Abstract
Sleep-wake disturbances are defined as perceived or actual alterations in sleep that result in impaired daytime functioning. Unlike other cancers, there is limited information about sleep-wake disturbances in adults with primary brain tumors throughout the illness trajectory. Sleep-wake disturbance is among the most severe and common symptoms reported by primary brain-tumor patients, particularly those undergoing radiation therapy. As with other cancers and neurologic illness, sleep-wake disturbance may also be clustered or related to other symptoms such as fatigue, depression, and cognitive impairment. There is increasing evidence for a genetic basis of normal sleep and sleep regulation in healthy adults. Specific mutations and single nucleotide variants have been reported to be associated with both fatigue and sleep-wake disorders, and both inflammation and alterations in circadian rhythms have been postulated to have a potential role. Guidelines for assessment and interventions have been developed, with cognitive behavioral therapy, exercise, and sleep hygiene demonstrating benefit in patients with other solid tumors. Further research is needed to identify risk and appropriate treatment in the brain-tumor patient population.
Keywords: brain tumor, hypersomnia, insomnia, radiation therapy, sleep-wake, symptoms
The illness trajectory in patients with primary brain tumors can be unpredictable, yet symptoms related to the tumor and its treatment are often present and persistent. Symptoms tend not to occur in isolation and often form clusters or complexes that can influence the occurrence and severity of symptoms. Two commonly co-occurring symptoms in solid tumor and primary brain-tumor patients are fatigue and sleep-wake disturbance.1–3 Although cancer-related fatigue and cancer-related sleep-wake disturbances are distinct concepts, the majority of studies in patients with cancer support a strong relationship between these 2 symptoms. The occurrence of both fatigue and sleep-wake disturbances influence the perceptions of the intensity and persistence of each symptom and impact the occurrence of other disease and treatment-related symptoms.4–8
Sleep-wake disturbances are defined as perceived or actual alterations in sleep that result in impaired daytime function.9 Sleep-wake disturbances encompass 2 distinct periods: the sleep period (referred to as sleep-wake) and the wake period (referred to as activity-rest). Patients with sleep-wake disturbances may complain of difficulty initiating or maintaining sleep, waking too early, waking up feeling unrefreshed as well as reporting fatigue, hypersomnia, somnolence, or excessive daytime sleepiness during the wake period. In the literature, these terms defining daytime sleepiness are often used interchangeably, leading to a lack of clarity. For the purpose of this review, we will use the term “hypersomnia” as defined by the American Academy of Sleep Medicine, to be constant or recurrent episodes of extreme sleepiness during the activity-rest period.9 These sleep-wake disturbances, which include both alterations in sleep (insomnia) and daytime hypersomnia, can occur in healthy and ill people and may recur or persist throughout the trajectory of an illness.10–13 Situational stress, aging, drug treatment, and illness are often associated with the report of sleep disturbances.14 As sleep occupies one- third of our life, sleep-wake disturbances that persist can impact daily living and be associated with serious health consequences.15
Unlike other cancers, there is limited information concerning sleep-wake disturbances in adults with primary brain tumors throughout the illness trajectory. A review article focused on management of both fatigue and sleep describes the omission of patient with primary brain tumors in intervention studies designed to reduce sleep-wake disturbance in other solid tumor patients.16 Despite the significance of the symptom in this patient population, this lack of inclusion may result from the unique differences and relative rarity of brain tumors. The purpose of this paper is to synthesize the knowledge of sleep-wake disturbances, with a focus on the prevalence and mechanisms of insomnia and hypersomnia in adult patients with primary intracranial tumors and to apply what is known from other solid tumors about screening, assessment, interventions, and implications to advance both research and practice. A comprehensive search of the literature related to sleep-wake disturbance and primary brain tumors was completed by authors TA and MS using PUBMED between the years 2000 and 2016. Authors AB and MS explored articles for sleep assessment and interventions in cancer. The key words searched were brain tumor, glioma, lesion, sleep disturbance, sleep-wake disturbance, intervention, adult, adult patients, and survivors.
Prevalence
Sleep-wake disturbances are common and are estimated to occur in 10%–20% of the general population.17,18 In patients with chronic illness, sleep-wake disturbances are more common and have been reported to occur in the majority of patients with illnesses such as HIV,19 neurologic illnesses,20,21 and cancer.13 Sleep-wake disturbances are reported to occur in > 70% of patients post stroke,22,23 in 80% of those with multiple sclerosis, 20,24 and in more than 40% of patients after mild traumatic brain injury.25 Insomnia is the most common sleep-wake disorder experienced by patients with neurologic illness.9 Characteristically, patients with insomnia not only report difficulty falling and staying asleep but also excessive daytime sleepiness (hypersomnia); these complaints are often associated with reports of depression, fatigue, anxiety, and pain.21,24,26,27
Insomnia has often been reported to be the most common sleep disturbance in cancer patients; however, a recent review determined that the prevalence of specific sleep-wake disturbances in cancer cannot be established from published literature.28,29 Studies that explored the prevalence of sleep-wake disturbances in cancer patients have focused on patients with breast and lung cancers. Women undergoing chemotherapy for breast cancer have had an estimated 80% prevalence of insomnia symptoms.30 Sleep-wake disturbances in patients with lung cancer have been estimated to be as high as 52%.31 Sleep-wake disturbances can occur throughout the illness trajectory in cancer patients, with 26% of cancer patients reporting poor sleep prior to treatment initiation.32 In cancer patients undergoing chemotherapy, prevalence estimates have been reported as 30%–50% of patients experiencing insomnia symptoms.8,29,30,33 Sleep-wake disturbances can also continue after therapy has been completed, with up to 65% of breast cancer survivors reporting sleep-wake disturbances and poor sleep quality as compared with women without breast cancer.28
The majority of evidence on the occurrence and risk of sleep-wake disturbances has been published in patients with other solid tumor malignancies. Table 1 provides an outline of the limited studies completed to date on the adult brain-tumor patient population. These reports indicate that sleep-wake disturbance is one of the 5 most common symptoms reported as moderate to severe by primary brain-tumor patients26,34 and occurs in anywhere between 17% and 54% of patients.35,36 Disturbed sleep has been reported to be significant across tumor grades and throughout the disease trajectory.37 A recent paper reported that disturbed nighttime sleep and hypersomnia were among the most severe symptoms noted by a large cross-sectional cohort of primary brain-tumor patients, were worse in those with poor performance status,26 and were associated with disease progression.27 As with other cancers and neurologic illness, sleep disturbance (both insomnia and hypersomnia) may also be clustered or related to other symptoms such as fatigue, depression, and cognitive impairment in the primary brain-tumor patient population.36,38,39
Table 1.
Studies exploring sleep-wake disturbance in the primary brain tumor patient population
| Author(s) | Purpose | Sample | Instruments | Results |
|---|---|---|---|---|
| Armstrong et al, 201526 | Descriptive cross-sectional study: Explore the CMTP 12 core symptoms in adult oncology studies | N = 621 adult primary brain-tumor patients | MDASI-BT KPS |
Insomnia and feeling drowsy (hypersomnia) were 2 of the most severe and frequent symptoms and were consistently reported. Symptoms were not different based on treatment status, tumor grade, or if the patient had recurrence but were worse in patients with poor KPS (P < .05). |
| Armstrong and Gilbert, 201216 | Review: Fatigue and sleep in primary brain tumor patients | None; review article | None | Primary brain tumor patients were not routinely included in studies evaluating management of sleep disturbance and fatigue. |
| Armstrong et al., 201134 | Descriptive study: Symptom prediction of tumor progression or recurrence | N = 294 primary brain tumor patients | MDASI-BT | Sleepiness was one of the 5 most common symptoms reported as moderate to severe and was not a predictor of tumor progression. |
| Armstrong et al., 201038 | Descriptive study: Prediction of fatigue severity and association with other symptoms | N = 201 patients with primary brain tumors in various stages | MDASI-BT | Fatigue was the most prevalent symptom, and severity was associated with difficulty sleeping, along with overall symptom severity and interference. |
| Brown et al., 200640 | Descriptive study: Prognostic importance of baseline QOL | N = 194 adult patients newly diagnosed with glioblastomas | LASA FACT-Br, Fatigue SDS, ESS, POMS-SF |
One-third of patients had clinically significant fatigue at baseline. Increased fatigue (P = .003), EDS (P = .01), and lower overall QOL scores (LASA, P = .001; FACT-Br, P = .0001) correlated with worse performance scores. |
| Cheng et al, 201037 | Descriptive study: Baseline QOL prior to surgery in Chinese patients | N = 92 adult patients with histologically confirmed glioma | EORTC QLQC30 BN20 MMSE KPS |
Fatigue (82%) and insomnia (39%) were 2 of the most frequently reported symptoms. Insomnia was worse in patients >50 years of age (P = .047) and with poor performance status (P = .01) |
| Fox et al., 200739 | Descriptive study: Co-occurring symptoms in high-grade glioma patients | N = 73 adult survivors of brain malignancy | BFI GSDS FACT-G |
Depression, fatigue, sleep disturbance, and cognitive impairment were significantly intercorrelated and correlated with functional status. Fatigue and sleep disturbance had minimal contributions to QOL, not a cause for distress, and explained 1% of the variance in functional status. |
| Gapstur et al., 200957 | Review: Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors |
N = 25 articles Categories: (1) Mixed samples of adult and pediatric survivors of pediatric brain tumors, (2) Pediatric survivors of brain tumors, or 3) Adult survivors of brain tumors. |
None | Patients with brain tumors experience HPA dysfunction, affecting both Process S (homeostasis) and Process C (circadian) of the Two-Process Model of Sleep Regulation. Factors associated with sleep issues were hypothalamic damage resulting in EDS, melatonin and hypocretin defects that contribute to poor arousal mechanisms, and changes in circadian rhythm related to damage to the SCN. |
| Gustafson et al., 200635 | Descriptive study: QOL and coping with illness-related problems | N = 39 adult patients diagnosed with low-grade gliomas. | EORTC-QLQ-C30 | Median values were higher for symptoms of fatigue, sleep disturbance and pain, and 21 patients (54%) reported sleep disturbances. There was a significant relationship between overall quality of life and fatigue (rho: −0.70, P < .001) |
| Mulrooney et al., 200836 | Retrospective study: Prevalence and risk factors for fatigue and sleep disturbance | N = 2645 adult survivors of mixed cancer types (including CNS) tumor with and without radiation | FACIT-Fatigue PSQI ESS |
16% had a CNS malignancy. Overall, 19% of survivors were most fatigued, 17% reported disrupted sleep, and 14% reported increased daytime sleepiness. Females were more likely to be fatigued. |
| Powell et al., 201141 | Descriptive study: Incidence pattern and severity of radiation somnolence | N = 70 adult patients receiving radical cranial radiation assessed at baseline, 10 weeks post therapy | VAS to measure somnolence FACT-G EORTC QLQ30 + 3 |
90% of patients experienced > grade 1 somnolence, correlated with VAS scores (r = 0.456, P < .001). The score increased from 3 to 12 weeks (P < .001) with peak at end of treatment and improvement 6 weeks later. |
| Van Someren et al., 200464 | Descriptive study: Childhood CRT relationship to altered sleep in adulthood |
N = 25 patients 8–29 years after CRT for medulloblastoma or other intracranial tumors N = 34 controls |
Endocrine levels Sleep and circadian rhythms using wrist actigraphy PSQI, Circadian Type inventory, flexibility scale of the Circadian Type inventory, The Composite morningness Questionnaire. |
CRT group had longer sleep duration than the control group. Sleep-wake rhythms showed greater amplitude, less fragmentation, and less tolerance for alterations in the timing of sleep. Regression showed both radiation dosage and neuroendocrine status to be predictors of sleep changes. |
| Yavas et al, 201227 | Descriptive study: Quality of life, cognitive and emotional distress in high-grade glioma patients | N = 118 patients with high-grade glioma followed longitudinally for the first 5 years | EORTC-C30 BN-20 MMSE HADS |
Global score, physical, role and emotional function, insomnia (all P < .001) and appetite loss (P = .008) were associated with disease progression. Symptom domain scores (including insomnia) increased over the disease trajectory. |
Abbreviations: BFI, Brief Fatigue Inventory; BN20, Brain Neoplasm; CMTP, Center for Medical Technology Policy; CNS, Central Nervous System; CRT, Cranial Radiation Therapy; EDS, Excessive Daytime Sleepiness; EORTC QLQC30, The European Organisation for Research and Treatment of Cancer Core Questionnaire; ESS, Epworth Sleepiness Scale; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy: Fatigue; FACT-Br, Functional Assessment of Cancer Therapy-Brain; Fatigue; FACT-G, Functional Assessment of Cancer Therapy: General; GSDS, General Symptom Distress Scale; HADS, Hospital Anxiety Depression Scale; KPS, Karnofsky Performance Status; LASA, Linear Analogue Scale Assessment; MDASI-BT, MD Anderson Symptom Inventory- Brain Tumor; MMSE, Mini-Mental State Examination; POMS-SF, Profile of Mood States Short Form; PSQI, Pittsburgh Sleep Quality Index; QOL, Quality of Life; SCN, Suprachiasmatic Nucleus; SDS, Symptom Distress Scale; VAS, Visual Analog Scale.
Insomnia is the most common sleep-wake disorder in patients with primary brain tumors, but additional sleep-wake disorders, including sleep-related breathing (eg, obstructive sleep apnea) and movement disorders (eg, restless legs syndrome), also may occur.16 Poor sleep can be particularly bothersome, especially when patients with brain tumors also report hypersomnia.36,40 Hypersomnia was reported in more than 90% of primary brain-tumor patients undergoing cranial radiation therapy.41 In another study, primary brain-tumor patients who reported hypersomnia also reported lower quality of life and had worse performance status and shorter survival.40
Other clinical and environmental factors may contribute to sleep-wake disturbances in the brain-tumor population. Clinicians frequently interrupt hospitalized patients who are sleeping and may alter their sleep-wake and activity-rest cycle.42,43 Additionally, side effects of cancer therapy and concomitant medications such as corticosteroids and anticonvulsants can alter sleep.16,44 Corticosteroids are commonly prescribed and have a well-documented impact on sleep, with a recent report indicating that more than one-third of patients experienced altered sleep and increased effect based on dose and time on therapy.45 Patients may experience mood disturbances and altered daily routines as a result of the tumor itself.46 Cognitive or neurological effects of the tumor or toxicities related to treatment may result in altered mood or the development of maladaptive behaviors such as excessive time in bed and reduced physical activity, which may also impact sleep.47
In summary, sleep-wake disturbance has been commonly reported in other solid-tumor malignancies and neurologic illnesses, but there is a paucity of studies in adult brain-tumor patients. These data would provide insights into the frequency and severity of these symptoms and their impact on patient outcomes as well as provide the foundation for future investigations of pathogenesis and treatment interventions.
Mechanisms
Genetic Predisposition
There is increasing evidence for a genetic basis of normal sleep and sleep regulation in healthy adults. Specific mutations and single nucleotide variants have been reported to be associated with both fatigue and sleep-wake disorders.15,48–50 Identification of candidate variants may help classify those who may be at higher risk and may help improve understanding of the mechanism(s) of sleep-wake disturbance. Polymorphisms in genes associated with regulation of the circadian system and those associated with inflammation have been proposed. However, sleep-wake is a complex process and involves the interaction of products of many genes as well as the influence of zeitgerber and other environmental influences.51 Furthermore, these environmental influences on sleep-wake may differ by cancer type and treatment received. The influence of gene-environment interactions on sleep outcomes has not been extensively investigated.
In breast cancer patients, variations in 3 cytokine genes (interleukin 1 receptor 2 [IL1R2], IL13, and NFKB2) predicted the development of more severe sleep-wake disturbances.52 Another polymorphism in IL4 has been reported in breast cancer patients to correlate with the cluster of pain, fatigue, sleep-wake disturbance, and depression.53 Similar findings were shown in patients with HIV, in which polymorphisms in IL1R2 and RNfA were associated with short sleep duration.54 In neurologic disease, mild traumatic brain injury and primary sleep disorders such as insomnia, sleep apnea, and RLS among others, clock genes including PER1, PER2, PER3, CYR1, and CRY2 polymorphisms have been associated with sleep onset, duration, and quality of recovery from sleep deficits.15,25,55 This relationship between inflammatory and clock genes/pathways and symptoms continues to be an area of active investigation in patients with other solid tumors and neurologic injury.
Mechanisms in Brain-tumor Patients
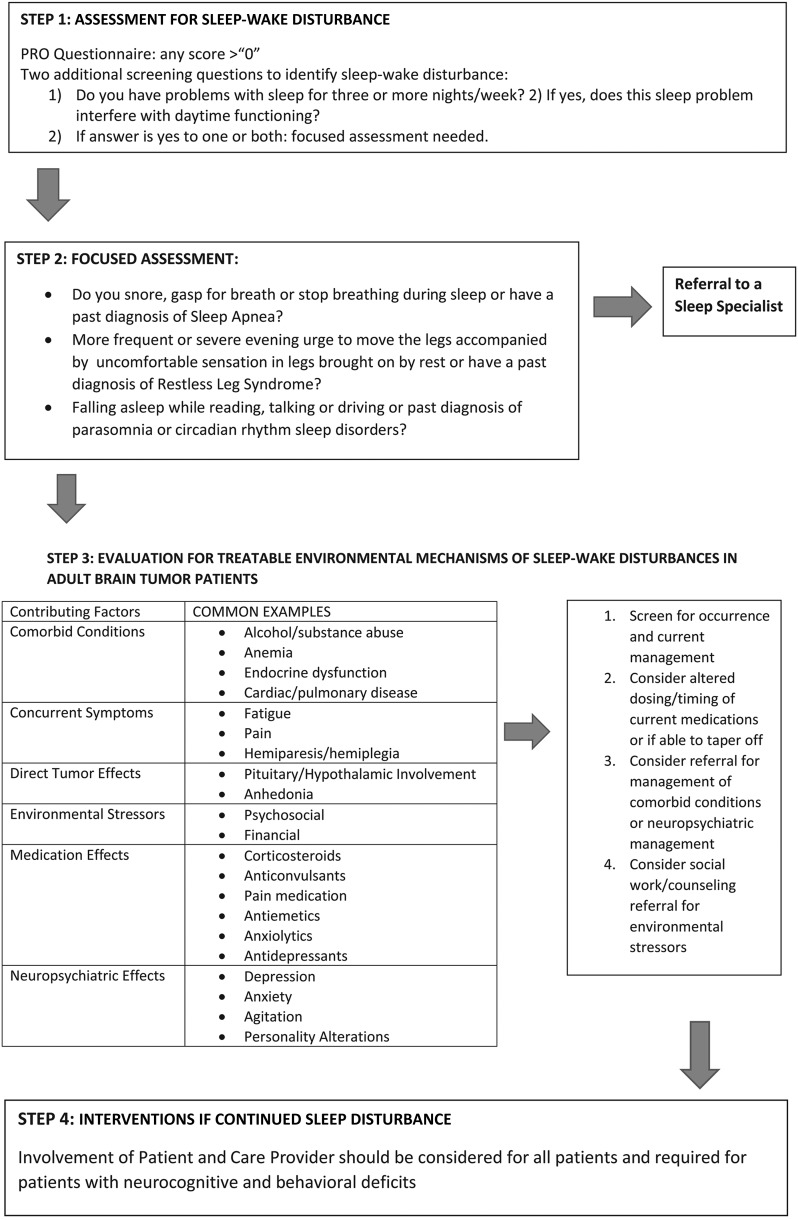
Environmental mechanisms in the brain-tumor patient population can be broadly classified into the following groups: direct effect by the tumor, effects of medications, effects of radiation therapy, and neuropsychiatric effects such as depression and anxiety. Fig. 1 includes a table of predisposing factors. In any individual patient, multiple etiologic factors may contribute to the type, occurrence, severity, and associated impact.
Fig. 1.
Assessment and management of sleep-wake disturbance in the primary brain tumor patient population.
Cranial radiation remains the most common and significant factor reported to be associated with hypersomnia and sleep disturbances in primary brain-tumor patients. Faithfull and Brandas56,57 described a somnolence syndrome in malignant glioma patients that occurred during and immediately following cranial radiation therapy. Symptoms included fatigue, excessive drowsiness, incoordination, and inability to concentrate that occurred in a cyclical pattern. Gapstur et al.58 identified dysfunction in the hypothalamic-pituitary adrenal (HPA) axis and defects in melatonin and hypocretin secretion that contributed to excessive daytime sleepiness and damage to the suprachiasmatic nuclei affecting arousal and circadian rhythms in patients with brain tumors.
Sickness models propose that increased secretion of IL-1 and IL-6 may lead to stimulation of the HPA, causing symptoms including sleep-wake disturbance, fatigue, and depression. In the case of brain radiation, inflammation may be the underlying mechanism leading to fatigue and the cascade of additional symptoms either directly or based on its disruption of underlying circadian rhythms.59 In a recent report exploring the impact of radiation in an animal model, whole brain irradiation increased non-REM sleep as well as levels of IL-1B protein expression in the hypothalamus.59 In a pilot study exploring sleep using actigraphy and self-report of sleep as well as evaluation of serum melatonin, it was hypothesized that this neuroinflammation may result in aberrant production of melatonin as an immunomodulating response, with secondary effects including excessive daytime sleepiness (hypersomnia) and worsening of inflammation-related fatigue.16 Altered melatonin secretion has also been reported to be associated with excessive daytime sleepiness in adult survivors who had been treated with cranial radiation as children60 and postulated to occur in adult glioma survivors.61 Animal models exploring the impact of cranial radiation on melatonin have demonstrated a 2-phase reaction, with immediate reduction in melatonin synthesis followed quickly by an increase in melatonin synthesis and circulating melatonin.62 This increase in melatonin secretion has also been reported in other inflammatory disease states known to affect the brain.63,64 Patients may experience sleep disturbance from damage of the hypothalamus, defects in melatonin and hypocretin, or injury to the suprachiasmatic nucleus resulting in poor arousal, hypersomnia, and disruption in circadian activity.58 In fact, Muller et al.60 found an inverse relationship between melatonin secretion and excessive daytime sleepiness (hypersomnia) in patients with craniopharyngioma. Those who receive cranial radiation may demonstrate longer sleep duration, circadian rhythms with greater amplitude, less fragmentation, and poorer tolerance for alterations in the timing of sleep.65 Adult survivors of childhood brain tumors were at increased risk of having significant problems with sleep as adults if they received a radiation dose >3500 cGy, were younger at the time of treatment, and had a diagnosis of craniopharyngioma.58
Assessment
Guidelines
Information regarding screening and/or assessment and management of sleep-wake disturbances in patients with cancer is available from 3 sources: the Pan-Canadian Practice Guideline (which also includes guidelines for assessment and management),66,67 the National Comprehensive Cancer Network (NCCN) guidelines for sleep-wake disturbances,68 and the Oncology Nursing Society Putting Evidence into Practice (PEP) resources on sleep-wake disturbances focusing on evidence-based interventions.69
Assessment Tools
No tool with established reliability and validity in adults with cancer is used routinely to screen for sleep-wake disturbances comorbid with cancer in primary care, oncology, or neuro-oncology clinics. The Pan-Canadian practice guideline suggests asking patients with cancer about the presence of sleep problems and then asking about the relationship between the problem and daily functioning.66 Questionnaires developed specifically for the brain-tumor patient population include the Functional Assessment of Cancer Therapy-Brain (FACT-BR), EORTC QLQ-C30, and BN20, and the MD Anderson Symptom Inventory-Brain Tumor (MDASI-BT), all of which include questions related to the severity of sleep and hypersomnia and use a Likert scale in which zero represents “not present.”70–72 The benefit of these instruments is that they ask about other symptoms that may co-occur or impact sleep.
In summary, screening and assessment for sleep-wake disturbances in brain-tumor patients is not conducted routinely and, when performed, a variety of measurements are available for use.
In the clinical setting, Fig. 1 includes assessment guidelines to screen for occurrence.66 Further clinical evaluation is warranted if a positive response is given. For patients with sleep-wake disturbance that is not responsive to intervention or if sleep disturbances other than insomnia or hypersomnia do not resolve, referral for further evaluation by a sleep specialist should be considered. Polysomnography, which is the gold standard for assessing and diagnosing sleep disorders such as sleep apnea and restless legs syndrome, may be performed.66
More detailed assessment may be needed in the research setting, and the choice of instrument is dependent on the type of sleep-wake disturbance of interest. Research studies focusing on cancer patients use the Epworth Sleepiness Scale frequently when patients report excessive daytime sleepiness/hypersomnia73,74 in addition to the multi-item Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Scale.75,76 Both scales were developed to assess insomnia that occurs when chronic conditions, such as cancer, are not present. Both have documented reliability and validity figures in patients with cancer, but not for brain-tumor patients. An advantage of the PSQI is that it screens for sleep apnea, restless legs syndrome, and other sleep-wake disorders in addition to insomnia. When sleep apnea is suspected, the STOP-BANG77 and Epworth Sleepiness Scale (ESS) are used.73 The Patient-Reported Outcomes Measurement Information System (PROMIS) for Sleep Disturbance has also begun to be used in cancer patients and includes a sleep-thermometer tool for use in self-report.78 Finally, actigraphy or polysomnography may complement subjective evaluation of sleep in the research setting, although cost and patient compliance sometimes ipact their feasibility in clinical research studies.
Interventions
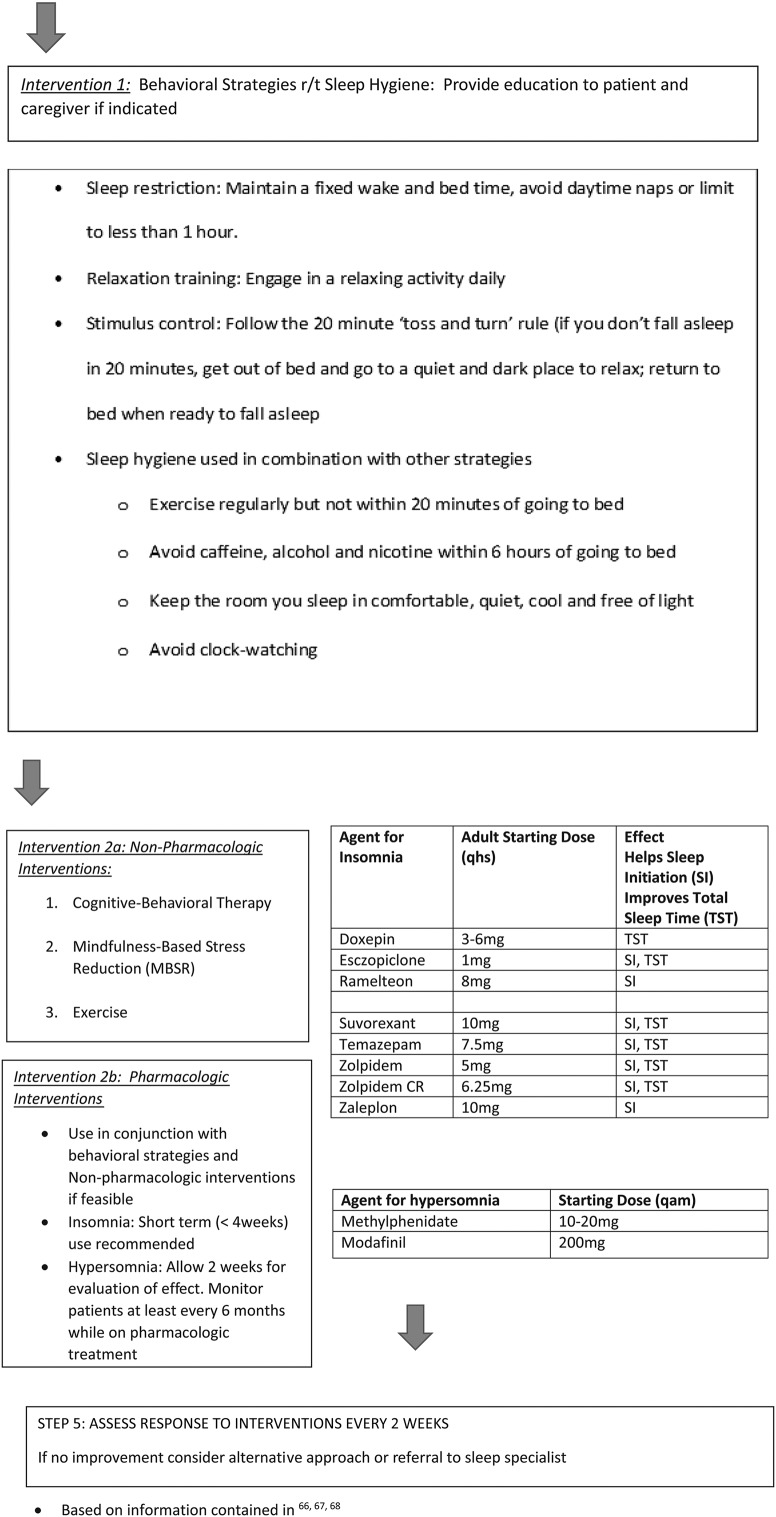
Management of altered sleep-wake is complex and includes assessment and interventions geared to promoting improvement in the fundamental causes of the sleep-wake disturbance. Interventions to improve sleep-wake disturbances in cancer patients have been primarily implemented in women with breast cancer and have not been tested in patients with brain tumors. Evidence related to specific interventions in the cancer population has been synthesized and is available at the Putting Evidence into Practice (PEP) online resources (https://www.ons.org/practice-resources/pep)79 and resultant review articles.13,80,81 These guidelines and the Pan-Canadian guidelines recommend prevention and supportive education about sleep for all patients.66 Although evaluation of interventions in the brain-tumor patient population is lacking, the approaches outlined in these guidelines are recommended in routine clinical care. Fig. 1 outlines specific considerations for brain-tumor patients with complaints of sleep-wake disturbances. Assessment for and modification of contributing factors and reinforcing sleep hygiene and sleep promotion activities (see Fig. 1 for behavioral strategies for sleep-wake disturbance82) are important first steps for all patients. These preventative and educational strategies can be used in conjunction with nonpharmacological or pharmacological interventions, depending on the suspected underlying etiology and considering individual patient needs and deficits. In general, pharmacological interventions should be initiated for a short duration and monitored due to unwanted adverse drug events. All interventions need follow-up and ongoing re-evaluation. The National Comprehensive Cancer Network (NCCN) survivorship guidelines provide a more detailed summary of the evidence supporting these interventions and complement the ONS recommendations and available guide for sleep management (http://www.nccn.org/professionals/physician_gls/pdf/survivorship).
Nonpharmacological Intervention
The initial step in treatment is based on assessment and diagnosis of the sleep disorder, as described above. Other sleep-wake disorders, such as central or obstructive sleep apnea and restless legs syndrome, need to be ruled out. Insomnia is thought to be a disorder of hyperarousal; other factors such as neurophysiological and neuroendocrine dysregulation, anxiety, cancer treatments, or altered circadian rhythmicity have also been reported as etiologic factors of insomnia in patients with cancer.8,58,83,84 Sleep hygiene should be assessed in any patient with suspected sleep-wake disturbances, and adaptive strategies should be considered prior to initiation of any additional interventions (see Fig. 1). Specific interventions have been found to be helpful in patients with other solid tumor malignancies and are described in detail below.
Cognitive Behavioral Therapy Insomnia (CBT-I), mindfulness-based stress reduction (MBSR), and exercise are the interventions with the most evidence for improving sleep and reducing other associated symptoms in patients with other solid tumors. CBT-I is a type of psychotherapy that has been used to help with a variety of behavioral changes. The main aim of CBT-I is to explore and gain understanding about how a person thinks and feels and the relationship with the resulting behavior, so that new strategies for healthy thinking, coping, and behaviors can be explored. For sleep disturbance, CBT-I strategies include sleep restriction, stimulus control, and sleep hygiene modification.
CBT-I has been shown to improve sleep-wake disturbances in patients with cancer (most extensively in women with breast cancer) with meta-analysis reporting significant improvements in sleep for patients with cancer who participate in CBT-I.80,85 CBT-I has been successful when delivered by a nurse with specialized training86 in group sessions.87,88 For those unable to participate in face-to-face sessions, self-administered CBT-I using video, MP-3 player, booklets, or phone consultation are other options.89,90,91 Compared with other nonpharmacological interventions, CBT-I has been considered the best strategy for treating insomnia.80 In patients with primary brain tumors and insomnia related to anxiety, CBT-I could prove to be an effective intervention for sleep-wake disturbances related to anxiety or cancer-related treatment. Sleep restriction during the day may be a component of CBT-I and are especially helpful in brain-tumor patients inclined to nap. In these patients who have cognitive deficits, involvement of a caregiver to utilize and inforce these strategies may be necessary. It is hypothesized that after CBT-I, patients may experience a change in neural network dysregulation and other biological mechanisms that could lead to a change in sleep behavior.92
Mindfulness based stress reduction (MBSR) is “a consciousness discipline that is grounded in Eastern philosophy and traditions” such as yoga and Buddhism that focuses on awareness of the present moment. It aims to teach people to deal more effectively with an experience through awareness of feelings, thoughts, and bodily sensations, using practices such as body scan and exercises for yoga and meditation. Meta-analyses have shown that MBSR interventions may be a novel approach for managing sleep-wake disturbances in patients with cancer.93 Formalized MSBR sessions have shown promising results in patients with breast cancer,94,95 but home-based MBSR may be more convenient and beneficial for sleep-wake disturbances.96 Self-reported sleep improved more in those who participated in MBSR when compared with sleep hygiene.97 In patients with brain tumors, MBSR could help with sleep-wake disturbance related to anxiety and, along with its yoga component, help regulate circadian rhythms. Evaluation of physical and cognitive deficits that would either preclude participation (eg, receptive aphasia) or require modifications (eg, hemiparesis) should be considered and communicated to the parties involved including patient, caregiver, and provider.
Exercise is defined as physical exertion imposed on the body with the aim of improving or maintaining physical and mental fitness level or health. A variety of activities can be performed as exercise, but the most important elements are frequency, intensity, time, and type.98 Exercise interventions have been shown to reduce sleep-wake disturbances in cancer patients undergoing active treatment and in survivors,99,100 including high doses of 50–60 minutes of exercise for women with breast cancer101,102 as well as home-based walking and strengthening activities103–105 or a combination of home-based and supervised or video exercise.106,107 Exercise frequency, intensity, time, and type may be modified based on the health of the patient with cancer. Aerobic exercise has been reported to improve mental health and promote structural changes in the brain. Exercise may help regulate disruption in the circadian rhythm in addition to building vasculature and promoting neurogenesis in patients with primary brain tumors who have suffered damage to these functions.108 Studies have shown feasibility of exercise and a relationship between exercise and survival and physical functioning in brain-tumor patients with KPS > 70%,109 but studies exploring the impact on sleep have not been reported.
The interventions noted above have not been fully evaluated in the brain-tumor patient population, but evidence supporting their utility for other solid tumor patients warrants consideration for their use in the clinical setting and further studies evaluating their utility for brain-tumor patients. Other nonpharmacological interventions (eg, light-therapy) need further study in order to determine their effectiveness on sleep-wake disturbances in patients with cancer.
Pharmacological Interventions
Pharmacological approaches may be important short-term adjuncts for the brain-tumor patient with sleep-wake disturbance because nonpharmacological interventions may take time and effort to implement or not be feasible in selected patients. CBT-I may also be used alone or in combination with low-risk pharmacological agents.110,111 For example, in breast cancer survivors, CBT-I and armodafinil reduced the severity of insomnia.112 Other agents may also be helpful, but studies exploring risks and benefits in the cancer patient population have not been completed.
The decision to use pharmacological agents must be made carefully based on patient assessment including history of sleep-wake disturbance, concomitant medications, and comorbid conditions. For instance, if insomnia is medication-induced (eg, corticosteroids), lowering the dose or altering the time taken during the day may improve sleep. If there are existing comorbid conditions or symptom clusters such as depression, anxiety, or seizures, one medication may be given to improve insomnia along with the other factors. In this situation, there are antidepressant, anxiolytic, or barbiturate medications that can be prescribed to be taken at bedtime for their sedative effects.113 Other medications that may be helpful for patients with sleep disturbances and other concurrent illnesses or symptoms include benzodiazepine hypnotics, benzodiazepine-receptor agonists, antidepressants, psychostimulants, and melatonin.114 Drug-drug interactions should be considered and patients monitored for treatment efficacy and side effects. Medications need to be initiated at a low dose, administered for short-term duration, and tapered slowly to prevent withdrawal symptoms.16 Randomized controlled trials with pharmacological interventions have not been conducted in the primary brain-tumor patient population.
Implications for Practice and Research
Clinicians need to recognize potential barriers to successful intervention implementation in patients with primary brain tumors. Sleep-wake disturbances such as insomnia and hypersomnia do not often occur in isolation but rather may be associated with disease and treatment-related symptoms. Adult brain-tumor patients may experience difficulty with patient activation, decision-making, or self-efficacy. This may be due to cognitive decline or mood disturbances.115 The long-term effects of treatment could result in problems with ambulation or balance, aphasia, vision and hearing deficits, or hemiparesis, thus making exercise or MBSR a challenge.116 Sleep-wake disturbances may be associated with irritability and inability to concentrate, impaired compliance, and difficulty making decisions. This impact may be even more critical in the primary brain-tumor population, a cohort already at risk for neurocognitive difficulties. To overcome these barriers, interventions may need to be modified or individualized for specific sleep-wake and activity-rest patterns in the brain-tumor patient population.
In addition to the physiological barriers there are psychological and system barriers. Acceptability, attitudes, and beliefs about sleep-wake disturbances and hypersomnia, perceived benefits, and perceived efficiency of the intervention may all influence the implementation of an intervention. There also are cost-related factors such as reimbursement for CBT-I, MBSR, and gym memberships, and provider-related barriers such as the lack of clinician or personal trainer knowledge on the ideologies of intervention implementation.117,118
There is a growing body of evidence that links quality of sleep with health and overall wellness. Recent reports in patients with systemic cancer are beginning to uncover important phenotypes associated with the risk of sleep-wake disturbances and improved guidelines on assessment within the clinic. In addition, there is evidence of benefit for CBT-I in patients with solid tumors. Research is limited, however, in the primary brain-tumor population. More extensive studies are needed to evaluate the influence of clinical and environmental factors and the association with candidate genes that may allow assignment of risk phenotypes. In addition, further exploration of the potential role of inflammation and altered circadian rhythms in primary brain-tumor patients are important for the development of mechanism-driven interventional studies.
In practice, it is important to assess patients for sleep complaints, contributing factors, and symptom clusters. Educating patients about sleep hygiene and the potential risk of sleep disturbance based on the disease and its treatment are just as important. The NCCN and Pan-Canadian guidelines are useful for identifying patients experiencing sleep-wake disturbances and the type of sleep disorder. Implementation of interventions such as CBT-I in general practice requires specialized training and may be limited by the real-life constraints of daily practice.119 Referrals to appropriate consultants may include a sleep medicine specialist for those patients with refractory insomnia or other sleep disorders, and psychologists for counseling and CBT-I.
Conclusions
As noted in the introduction, the purpose of this paper was to synthesize the knowledge of sleep-wake disturbances, with a focus on the prevalence and mechanisms of insomnia and hypersomnia in adult brain-tumor patients and to apply what is known from other solid tumors about screening, assessment, interventions, and implications to advance both research and practice. This report has several limitations including the paucity of literature related to describing the incidence in the primary brain-tumor population despite its wide clinical recognition and absence of studies exploring management. This report is also limited in that it was not a systematic review, and guidelines specific to this population could not be generated as a result. Nevertheless, sleep-wake disturbances are common in adult patients with solid tumors including primary brain tumors. Guidelines for assessment and management of sleep-wake disturbance have been identified, and studies focused on the underlying biologic mechanisms are beginning to be explored. However, the data specific to patients with primary brain tumors are quite limited. Sleep research on brain-tumor patients has focused primarily on those receiving radiation therapy and on adult survivors of childhood brain cancer. Patients may experience a fatigue-sleep cluster, most commonly with insomnia and hypersomnia, but also with severe sleep-wake disorders; the risk may be highest in those undergoing or who have previously undergone cranial radiation. This is especially concerning because of the relative frequency and severity in studies reported to date and the potential implications in a patient population at increased risk for neurocognitive symptoms and functional limitations. Studies exploring genomic risk and mechanisms are needed. Interventions to reduce sleep-wake disturbances in patients with other solid tumors have been developed but need to be tested in adults with malignant brain tumors to determine their effectiveness in this vulnerable population.
Funding
None declared.
Conflict of interest statement. T. Armstrong (research support ABBvie).
References
- 1. Dodd M, Janson S, Facione N et al. . Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. [DOI] [PubMed] [Google Scholar]
- 2. Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 3. Williams LA. Clinical management of symptom clusters. Semin Oncol Nurs. 2007;23(2):113–120. [DOI] [PubMed] [Google Scholar]
- 4. Banthia R, Malcarne VL, Ko CM, Varni JW, Sadler GR. Fatigued breast cancer survivors: the role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24(8):965–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zee PC, Ancoli-Israel S. Does effective management of sleep disorders reduce cancer-related fatigue? Drugs. 2009;69Suppl 2:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 7. Mendoza TR, Wang XS, Cleeland CS et al. . The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 8. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. [DOI] [PubMed] [Google Scholar]
- 9. Medicine AAoS. International Classification of Sleep Disorders. 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berger AM, Parker KP, Young-McCaughan S et al. . Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005;32(6):E98–126. [DOI] [PubMed] [Google Scholar]
- 11. Berger AM. Update on the state of the science: sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009;36(4):E165–E177. [DOI] [PubMed] [Google Scholar]
- 12. Johnson SS, Castle PH. How do sleep, food, mood, and exercise relate to well-being? Am J Health Promot. 2015;29(4):TAHP4–5. [PubMed] [Google Scholar]
- 13. Johnson JA, Rash JA, Campbell TS et al. . A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2015;27:20–28. [DOI] [PubMed] [Google Scholar]
- 14. Sateia MJ, Pigeon WR. Identification and management of insomnia. Med Clin North Am. 2004;88(3):567–596, vii. [DOI] [PubMed] [Google Scholar]
- 15. Hamet P, Tremblay J. Genetics of the sleep-wake cycle and its disorders. Metabolism. 2006;55(10 Suppl 2):S7–12. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro Oncol. 2012;14Suppl 4:iv65–iv72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford ES, Wheaton AG, Cunningham TJ, Giles WH, Chapman DP, Croft JB. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care survey 1999–2010. Sleep. 2014;37(8):1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dauvilliers Y, Buguet A. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Darko DF, McCutchan JA, Kripke DF, Gillin JC, Golshan S. Fatigue, sleep disturbance, disability, and indices of progression of HIV infection. Am J Psychiatry. 1992;149(4):514–520. [DOI] [PubMed] [Google Scholar]
- 20. Carnicka Z, Kollar B, Siarnik P, Krizova L, Klobucnikova K, Turcani P. Sleep disorders in patients with multiple sclerosis. J Clin Sleep Med. 2015;11(5):553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fogelberg DJ, Hoffman JM, Dikmen S, Temkin NR, Bell KR. Association of sleep and co-occurring psychological conditions at 1 year after traumatic brain injury. Arch Phys Med Rehabil. 2012;93(8):1313–1318. [DOI] [PubMed] [Google Scholar]
- 22. Siarnik P, Kollar B, Carnicka Z et al. . Association of sleep disordered breathing with wake-up acute ischemic stroke: a full polysomnographic study. J Clin Sleep Med. 2015;12(4):549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siarnik P, Kollar B, Carnicka Z, Sutovsky S, Klobucnikova K, Turcani P. Characteristics of sleep-disordered breathing in etiologic subtypes of minor-to-moderate acute ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24(5):1087–1093. [DOI] [PubMed] [Google Scholar]
- 24. Strober LB. Fatigue in multiple sclerosis: a look at the role of poor sleep. Front Neurol. 2015;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hong CT, Wong CS, Ma HP et al. . PERIOD3 polymorphism is associated with sleep quality recovery after a mild traumatic brain injury. J Neurol Sci. 2015;358(1–2):385–389. [DOI] [PubMed] [Google Scholar]
- 26. Armstrong TS, Vera-Bolanos E, Acquaye AA, Gilbert MR, Ladha H, Mendoza T. The symptom burden of primary brain tumors: evidence for a core set of tumor and treatment-related symptoms. Neuro Oncol. 2015;18(2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yavas C, Zorlu F, Ozyigit G et al. . Health-related quality of life in high-grade glioma patients: a prospective single-center study. Support Care Cancer. 2012;20(10):2315–2325. [DOI] [PubMed] [Google Scholar]
- 28. Otte JL, Carpenter JS, Russell KM, Bigatti S, Champion VL. Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. J Pain Symptom Manage. 2010;39(3):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Med Rev. 2004;8(3):199–212. [DOI] [PubMed] [Google Scholar]
- 30. Palesh OG, Roscoe JA, Mustian KM et al. . Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008;62(3):391–400. [DOI] [PubMed] [Google Scholar]
- 32. Phillips KM, Jim HS, Donovan KA, Pinder-Schenck MC, Jacobsen PB. Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer. 2012;20(2):357–365. [DOI] [PubMed] [Google Scholar]
- 33. Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54(9):1309–1321. [DOI] [PubMed] [Google Scholar]
- 34. Armstrong TS, Vera-Bolanos E, Gning I et al. . The impact of symptom interference using the MD Anderson Symptom Inventory-Brain Tumor Module (MDASI-BT) on prediction of recurrence in primary brain tumor patients. Cancer. 2011;117(14):3222–3228. [DOI] [PubMed] [Google Scholar]
- 35. Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14(12):1205–1212. [DOI] [PubMed] [Google Scholar]
- 36. Mulrooney DA, Ness KK, Neglia JP et al. . Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep. 2008;31(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng JX, Liu BL, Zhang X et al. . Health-related quality of life in glioma patients in China. BMC Cancer. 2010;10:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armstrong TS, Cron SG, Bolanos EV, Gilbert MR, Kang DH. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715. [DOI] [PubMed] [Google Scholar]
- 39. Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. [DOI] [PubMed] [Google Scholar]
- 40. Brown PD, Ballman KV, Rummans TA et al. . Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. [DOI] [PubMed] [Google Scholar]
- 41. Powell C, Guerrero D, Sardell S et al. . Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: a prospective study. Radiother Oncol. 2011;100(1):131–136. [DOI] [PubMed] [Google Scholar]
- 42. Sheely LC. Sleep disturbances in hospitalized patients with cancer. Oncol Nurs Forum. 1996;23(1):109–111. [PubMed] [Google Scholar]
- 43. Monas L, Csorba S, Kovalyo M, Zeligman R, Dror YF, Musgrave CF. The relationship of sleep disturbance and symptom severity, symptom interference, and hospitalization among Israeli inpatients with cancer. Oncol Nurs Forum. 2012;39(4):E361–E372. [DOI] [PubMed] [Google Scholar]
- 44. Valko PO, Siddique A, Linsenmeier C, Zaugg K, Held U, Hofer S. Prevalence and predictors of fatigue in glioblastoma: a prospective study. Neuro Oncol. 2015;17(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armstrong TS, Ying Y, Wu J et al. . The relationship between corticosteroids and symptoms in patients with primary brain tumors: utility of the Dexamethasone Symptom Questionnaire-Chronic. Neuro Oncol. 2015;17(8):1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Acquaye AA, Vera-Bolanos E, Armstrong TS, Gilbert MR, Lin L. Mood disturbance in glioma patients. J Neurooncol. 2013;113(3):505–512. [DOI] [PubMed] [Google Scholar]
- 47. Jones LW, Guill B, Keir ST et al. . Patterns of exercise across the cancer trajectory in brain tumor patients. Cancer. 2006;106(10):2224–2232. [DOI] [PubMed] [Google Scholar]
- 48. Miaskowski C. Pharmacologic management of sleep disturbances in noncancer-related pain. Pain Manag Nurs. 2009;10(1):3–13. [DOI] [PubMed] [Google Scholar]
- 49. Cirelli C. The genetic and molecular regulation of sleep: from fruit flies to humans. Nat Rev Neurosci. 2009;10(8):549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146(2):194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang B, Veasey SC, Wood MA et al. . Impaired rapid eye movement sleep in the Tg2576 APP murine model of Alzheimer's disease with injury to pedunculopontine cholinergic neurons. Am J Pathol. 2005;167(5):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alfaro E, Dhruva A, Langford DJ et al. . Associations between cytokine gene variations and self-reported sleep disturbance in women following breast cancer surgery. Eur J Oncol Nurs. 2014;18(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Illi J, Miaskowski C, Cooper B et al. . Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58(3):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee KA, Gay C, Byun E, Lerdal A, Pullinger CR, Aouizerat BE. Circadian regulation gene polymorphisms are associated with sleep disruption and duration, and circadian phase and rhythm in adults with HIV. Chronobiol Int. 2015;32(9):1278–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hida A, Kitamura S, Katayose Y et al. . Screening of clock gene polymorphisms demonstrates association of a PER3 polymorphism with morningness-eveningness preference and circadian rhythm sleep disorder. Sci Rep. 2014;4:6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faithfull S. Patients’ experiences following cranial radiotherapy: a study of the somnolence syndrome. J Adv Nurs. 1991;16(8):939–946. [DOI] [PubMed] [Google Scholar]
- 57. Faithfull S, Brada M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol (R Coll Radiol). 1998;10(4):250–254. [DOI] [PubMed] [Google Scholar]
- 58. Gapstur R, Gross CR, Ness K. Factors associated with sleep-wake disturbances in child and adult survivors of pediatric brain tumors: a review. Oncol Nurs Forum. 2009;36(6):723–731. [DOI] [PubMed] [Google Scholar]
- 59. Ballesteros-Zebadua P, Chavarria A, Celis MA, Paz C, Franco-Perez J. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS Neurol Disord Drug Targets. 2012;11(7):937–949. [DOI] [PubMed] [Google Scholar]
- 60. Muller HL, Handwerker G, Wollny B, Faldum A, Sorensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab. 2002;87(8):3993–3996. [DOI] [PubMed] [Google Scholar]
- 61. Wion D, Berger F, Wion-Barbot N. Glioma, melatonin, and radiotherapy. Cancer Res. 2006;66(12):6457. [DOI] [PubMed] [Google Scholar]
- 62. Kassayova M, Ahlersova E, Ahlers I. Two-phase response of rat pineal melatonin to lethal whole-body irradiation with gamma rays. Physiol Res. 1999;48(3):227–230. [PubMed] [Google Scholar]
- 63. Ozkan E, Yaman H, Cakir E et al. . Plasma Melatonin and Urinary 6-Hydroxymelatonin Levels in Patients with Pulmonary Tuberculosis. Inflammation. 2012;35(4):1429–1434. [DOI] [PubMed] [Google Scholar]
- 64. Bagci S, Yildizdas D, Horoz OO, Reinsberg J, Bartmann P, Mueller A. Use of nocturnal melatonin concentration and urinary 6-sulfatoxymelatonin excretion to evaluate melatonin status in children with severe sepsis. J Pediatr Endocrinol Metab. 2011;24(11-12):1025–1030. [DOI] [PubMed] [Google Scholar]
- 65. Van Someren EJ, Swart-Heikens J, Endert E et al. . Long-term effects of cranial irradiation for childhood malignancy on sleep in adulthood. Eur J Endocrinol. 2004;150(4):503–510. [DOI] [PubMed] [Google Scholar]
- 66. Howell D, Oliver TK, Keller-Olaman S et al. . A Pan-Canadian practice guideline: prevention, screening, assessment, and treatment of sleep disturbances in adults with cancer. Support Care Cancer. 2013;21(10):2695–2706. [DOI] [PubMed] [Google Scholar]
- 67. Howell D, Keller-Olaman S, Oliver TK et al. . A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233–e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Denlinger CS. NCCN Guidelines Version 1.2015 survivorship. 2015 https://www.nccn.org (accessed February 3 2016).
- 69. Acquaye AA, Lin L, Vera-Bolanos E, Gilbert MR, Armstrong TS. Hope and mood changes throughout the primary brain tumor illness trajectory. Neuro Oncol. 2015;18(1):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Armstrong TS, Mendoza T, Gning I et al. . Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 71. Taphoorn MJ, Claassens L, Aaronson NK et al. . An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 72. Thavarajah N, Bedard G, Zhang L et al. . Psychometric validation of the functional assessment of cancer therapy--brain (FACT-Br) for assessing quality of life in patients with brain metastases. Support Care Cancer. 2014;22(4):1017–1028. [DOI] [PubMed] [Google Scholar]
- 73. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 74. Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep. 1992;15(4):376–381. [DOI] [PubMed] [Google Scholar]
- 75. Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 76. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 77. Chung F, Yegneswaran B, Liao P et al. . STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. [DOI] [PubMed] [Google Scholar]
- 78. Cella D, Riley W, Stone A et al. . The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. ONS. Putting evidence into practice online resources for sleep-wake disturbances 2015. http://www.ons.org/practice-resources/pep (accessed February 3 2016).
- 80. Garland SN, Johnson JA, Savard J et al. . Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Langford DJ, Lee K, Miaskowski C. Sleep disturbance interventions in oncology patients and family caregivers: a comprehensive review and meta-analysis. Sleep Med Rev. 2012;16(5):397–414. [DOI] [PubMed] [Google Scholar]
- 82. Morgenthaler T, Kramer M, Alessi C et al. . Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American academy of sleep medicine report. Sleep. 2006;29(11):1415–1419. [PubMed] [Google Scholar]
- 83. Roth T. Understanding neuronal pathways: novel targets for the management of insomnia. J Clin Psychiatry. 2007;68Suppl 5:4–5. [PubMed] [Google Scholar]
- 84. Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583–590. [DOI] [PubMed] [Google Scholar]
- 85. Johnson JA, Rash JA, Campbell TS et al. . A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. [DOI] [PubMed] [Google Scholar]
- 86. Matthews EE, Berger AM, Schmiege SJ et al. . Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241. [DOI] [PubMed] [Google Scholar]
- 87. Fleming L, Randell K, Harvey CJ, Espie CA. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. 2014;23(6):679–684. [DOI] [PubMed] [Google Scholar]
- 88. Vargas S, Antoni MH, Carver CS et al. . Sleep quality and fatigue after a stress management intervention for women with early-stage breast cancer in Southern Florida. Int J Behav Med. 2014;21(6):971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Casault L, Savard J, Ivers H, Savard MH. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behav Res Ther. 2015;67:45–54. [DOI] [PubMed] [Google Scholar]
- 90. Kwekkeboom KL, Abbott-Anderson K, Cherwin C, Roiland R, Serlin RC, Ward SE. Pilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J Pain Symptom Manage. 2012;44(6):810–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P. Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. J Neuropsychiatry Clin Neurosci. 2009;21(2):114. [DOI] [PubMed] [Google Scholar]
- 93. Chiu HY, Chiang PC, Miao NF, Lin EY, Tsai PS. The effects of mind-body interventions on sleep in cancer patients: a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2014;75(11):1215–1223. [DOI] [PubMed] [Google Scholar]
- 94. Bower JE, Crosswell AD, Stanton AL et al. . Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lengacher CA, Shelton MM, Reich RR et al. . Mindfulness based stress reduction (MBSR(BC)) in breast cancer: evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT). J Behav Med. 2014;37(2):185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Andersen SR, Wurtzen H, Steding-Jessen M et al. . Effect of mindfulness-based stress reduction on sleep quality: results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52(2):336–344. [DOI] [PubMed] [Google Scholar]
- 97. Nakamura Y, Lipschitz DL, Kuhn R, Kinney AY, Donaldson GW. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: a pilot randomized controlled trial. J Cancer Surviv. 2013;7(2):165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46(13):909–916. [DOI] [PubMed] [Google Scholar]
- 99. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Clin Otolaryngol. 2012;37(5):390–392. [DOI] [PubMed] [Google Scholar]
- 100. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;8:CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Courneya KS, Karvinen KH, McNeely ML et al. . Predictors of adherence to supervised and unsupervised exercise in the Alberta Physical Activity and Breast Cancer Prevention Trial. J Phys Act Health. 2012;9(6):857–866. [DOI] [PubMed] [Google Scholar]
- 102. Courneya KS, Segal RJ, Mackey JR et al. . Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144(2):361–369. [DOI] [PubMed] [Google Scholar]
- 103. Cheville AL, Kollasch J, Vandenberg J et al. . A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sprod LK, Hsieh CC, Hayward R, Schneider CM. Three versus six months of exercise training in breast cancer survivors. Breast Cancer Res Treat. 2010;121(2):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Donnelly CM, Blaney JM, Lowe-Strong A et al. . A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecol Oncol. 2011;122(3):618–624. [DOI] [PubMed] [Google Scholar]
- 106. Rogers LQ, McAuley E, Anton PM et al. . Better exercise adherence after treatment for cancer (BEAT Cancer) study: rationale, design, and methods. Contemp Clin Trials. 2012;33(1):124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wenzel JA, Griffith KA, Shang J et al. . Impact of a home-based walking intervention on outcomes of sleep quality, emotional distress, and fatigue in patients undergoing treatment for solid tumors. Oncologist. 2013;18(4):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol. 2012;3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ruden E, Reardon DA, Coan AD et al. . Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29(21):2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Morin CM, Beaulieu-Bonneau S, Ivers H et al. . Speed and trajectory of changes of insomnia symptoms during acute treatment with cognitive-behavioral therapy, singly and combined with medication. Sleep Med. 2014;15(6):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Morin CM, Vallieres A, Guay B et al. . Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Roscoe JA, Garland SN, Heckler CE et al. . Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. 2015;17(4):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia-NHANES 1999–2010. Sleep. 2014;37(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hottinger AF, Yoon H, DeAngelis LM, Abrey LE. Neurological outcome of long-term glioblastoma survivors. J Neurooncol. 2009;95(3):301–305. [DOI] [PubMed] [Google Scholar]
- 117. Borneman T, Koczywas M, Sun VC, Piper BF, Uman G, Ferrell B. Reducing patient barriers to pain and fatigue management. J Pain Symptom Manage. 2010;39(3):486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J Clin. 2015;65(3):190–211. [DOI] [PubMed] [Google Scholar]
- 119. Manber R, Carney C, Edinger J et al. . Dissemination of CBTI to the non-sleep specialist: protocol development and training issues. J Clin Sleep Med. 2012;8(2):209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]