Abstract
Background.
Our aim was to review MRI characteristics of patients with primary CNS lymphoma (PCNSL) enrolled in a randomized phase II trial and to evaluate their potential prognostic value and patterns of relapse, including T2 fluid attenuated inversion recovery (FLAIR) MRI abnormalities.
Methods.
Neuroimaging findings in 85 patients with PCNSL enrolled in a prospective trial were reviewed blinded to outcomes. MRI characteristics and responses according to International PCNSL Collaborative Group (IPCG) criteria were correlated with progression-free survival (PFS) and overall survival (OS).
Results.
Multivariate analysis showed that objective response at 2 months (P < .001) and at end of treatment (P = .015) were predictors of prolonged OS. Infratentorial location (P = .008) and large (>11.4 cm3) enhancing tumor volume (P = .006) were associated with poor OS and PFS, respectively. Ratio of change in product of largest diameters at early MRI evaluation but not timing of complete response achievement (early vs delayed) was prognostic for OS. Sixty-nine patients relapsed. Relapse in the brain (n = 52) involved an initial enhancing site, a different site, or both in 46%, 40%, and 14% of patients, respectively. At baseline, non-enhancing T2-FLAIR hypersignal lesions distant from the enhancing tumor site were detected in 18 patients. These lesions markedly decreased (>50%) in 16 patients after chemotherapy, supporting their neoplastic nature. Of these patients, 10/18 relapsed, half (n = 5) in the initially non-enhancing T2-FLAIR lesions.
Conclusions.
Baseline tumor size and infratentorial localization are of prognostic value in PCNSL. Our findings provide evidence that non-enhancing FLAIR abnormalities may add to overall tumor burden, suggesting that response criteria should be refined to incorporate evaluation of T2-weighted/FLAIR sequences.
Keywords: magnetic resonance imaging, primary CNS lymphoma, prognostic factor, response criteria, T2-weighted/FLAIR sequences
Importance of the study
This study suggests that baseline imaging characteristics such as initial tumor size and infratentorial involvement are of prognostic value in primary CNS lymphoma. Radiographic response as determined by IPCG response criteria is also a valuable predictor of patient survival. However, the study provides evidence that T2-FLAIR hyperintense lesions without contrast enhancement may constitute additional foci of disease that require attentive follow-up, potentially constituting eventual sites of disease relapse. Response criteria could be further refined by inclusion of T2-FLAIR sequence assessments in order to improve baseline disease assessment, response evaluation, and progression determination.
Primary central nervous system lymphoma (PCNSL) is a rare subtype of malignant extranodal diffuse large B-cell lymphoma (DLBCL) confined to the CNS. High-dose methotrexate (hd MTX)–based chemotherapy is the standard initial treatment for PCNSL. However, consolidation with whole-brain radiotherapy is controversial, particularly in elderly patients who are at high risk for severe neurotoxicity.1 Despite the high rate of response to chemotherapy, relapse occurs in a majority of patients after several months or years, leading to a poor overall outcome. Neuroimaging of PCNSL shows typical intense and homogeneously enhancing mass lesions that are likely located deep in periventricular areas.2–5 However, the impact of baseline neuroimaging characteristics on patient outcome is unclear. The most commonly used response criteria are those published by the International PCNSL Collaborative Group (IPCG), which combines MRI findings, eye examination, cerebrospinal fluid (CSF) analysis, and steroid dose.6 Of note, MRI response criteria rely on contrast enhancement measure changes and does not take into consideration non-enhancing lesions typically visualized on T2 fluid attenuated inversion recovery (FLAIR) MRI. Finally, neuroimaging features at relapse are poorly documented.7 Recently, we completed a phase II trial evaluating 2 hd MTX–based polychemotherapy regimens in the absence of radiotherapy in elderly patients with PCNSL.8 Our objective was to review MRI characteristics of patients with PCNSL enrolled in this trial and to evaluate their potential prognostic value and patterns of relapse, including T2-FLAIR MRI abnormalities.
Methods
Study Design
The present study is a post-hoc analysis of patients enrolled in an open-label, randomized, phase II trial conducted by the intergroup of the Association des Neuro-Oncologue d’Expression Française (ANOCEF) and the Groupe Ouest-Est d’Etude des Leucémies et Autres Maladies du Sang (GOELAMS). Study design, inclusion criteria, patient characteristics, procedures, treatment, efficacy, and safety results have been published previously.8 In brief, this phase II trial evaluated 2 MTX-based regimens in the absence of brain radiotherapy in immunocompetent patients aged >60 years and with a Karnofsky performance status of >40%. Patients were randomized to 2 groups: one group received a combination of hd MTX, vincristine, procarbazine, and cytarabine (MPV-A) and the other group received hd MTX and temozolomide (MT). The study used a noncomparative randomized phase II design, with a total of 47 patients enrolled in the MPV-A group and 48 in the MT group. The study was approved by the institutional review board of the Pitie-Salpetriere Hospital and the French Agency for the Safety of Health Products and was conducted according to the Declaration of Helsinki and Good Clinical Practice (ClinicalTrials.gov: NCT00503594). All patients or their guardians provided written informed consent.
Neuroimaging Evaluation
Only patients followed with MRI were included in this study; patients exclusively followed with CT scans were excluded. MRI evaluation was performed at baseline, before histological diagnosis and initiation of corticosteroid treatment, after administration of 2 treatment cycles (2 mo), after completion of chemotherapy (4 mo), and every 2 months until progression. The MRI parameters included at least T1, T1 with gadolinium injection, and T2 or FLAIR sequences. Clinical examinations were systematically performed at the same time. All MRIs were reviewed by 2 neuro-oncologists blinded to individual patient outcomes (E.T. and C.H.).
At baseline and at all evaluation time points until relapse, the following characteristics were recorded: number, anatomical site, and measures (largest diameter, product of largest perpendicular diameters, and volume [calculated based on ellipsoid volume formula]) of enhancing and non-enhancing T2-FLAIR lesions. Distinct lobes and lateralization were based on the Talairach atlas; deep lesions were defined according to Ferreri et al9 as periventricular regions, basal ganglia, brainstem, and/or cerebellum. Infratentorial localization was defined as lesions in the brainstem and/or the cerebellum. The response rates were analyzed using the IPCG criteria.6 These response criteria define complete response (CR) as complete disappearance of contrast enhancement on MRI, no evidence of ocular lymphoma, negative CSF cytology, and discontinuation of corticosteroid use for at least 2 weeks prior to the evaluation of response. Unconfirmed CR (Cru) is used to characterize radiographic findings that fulfill criteria for a CR, but the patient remains on corticosteroids, or MRI that continues to show small but persistent enhancing abnormalities possibly related to biopsy or surgery. Partial response (PR) is defined as a 50% decrease in enhancing tumor or residual disease on eye examinations, or persistent or suspicious CSF cytology. Progressive disease (PD) is defined as 25% increase in the enhancing lesion, appearance of any new CNS or non-CNS site of disease, recurrent or new ocular disease, or recurrent or positive CSF cytology. Any other situation is characterized as stable disease (SD).
Statistical Analysis
Survival times were calculated from the date of randomization for analyses of the prognostic impact of baseline MRI characteristics, and from the date of the first MRI (at 2 mo) and the second MRI (at the end of treatment) for corresponding landmark analyses. For analysis of relapse characteristics, survival was calculated from the date of first relapse. Survival was analyzed with Kaplan–Meier and log-rank test methodology. Cox proportional hazards models were used for performing multivariate analyses and for estimating hazard ratios in survival regression models. Multivariate analyses included the variables that had a significant prognostic value in the clinical trial: KPS and Mini-Mental State (MMS) scores.8 Comparisons of categorical variable distribution were performed using the χ2 or Fisher’s exact test. Comparisons of continuous variable distribution were performed using the nonparametric Mann–Whitney U test and receiver operating characteristic (ROC) curve analysis. Initial and relapse continuous characteristics were compared using the paired t-test. Correlations were analyzed using the Spearman test. All reported P-values are 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed using PASW statistics v21.
Results
Patient Characteristics
A total of 85 out of 95 patients enrolled in the trial were included in this neuroimaging study. Excluded were 7 patients followed with CT scan and 3 patients with missing baseline MRI. Relevant patient characteristics are summarized in Table S1.
Baseline MRI Characteristics and Their Prognostic Impact
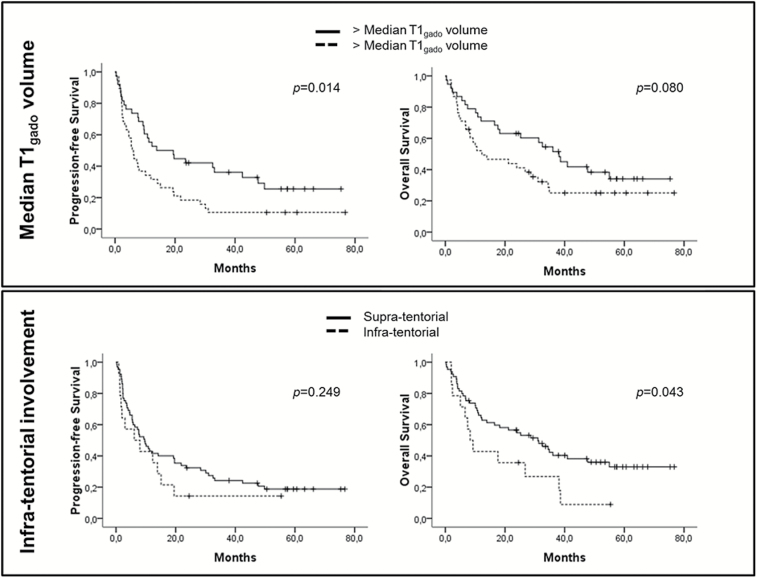
Baseline MRI characteristics are summarized in Table 1. Overall, our series was similar to previous studies, with a majority of patients showing deeply located homogeneous contrast-enhancing mass lesions. In total, 55% of patients showed a single enhancing lesion. Infratentorial location was observed in 18% of patients. Multivariate analysis, including KPS and MMS, showed poor progression-free survival (PFS) for patients with a large (>11.4 cm3) initial T1 enhancing volume (P = .006, hazard ratio [HR] = 2.176) and large initial FLAIR diameter (>40 mm) (P = .033, HR = 1.834) (Table 2). Multivariate analysis showed shorter overall survival (OS) for patients with infratentorial enhancing lesion(s) (P = .008, HR =2.693) and large initial FLAIR diameter (P = .006, HR =2.381; Fig. 1 and Fig. S1). The number of enhancing lesions, meningeal involvement, and supratentorial deep locations did not correlate with patient outcome.
Table 1.
Initial and recurrent MRI characteristics
| Neuroimaging Characteristics | Baseline | Relapse | Initial- Recurrence Comparison | ||
|---|---|---|---|---|---|
| N | % | N | % | P | |
| T1 without enhancement | |||||
| Hypointensity | 46 | 73.0 | |||
| Normointensity | 13 | 20.6 | |||
| Hyperintensity | 4 | 6.4 | |||
| Number of lesion(s) (range) | 1 (1–7) | 1 (1–6) | .534 | ||
| Single | 44 | 55.7 | 24 | 53.3 | |
| 2 | 9 | 24.1 | 11 | 24.4 | |
| 3 of more | 13 | 20.2 | 10 | 22.3 | |
| Lateralization | .868 | ||||
| Right | 26 | 32.9 | 11 | 24.4 | |
| Left | 12 | 15.2 | 9 | 20 | |
| Bilateral | 35 | 44.3 | 23 | 51.1 | |
| Median | 6 | 7.6 | 2 | 4.4 | |
| Location | .692 | ||||
| Non-deep (superficial) | 11 | 13.9 | 34 | 75.6 | |
| Deep (corpus callosum, basal ganglia, brainstem and/or cerebellum) | 56 | 70.9 | 4 | 8.9 | |
| Both | 12 | 15.2 | 7 | 15.6 | |
| Infratentorial lesions (brainstem and/or cerebellum) | 14 | 17.7 | |||
| Median T1 diameter, cm, median (range) | 4 (0–16.4) | 4.9 (1–16.5) | .951 | ||
| Median T1 product, cm2, median (range) | 10.06 (0–39.19) | 10 (0.8–49.14) | .128 | ||
| Median T1 volume, cm3, median (range) | 11.4 (0–96.2) | 8.0 (0.2–123.5) | .137 | ||
| Enhancement type | .247 | ||||
| Homogeneous | 65 | 87.8 | 36 | 80 | |
| Heterogeneous | 9 | 12.2 | 9 | 20 | |
| Meningeal enhancement | 18 | 23.4 | 13 | 29.5 | .382 |
| Local | 11 | 61.1 | 7 | 53.8 | |
| Diffuse | 7 | 38.9 | 6 | 46.2 | |
| “Distant” FLAIR | 18 | 23.7 | |||
| Median FLAIR Diameter, mm, median (range) | 83.5 (20–290) | 90 (10-10-237) | .266 | ||
| Median FLAIR product, mm2, median (range) | 4605 (320–12700) | 3853.5 (100–10125) | .021 | ||
| Median FLAIR Volume, cm3, median (range) | 94.8 (2.7–447.7) | 45.5 (0.4–300.4) | .015 | ||
| FLAIR type of enhancing lesion | .649 | ||||
| Hypointensity | 14 | 19.2 | 8 | 19.0 | |
| Normointensity | 23 | 31.5 | 10 | 23.8 | |
| Hyperintensity | 36 | 49.3 | 24 | 57.1 | |
Table 2.
Prognostic impact of MRI characteristics at baseline, HR (95% CI)
| Univariate | Multivariate* | Univariate | Multivariate* | |||||
|---|---|---|---|---|---|---|---|---|
| Factors | P | HR | P | HR | P | HR | P | HR |
| T1 without enhancement (hypo vs normo vs hyper-intensity) | .072 | .372 | ||||||
| Number of lesion(s) (single vs multiple) | .356 | .238 | ||||||
| Deep vs non-deep lesion | .254 | .158 | ||||||
| Median T1 diameter (cutoff: median) | .112 | .079 | .458 | |||||
| Median T1 product (cutoff: median) | .233 | .799 | ||||||
| Median T1 volume (cutoff: median) | .014 | 1.877 (1.124–3.133) |
.006 | 2.176 (1.245–3.803) |
.080 | .058 | ||
| Meningeal enhancement | .378 | .933 | ||||||
| “Distant” FLAIR infiltration | .093 | .051 | .468 | |||||
| Median FLAIR diameter (cutoff: median) | .086 | .033 | 1.834 (1.051–3.200) |
.037 | 1.799 (1..029-3.145) |
.008 | 2.381 (1.254–4.523) |
|
| Median FLAIR product (cutoff: median) | .417 | .117 | .076 | |||||
| Median FLAIR volume (cutoff: median) | .443 | .235 | ||||||
| FLAIR intensity (hypo vs normo vs hyper-intensity) | .046 | 0.493 (0.319–0.763) |
0.349 | .435 | ||||
| Infratentorial involvement vs only supratentorial lesions | .249 | .043 | 1.931 (1.008–3.698) |
.008 | 2.693 (1.299–5.582) |
|||
adjusted by the KPS and the MMS
Fig. 1.
Progression-free survival and OS according to the T1 enhancement volume and posterior fossa involvement.
Objective Response and Survival Impact
Best objective responses (ORs) based on the IPCG criteria were as follows: CR: 56%, CRu: 4%, PR: 18%, SD: 7%, and PD: 15%. The median time from diagnosis to the achievement of CR was 81 days (range, 22–538). ORs at the first MRI were as follows: CR: 30%, PR: 43%, SD: 10%, and PD: 17%. At 4 months, ORs of patients were CR: 51%, CRu: 4%, PR: 20%, and PD: 25%. Among patients showing PR at the first MRI evaluation (N = 30), 13 (43%) achieved CR, 10 (33%) achieved PR, and 7 (24%) progressed at the end of the treatment. Similarly, among patients with a stable disease at the first MRI evaluation (N = 7), 5 (72%) progressed and 2 (28%) showed PR at the end of the treatment. Among patients showing CR at the end of the treatment, 22 (69%) relapsed at a median time of 19.4 months (95% CI: 6.5–32.4). Among patients who showed PR at the end of the treatment (N = 12), 50% showed PFS of >12 months, although they did not undergo further treatment after the completion of the protocol. Both OR at the first MRI (2 mo) and at the end of the treatment (4 mo) were correlated with OS (P < .001 for both). Notably, early (at the first MRI evaluation) versus delayed achievement of CR did not affect survival (PFS: P = .701; OS: P = .493).
The product of perpendicular diameters seemed to be more discriminative at the first MRI (HR: 1.970, 95% CI: 1.401–2.769) and MRI at the end of the treatment (HR: 2.012, 95% CI: 1.144–3.540) than the larger diameter alone (HR = 1.750, 95% CI: 1.249–2.453 and HR = 1.599, 95% CI: 0.967–2.644, respectively) (Fig. S2).
Continuous analysis of percentage decrease in T1 enhancement (product of perpendicular diameter) between the baseline and the first MRI evaluations (ie, the ratio of change in product of largest diameters) indicated a correlation with OS according to Cox analysis (P = .052). Moreover, it was associated with prolonged OS (cutoff: median) according to ROC analysis (P = .031, area under the curve: 0.665), which identified the best discriminative cutoff of a decrease of 88.5% and was associated with the response at the end of the treatment (P < .001).
Relapse
At the time of analysis, 69 patients had progressed or relapsed. Brain MRI at the time of recurrence was available for 52 patients. Relapse in the brain involved the initial enhancing site, a site at distance, or both in 21 (46%), 18 (40%), and 6 (14%) patients, respectively. In 7 patients, there was no brain involvement at time of recurrence, with disease restricted to the eyes (n = 6) or lymph node (n = 1). The relapse characteristics did not differ from those observed in the initial MRI in terms of topography, T1 volume, and number of lesions (Table 1). At relapse, large enhancing T1 and FLAIR volumes were associated with poor outcome (P = .001 and P < .001, respectively).
Non-enhancing T2-FLAIR Hypersignal Lesions
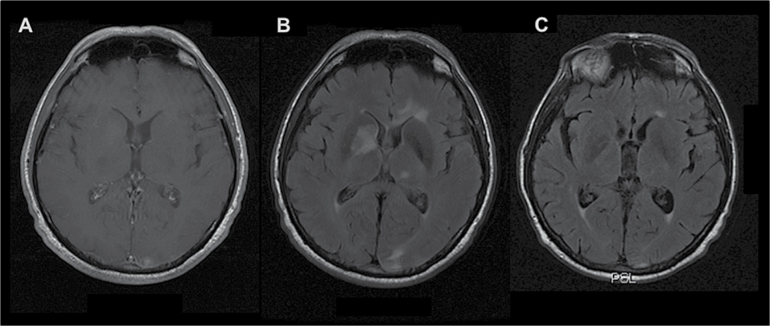
At baseline, non-enhancing T2-FLAIR hypersignal lesions (>10 mm) at a distance from the enhancing tumor site were detected in 18 patients (23%). Of these, 16 (89%) showed a marked decrease of the non-enhancing lesions (>50%) after chemotherapy, in favor of their neoplastic nature (Fig. 2). Among these 18 patients, 10 relapsed, half of them (n= 5) in the initially non-enhancing T2-FLAIR lesions.
Fig. 2.
Distant FLAIR involvement without T1 post-gadolinium enhancement. (A) Initial T1-weighted image with gadolinium injection. (B) Initial FLAIR image with non-enhancing lesions. (C) FLAIR image evaluation at the end of the treatment; marked response of the initial FLAIR lesions.
Discussion
Data on MRI characteristics in the assessment of PCNSL patients treated with standardized polychemotherapy in prospective trials are scant.7 Among the initial radiological findings, which are in accordance with those reported previously,2–5,10 we observed that baseline tumor size (diameter and volume) and infratentorial involvement are of prognostic value. While initial maximum tumor size (bulk) has been reported as an independent adverse prognostic factor in patients with systemic DLBCL,11 to our knowledge our report is the first documentation of a relationship between a radiographic measure of tumor burden and prognosis in PCNSL. Recent PET scan studies in patients with systemic DLBCL suggested a better prognostic value of metabolic tumor volume than that of standardized uptake value, further emphasizing the value of baseline tumor burden evaluations.12,13 Although deep locations have been described as associated with poor outcome in several publications, including the scoring system published by Ferreri et al,9,14,15 infratentorial location has never been specifically evaluated. In the present study, deep location was not a prognostic factor, whereas infratentorial involvement independently predicted survival. Of note, this absence of prognostic impact of deep locations was in line with the Memorial Sloan-Kettering Cancer Center prognostic model,16 where no neuroradiological characteristics were prognostic.
With respect to the prognostic impact of the IPCG response criteria, we observed a significant correlation between OR to chemotherapy and outcome and confirmed that the product of diameters, as utilized in the IPCG criteria, is a superior assessment tool compared with largest diameter, as used in other response criteria such as RECIST (Response Evaluation Criteria In Solid Tumors). Although we observed a better prognostic outcome in patients showing high percentage decrease in T1 enhancement in the first MRI, we did not observe any difference in prognosis between patients showing early CR at 2 months versus those showing delayed CR at the termination of the treatment, suggesting that the kinetics of treatment response were not relevant. This result differs from that of a study by Pels et al,17 which showed that patients showing delayed CR had a poor outcome. However, marked differences between the 2 studies may explain this discordance, including the chemotherapy regimen, which was more intensive in the Pels study, and the target population, younger in that study compared with ours. Otherwise, our results do suggest that stable disease at the first MRI evaluation may constitute an early sign of treatment failure. We also observed varying outcomes in the small group of patients showing persistent PR at the end of treatment. Some patients relapsed shortly afterward, while others (50%) achieved prolonged disease control, underlining the difficulty in assessing disease remission. Among other tools potentially useful to better define CR to treatment, 2-fluoro-2-deoxy-d-glucose PET has been recently incorporated in systemic non-Hodgkin lymphoma response criteria.18 However, metabolic imaging of brain disease is limited by the high basal brain glucose metabolism, highlighting a need for the development of new tracers for PCNSL. CSF concentrations of interleukin-10 have been proposed as another potential biomarker to aid in the radiographic assessment of residual disease in PCNSL.19
At recurrence, we observed that >50% of patients relapsed at a distance from the initial tumor site. This result is in line with the preliminary results of Schulte-Altedorneburg et al, 7 who evaluated the pattern of recurrence in 16 patients with PCNSL and showed that 12 patients relapsed at distance. Taken together, these results support the use of therapies that can cross the blood–brain barrier to widely reach all areas infiltrated by PCNSL, and argue against the use of therapies that increase focal control on enhancing areas, such as focal radiotherapy, tumor bed boost, and surgical resection.
Finally, we observed MRI baseline non-enhancing T2-FLAIR weighted hypersignal lesions at a distance from the enhancing lesion(s) in 23% of patients. Although small and scattered T2-FLAIR hypersignal lesions are common in elderly patients, presence of larger lesions that reduce after chemotherapy strongly support their neoplastic nature. Despite their relatively high frequency, T2-FLAIR hypersignal lesions have been described in rare non-enhancing presentations of PCNSL,20,21 including a diffuse form presenting as leukoencephalopathy termed “lymphomatosis cerebri.”3,21 However, non-enhancing lesions are not taken into account in the current IPCG response criteria. Moreover, these T2-weighted/FLAIR locations could constitute distinct sites of relapse, and lymphoma relapse could occur without any contrast enhancement, as reported in autopsy cases, suggesting the monitoring of T2-weighted/FLAIR sequences.22 Distinguishing non-enhancing lymphomatous infiltration from vascular leukoencephalopathy and MTX-related leukoencephalopathy is challenging, particularly in elderly patients. In this setting, MRI spectroscopy23 and PET scan24 should be investigated as complementary tools.
Conclusion
The present study suggests that baseline tumor size and infratentorial localization are prognostic factors in patients with PCNSL. It provides prospective validation of IPCG radiographic response criteria, to be favored over RECIST as relevant predictors of outcome in this disease. However, our findings indicate that a substantial rate of PCNSL display non-contrast enhancing lesions, suggesting that assessment of T2-weighted/FLAIR sequences is of importance and should be incorporated in refined criteria defining response and progression in PCNSL. Further validation of these results in an independent population, preferably including patients of all ages, is warranted.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by Direction de la recherche clinique de l’APHP-PHRC AOM06175/P06239.
Conflict of interest statement
Authors report no relevant conflict of interest.
Supplementary Material
Acknowledgments
This work was completed in the LOC (lymphome oculo-cérébraux) network supported by the INCa. To David Baro, for his advice.
References
- 1. Hoang-Xuan K, Bessell E, Bromberg J, et al. European Association for Neuro-Oncology Task Force on Primary CNS Lymphoma. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16(7):e322–e332. [DOI] [PubMed] [Google Scholar]
- 2. Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002;12(2):329–340. [DOI] [PubMed] [Google Scholar]
- 3. Küker W, Nägele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72(2):169–177. [DOI] [PubMed] [Google Scholar]
- 4. Partovi S, Karimi S, Lyo JK, et al. Multimodality imaging of primary CNS lymphoma in immunocompetent patients. Br J Radiol. 2014;87(1036):20130684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang YZ, Booth TC, Bhogal P, et al. Imaging of primary central nervous system lymphoma. Clin Radiol. 2011;66(8):768–777. [DOI] [PubMed] [Google Scholar]
- 6. Abrey LE, Batchelor TT, Ferreri AJ, et al. International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. [DOI] [PubMed] [Google Scholar]
- 7. Schulte-Altedorneburg G, Heuser L, Pels H. MRI patterns in recurrence of primary CNS lymphoma in immunocompetent patients. Eur J Radiol. 2012;81(9):2380–2385. [DOI] [PubMed] [Google Scholar]
- 8. Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251–e259. [DOI] [PubMed] [Google Scholar]
- 9. Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266–272. [DOI] [PubMed] [Google Scholar]
- 10. Haldorsen IS, Kråkenes J, Krossnes BK, et al. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989–2003. AJNR Am J Neuroradiol. 2009;30(4):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfreundschuh M, Ho AD, Cavallin-Stahl E, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008;9(5):435–444. [DOI] [PubMed] [Google Scholar]
- 12. Ceriani L, Martelli M, Zinzani PL, et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood. 2015;126(8):950–956. [DOI] [PubMed] [Google Scholar]
- 13. Sasanelli M, Meignan M, Haioun C, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(11):2017–2022. [DOI] [PubMed] [Google Scholar]
- 14. Ghesquières H, Drouet Y, Sunyach MP, et al. Evidence of time-dependent prognostic factors predicting early death but not long-term outcome in primary CNS lymphoma: a study of 91 patients. Hematol Oncol. 2013;31(2):57–64. [DOI] [PubMed] [Google Scholar]
- 15. Iwadate Y, Suganami A, Ikegami S, et al. Non-deep-seated primary CNS lymphoma: therapeutic responses and a molecular signature. J Neurooncol. 2014;117(2):261–268. [DOI] [PubMed] [Google Scholar]
- 16. Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5715. [DOI] [PubMed] [Google Scholar]
- 17. Pels H, Juergens A, Schirgens I, et al. Early complete response during chemotherapy predicts favorable outcome in patients with primary CNS lymphoma. Neuro Oncol. 2010;12(7):720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheson BD, Fisher RI, Barrington SF, et al. Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen-Them L, Costopoulos M, Tanguy ML, et al. French LOC Network for CNS Lymphoma. The CSF IL-10 concentration is an effective diagnostic marker in immunocompetent primary CNS lymphoma and a potential prognostic biomarker in treatment-responsive patients. Eur J Cancer. 2016;61:69–76. [DOI] [PubMed] [Google Scholar]
- 20. DeAngelis LM. Cerebral lymphoma presenting as a nonenhancing lesion on computed tomographic/magnetic resonance scan. Ann Neurol. 1993;33(3):308–311. [DOI] [PubMed] [Google Scholar]
- 21. Kitai R, Hashimoto N, Yamate K, et al. Lymphomatosis cerebri: clinical characteristics, neuroimaging, and pathological findings. Brain Tumor Pathol. 2012;29(1):47–53. [DOI] [PubMed] [Google Scholar]
- 22. Lai R, Rosenblum MK, DeAngelis LM. Primary CNS lymphoma: a whole-brain disease? Neurology. 2002;59(10):1557–1562. [DOI] [PubMed] [Google Scholar]
- 23. Adachi K, Yamaguchi F, Node Y, et al. Neuroimaging of primary central nervous system lymphoma in immunocompetent patients: comparison of recent and previous findings. J Nippon Med Sch. 2013;80(3):174–183. [DOI] [PubMed] [Google Scholar]
- 24. Palmedo H, Urbach H, Bender H, et al. FDG-PET in immunocompetent patients with primary central nervous system lymphoma: correlation with MRI and clinical follow-up. Eur J Nucl Med Mol Imaging. 2006;33(2):164–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.