Europe, Oceania, and Africa by sex (black and gray lines: males and females, respectively).
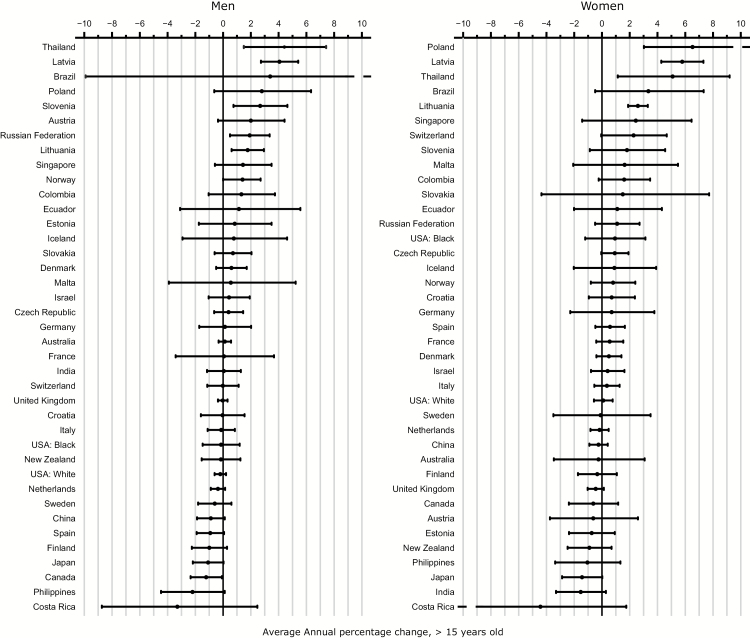
The AAPC for the last 10 years (commonly 1998–2007) is shown in Fig. 5. Generally about half of the countries exhibited an increasing incidence of brain and CNS cancer. Elsewhere, brain and CNS cancer rates have been quite stable or showing minor declines; only among Japanese men was there a significant decrease in rates over time (AAPC: ‒1.2%). In contrast, a marked annual increase was found in many South American countries: in Brazil, Colombia, and Ecuador with mean increases of 3.3%, 1.3%, and 1.1% per annum, respectively. Two countries in eastern Europe (Poland and the Russian Federation) and one in southern Europe (Slovenia) also exhibited increasing average annual rates, of 2.7%, 1.9%, and 2.6%, respectively. In northern Europe, rising rates were also found in Norway (AAPC: 1.4%) and in the Baltic countries of Latvia and Lithuania, with AAPC of 4.0% and 1.7%, respectively. In Asia, the rates increased in Singapore and more rapidly in Thailand and over the last decade (AAPC: 1.4% and 4.4%, respectively).
Fig. 5.
Average annual percentage change (with 95% CIs) based on trends in truncated age-standardized incidence rates of brain and CNS cancers (per 100,000, ages ≥15 y, world standard), 1993–2007 by sex.
Among women, trends over time were similar to those in men. The greatest increases were seen in Thailand with an AAPC of 5.0% annually over the last 10 years, respectively. In Europe, the Baltic countries including Latvia (5.7%) and Lithuania (2.5%) had large increases, followed by Slovenia (1.8%). Similar to men, increasing incidence rates in women were observed in Norway with an AAPC of 0.8%. In South America, rates in Brazil, Colombia and Ecuador increased annually by 3.3%, 1.5% and 1.1% respectively (Table 2. As with men, significantly decreasing trends in women were only seen in Japan (AAPC: -1.4%).
Discussion
This study describes the most recent patterns and trends in the incidence of brain and nervous system cancers in adults worldwide. Globally, a large variability in the magnitude of the diagnosis of new cases of brain and CNS cancer was found, with a 5-fold difference between the highest and lowest rates in the populations represented in this study. By regions, the highest rates were found mainly in European countries, while the lowest rates were seen predominantly in Asian countries. Such differences may be partially related to the different genetic background of these populations. Common gene variants have recently been suggested24 as influencing the risk of glioma, but this needs to be confirmed, while the prevalence of these variants in different world populations is not known.25 Varying trends were observed globally but are sufficiently diffuse in different countries across continents to warrant a cautious approach to interpretation.
Trends in brain cancer incidence are complex to interpret given the vast array of different histological subtypes and potential artefacts linked to changing diagnostic approaches and registry practices.26 The factors driving most of the temporal changes therefore appear to be common and have been likely driven by differences in reporting and completeness of registries in different countries. In addition, the limited number of known causative risk factors and the scarce related information on them at a country level makes interpretation of trends problematic.27 In contrast to the marked geographical differences, temporal changes tended to be similar in both sexes, a gender pattern confirmed in previous studies.17,28–30 Decreasing incidence rates were observed only in Japan.
We observed increasing brain and CNS incidence in most South American countries. In Colombia, one of the countries where the largest increase was observed, a parallel increase in mortality has been reported.31,32 Though no data are available on the introduction and access to diagnostic technology for Colombia and other South American countries, the increasing patterns could be partially related to the improvement in access to diagnostic technology.6 While the radiological accident with caesium-137 in Goiania (Brazil) in 1987 may have impacted on the rate of diagnosis in this region,33,34 it is noteworthy that compared with other world regions, testing of diagnostic radiological equipment prior to clinical use has been shown to be lower in Latin America.35 Given the reported higher risk of death from CNS cancers in Brazil, a Brazilian study has assessed the role of occupational exposures, particularly to pesticides.36 Colombia, Bolivia, and Ecuador have also been reported as being the highest users of pesticides relative to other Latin American countries,37 although their role remains inconclusive.11
We also found increasing incidence rates in most of the former Soviet Union countries. Radiofrequency emissions have been investigated as a risk factor for brain tumors. Although some studies have reported positive associations,38–40 others have not.41–44 A recent study conducted in the Nordic countries (Denmark, Finland, Norway, and Sweden) revealed that although mobile phone use increased dramatically, the incidence of glioma remained almost constant between 1979 and 2008 in all 4 countries.45 Due to the emerging findings on radiofrequency emission, it has been recently classified as a possible carcinogen in humans by the International Agency for Research on Cancer. Yet, the carcinogenic effect of non-ionizing radiofrequency remains unclear.46
Incidence rates decreased significantly over the last decade in both sexes in Japan. A previous study by Kim et al28 suggested that classification issues and exclusion of nonmalignant tumors from registry reporting may have been major contributors to this change over time. In Japan, a decreasing incidence has been reported over the period 1995–2004 in the Osaka Cancer Registry, and for the whole country, a decreasing trend has been observed since 1987.47 Both these studies highlight the importance of comparable inclusion criteria to enable valid comparison between registration systems. CT scan examinations have significantly higher radiation than normal X-rays48 and have been associated with brain cancer.49 The decreasing trends observed in Japan contrast strongly with the available information on the use of CT scan; Japan is one of the major users of CT scans worldwide, and their number has increased significantly over the last years.48 This supports the prospects of possible underlying reporting and classification issues for the explanation of the observed decreasing trends.
Asides from the possibility of genuine differences in risk among different ethnic groups, reported difference in incidence rates by race/ethnicity in the US might be explained in part by access to health care, reflecting social inequalities.50 Wrensch and colleagues (2002)51 suggested that variations in socioeconomic status may in part explain the higher rates among US whites relative to US blacks. Other studies have observed an elevated rate of brain and nervous system cancers among individuals with higher education levels.52 From a global perspective, the recent increase in incidence rates in countries in transition (eg, Brazil, Colombia, Costa Rica, Thailand, Ecuador, the former Eastern bloc countries) may be partly due to economic growth and consequently increased access to health care and improved diagnosis of this cancer.17
We observed that men had on average a 40% higher incidence of brain and CNS cancer than women, which was consistent with data published from Japan47 and the United States.8 It has been suggested that gender differences related to sex hormones and genetic features53 may be causative factors. In addition, adverse working conditions, including a higher exposure to pesticides, chemicals, and biological agents, among men may contribute.54
This is the first contemporary report providing a global analysis examining the geographic and temporal variations in brain and CNS cancer. Although great care has been taken to include only high (CI5) quality registration data, variations in the practices with regard to the reporting of these cancers persist. This renders comparison of temporal trends of brain and CNS cancers between population-based cancer registries particularly challenging. We noted different incidence trends (Supplementary Figure 1) in Sweden, Denmark, and Norway on comparing CI518 with other datasets obtained from the European Cancer Observatory (EUREG19 data that includes only malignant brain and CNS tumors), and NORDCAN,55 includes both malignant and nonmalignant tumors). Nonmalignant tumors were possibly included in Sweden in the CI5 data from 2001, while in Denmark nonmalignant tumors may have been included until 1998; this calls for a careful assessment of international comparisons of secular trends in incidence of brain tumors.
The results presented in this study indicate important regional and country variations in brain and CNS cancers worldwide. They provide important insights into further etiological research and put a focus on the role of environmental risk factors, including radiofrequency fields and occupational exposures, particularly pesticides and chemicals. The observed variations in countries with differing levels of resources could partly be explained by contrasts in health systems infrastructure, access to care, and the availability of diagnostic services. Special attention should be given to addressing quality and availability of incidence data in low and medium income countries. Better documentation of the recording process and the specific inclusion or exclusion criteria of nonmalignant neoplasms is also called for. Finally, comparative studies by histological type and birth cohort analyses could provide further insight into the epidemiology of these complex and life-threatening groups of cancers.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
Adalberto Miranda-Filho was supported by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, Brazil.
Conflict of interest statement. None declared
Supplementary Material
Acknowledgment
The authors gratefully acknowledge all cancer registries and their staff who have contributed by sharing their data through Cancer Incidence in Five Continents, the European Cancer Observatory and NORDCAN, thus enabling this study to take place.
References
- 1.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–2. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Seorjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. Accessed May 30, 2016. [Google Scholar]
- 3. Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol Clifton NJ. 2009;472:323–342. doi:10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 4. Fisher JL, Schwartzbaum JA, Wrensch M, et al. Epidemiology of Brain Tumors. Neurol Clin. 2007;25(4):867–890. [DOI] [PubMed] [Google Scholar]
- 5. Ho VKY, Reijneveld JC, Enting RH, et al. Changing incidence and improved survival of gliomas. Eur J Cancer Oxf Engl 1990. 2014;50(13):2309–2318. [DOI] [PubMed] [Google Scholar]
- 6. Davis D, Hoel D, Percy C, et al. Is Brain Cancer Mortality Increasing in Industrial Countries? Ann N Y Acad Sci. 1990;609(1 Trends in Can):191–204. [DOI] [PubMed] [Google Scholar]
- 7. Grieg NH, Ries LG, Yancik R, et al. Increasing annual incidence of primary malignant brain tumors in the elderly. JNCI J Natl Cancer Inst. 1990;82(20):1621–1624. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman S. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro-Oncol. 2006;8(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wigertz A, Lonn S, Schwartzbaum J, et al. Allergic Conditions and Brain Tumor Risk. Am J Epidemiol. 2007;166(8):941–950. [DOI] [PubMed] [Google Scholar]
- 10. Cahoon EK, Inskip PD, Gridley G, et al. Immune-related conditions and subsequent risk of brain cancer in a cohort of 4.5 million male US veterans. Br J Cancer. 2014;110(7):1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: Consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113(S7):1953–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zigmont V, Garrett A, Peng J, et al. Association between prediagnostic serum 25-hydroxyvitamin D concentration and glioma. Nutr Cancer. 2015;67(7):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18(1):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niederhuber JE, Armitage JO, Doroshow JH, et al. Abeloff’s Clinical Oncology.Fifth edition Philadelphia, Pennsylvania: Elsevier; 2014. [Google Scholar]
- 15. Davis FG, Malmer BS, Aldape K, et al. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2008;17(3):484–489. [DOI] [PubMed] [Google Scholar]
- 16. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncol. 2013;15(suppl 2): ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muir CS, Storm HH, Polednak A. Brain and other nervous system tumours. Cancer Surv. 1994;19–20:369–392. [PubMed] [Google Scholar]
- 18. Ferlay J, Bray F, Steliarova-Foucher E, et al. Cancer Incidence in Five Continents, CI5plus. IARC CancerBase No. 9, Lyon, France: International Agency for Research on Cancer; Available at: http://ci5.iarc.fr 2014. [Google Scholar]
- 19. Steliarova-Foucher E, O’Callaghan M, Ferlay J, Masuyer E, et al. : European Cancer Observatory: Cancer Incidence, Mortality, Prevalence and Survival in Europe. Version 1.0 (September 2012) European Network of Cancer Registries, International Agency for Research on Cancer Available from http://eco.iarc.fr/EUREG/Default.aspx. Accessed May 30, 2016.
- 20. Segi M, Fujisaku S, Kurihara M, et al. The age-adjusted death rates for malignant neoplasms in some selected sites in 23 countries in 1954–1955 and their geographical correlation. Tohoku J Exp Med. 1960;72:91–103. [DOI] [PubMed] [Google Scholar]
- 21. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 22. Joinpoint Regression Program, Version 4.2.0. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute.; 2015. [Google Scholar]
- 23. Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 24. Li J, Chen Q, Liu B, et al. Association between X-ray repair cross-complementing group 1 gene polymorphisms and glioma risk: a systematic review and meta-analysis based on 22 case-control studies. Int J Clin Exp Med. 2015;8(8):11863–11880. [PMC free article] [PubMed] [Google Scholar]
- 25. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro-Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bray F, Engholm G, Hakulinen T, et al. Trends in survival of patients diagnosed with cancers of the brain and nervous system, thyroid, eye, bone, and soft tissues in the Nordic countries 1964–2003 followed up until the end of 2006. Acta Oncol. 2010;49(5):673–693. [DOI] [PubMed] [Google Scholar]
- 27. Arora RS, Alston RD, Eden TOB, et al. Are reported increases in incidence of primary CNS tumours real? An analysis of longitudinal trends in England, 1979–2003. Eur J Cancer. 2010;46(9):1607–1616. [DOI] [PubMed] [Google Scholar]
- 28. Kim SJ, Ioannides SJ, Elwood JM. Trends in incidence of primary brain cancer in New Zealand, 1995 to 2010. Aust N Z J Public Health. 2015;39(2):148–152. [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Tovar M, López-Abente G, Pollán M, et al. Brain cancer incidence in the provinces of Zaragoza and Navarre (Spain): effect of age, period and birth cohort. J Neurol Sci. 1999;164(1):93–99. [DOI] [PubMed] [Google Scholar]
- 30. Yeole BB. Trends in the brain cancer incidence in India. Asian Pac J Cancer Prev APJCP. 2008;9(2):267–270. [PubMed] [Google Scholar]
- 31. Monteiro GTR, Koifman S. Mortalidade por tumores de cérebro no Brasil, 1980–1998. Cad Saúde Pública. 2003;19(4):1139–1151. [DOI] [PubMed] [Google Scholar]
- 32. Piñeros M, Gamboa O, Hernández-Suárez G, et al. Patterns and trends in cancer mortality in Colombia 1984–2008. Cancer Epidemiol. 2013;37(3):233–239. [DOI] [PubMed] [Google Scholar]
- 33. Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publication No. 164. Lyon: International Agency for Research on Cancer; 2014. [Google Scholar]
- 34. Anjos RM, Umisedo NK, Facure A, et al. Goiânia: 12 years after the 137Cs radiological accident. Radiat Prot Dosimetry. 2002;101(1–4):201–204. [DOI] [PubMed] [Google Scholar]
- 35. Martin CJ, Le Heron J, Borrás C, et al. Approaches to aspects of optimisation of protection in diagnostic radiology in six continents. J Radiol Prot Off J Soc Radiol Prot. 2013;33(4):711–734. [DOI] [PubMed] [Google Scholar]
- 36. Miranda-Filho AL, Monteiro GTR, Meyer A. Brain cancer mortality among farm workers of the State of Rio de Janeiro, Brazil: a population-based case-control study, 1996–2005. Int J Hyg Environ Health. 2012;215(5):496–501. doi:10.1016/j.ijheh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 37. Wesseling C, Aragón A, Castillo L, et al. Hazardous pesticides in Central America. Int J Occup Environ Health. 2001;7(4):287–294. [DOI] [PubMed] [Google Scholar]
- 38. Coureau G, Bouvier G, Lebailly P, et al. Mobile phone use and brain tumours in the CERENAT case-control study. Occup Environ Med. 2014;71(7):514–522. [DOI] [PubMed] [Google Scholar]
- 39. Hardell L. Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol. September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hardell L, Carlberg M. Increasing rates of brain tumours in the Swedish national inpatient register and the causes of death register. Int J Environ Res Public Health. 2015;12(4):3793–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pettersson D, Mathiesen T, Prochazka M, et al. Long-term mobile phone use and acoustic neuroma risk. Epidemiology. 2014;25(2):233–241. [DOI] [PubMed] [Google Scholar]
- 42. Deltour I, Johansen C, Auvinen A, et al. Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. JNCI J Natl Cancer Inst. 2009;101(24):1721–1724. [DOI] [PubMed] [Google Scholar]
- 43. INTERPHONE Study Group. Acoustic neuroma risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Cancer Epidemiol. 2011;35(5):453–464. [DOI] [PubMed] [Google Scholar]
- 44. INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol. 2010;39(3):675–694. [DOI] [PubMed] [Google Scholar]
- 45. Deltour I, Auvinen A, Feychting M, et al. Mobile phone use and incidence of glioma in the Nordic countries 1979–2008: consistency check. Epidemiol Camb Mass. 2012;23(2):301–307. [DOI] [PubMed] [Google Scholar]
- 46. Baan R, Grosse Y, Lauby-Secretan B, et al. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12(7):624–626. [DOI] [PubMed] [Google Scholar]
- 47. Nomura E, Ioka A, Tsukuma H. Trends in the incidence of primary intracranial tumors in Osaka, Japan. Jpn J Clin Oncol. 2011;41(2):291–294. [DOI] [PubMed] [Google Scholar]
- 48. Ghotbi N, Morishita M, Ohtsuru A, et al. Evidence-based guidelines needed on the use of CT scanning in Japan. Jpn Med Assoc J. 2005;48(9):451. [Google Scholar]
- 49. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346(may21 1):f2360–f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curry WT, Barker FG. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(1):25–39. [DOI] [PubMed] [Google Scholar]
- 51. Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncol. 2002;4(4):278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49(21):6137–6143. [PubMed] [Google Scholar]
- 53. McKinley BP, Michalek AM, Fenstermaker RA, et al. The impact of age and sex on the incidence of glial tumors in New York state from 1976 to 1995. J Neurosurg. 2000;93(6):932–939. [DOI] [PubMed] [Google Scholar]
- 54. Edgren G, Liang L, Adami H-O, et al. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol. 2012;27(3):187–196. [DOI] [PubMed] [Google Scholar]
- 55. Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.1 (09.07.2015). Association of the Nordic Cancer Registries Danish Cancer Society. Available from http://www-dep.iarc.fr/nordcan.htm. Accessed May 30, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.