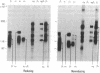
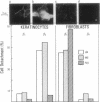
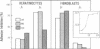Abstract
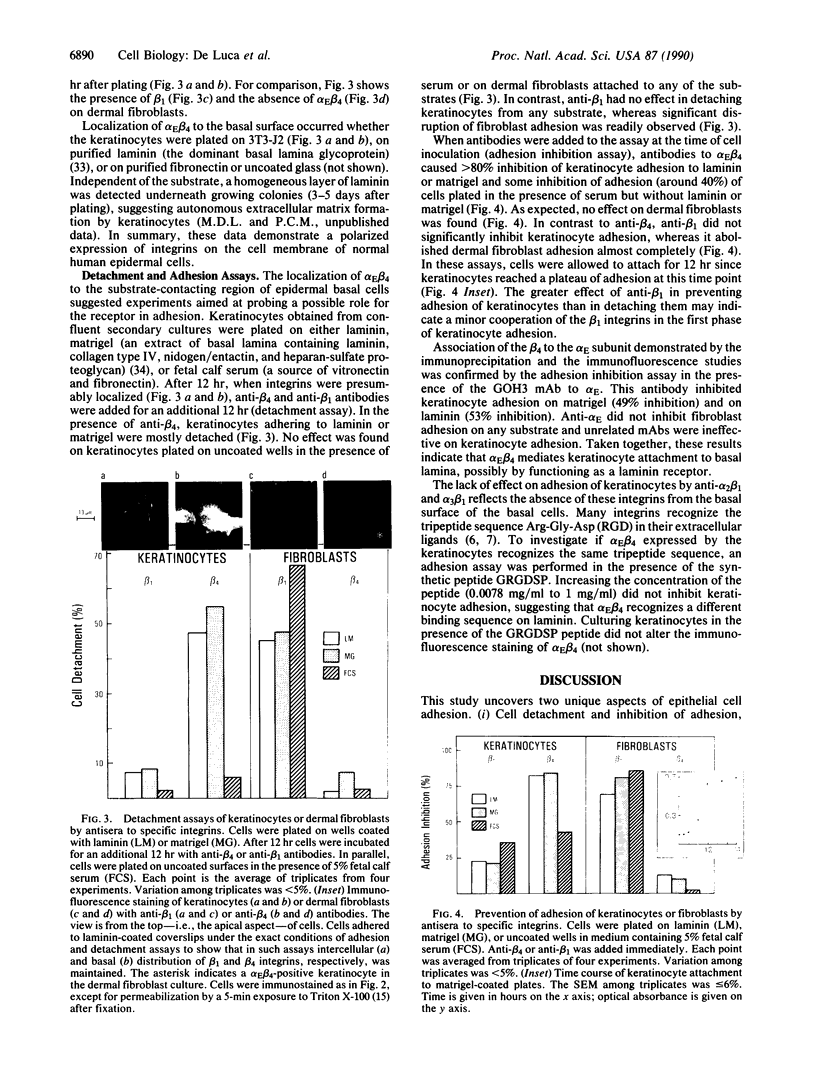
Epithelial cell interactions with matrices are critical to tissue organization. Indirect immunofluorescence and immunoprecipitations of cell lysates prepared from stratified cultures of human epidermal cells showed that the major integrins expressed by keratinocytes are alpha E beta 4 (also called alpha 6 beta 4) and alpha 2 beta 1. The alpha E beta 4 integrin is localized at the surface of basal cells in contact with the basement membrane, whereas alpha 2 beta 1/alpha 3 beta 1 integrins are absent from the basal surface and are localized only on the lateral surface of basal and spinous keratinocytes. Anti-beta 4 antibodies potently inhibited keratinocyte adhesion to matrigel or purified laminin, whereas anti-beta 1 antibodies were ineffective. Only anti-beta 4 antibodies were able to detach established keratinocyte colonies. These data suggest that alpha E beta 4 mediates keratinocyte adhesion to basal lamina, whereas the beta 1 subfamily is involved in cell-cell adhesion of keratinocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Compton C. C., Gill J. M., Bradford D. A., Regauer S., Gallico G. G., O'Connor N. E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989 May;60(5):600–612. [PubMed] [Google Scholar]
- Conforti G., Zanetti A., Colella S., Abbadini M., Marchisio P. C., Pytela R., Giancotti F., Tarone G., Languino L. R., Dejana E. Interaction of fibronectin with cultured human endothelial cells: characterization of the specific receptor. Blood. 1989 May 1;73(6):1576–1585. [PubMed] [Google Scholar]
- Cuono C., Langdon R., McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986 May 17;1(8490):1123–1124. doi: 10.1016/s0140-6736(86)91838-6. [DOI] [PubMed] [Google Scholar]
- De Luca M., Albanese E., Bondanza S., Megna M., Ugozzoli L., Molina F., Cancedda R., Santi P. L., Bormioli M., Stella M. Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns. 1989 Oct;15(5):303–309. doi: 10.1016/0305-4179(89)90007-7. [DOI] [PubMed] [Google Scholar]
- De Luca M., D'Anna F., Bondanza S., Franzi A. T., Cancedda R. Human epithelial cells induce human melanocyte growth in vitro but only skin keratinocytes regulate its proper differentiation in the absence of dermis. J Cell Biol. 1988 Nov;107(5):1919–1926. doi: 10.1083/jcb.107.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Vestweber D., Kemler R. Cell-matrix interactions and cell adhesion during development. Annu Rev Cell Biol. 1986;2:27–47. doi: 10.1146/annurev.cb.02.110186.000331. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Johnson M. H. From egg to epithelium. Annu Rev Cell Biol. 1988;4:459–485. doi: 10.1146/annurev.cb.04.110188.002331. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 Aug 16;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Remensnyder J. P., Kehinde O., Green H. Cultured epithelial autografts for giant congenital nevi. Plast Reconstr Surg. 1989 Jul;84(1):1–9. doi: 10.1097/00006534-198907000-00001. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. The keratinocyte as differentiated cell type. Harvey Lect. 1980;74:101–139. [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kajiji S. M., Davceva B., Quaranta V. Six monoclonal antibodies to human pancreatic cancer antigens. Cancer Res. 1987 Mar 1;47(5):1367–1376. [PubMed] [Google Scholar]
- Kajiji S., Tamura R. N., Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989 Mar;8(3):673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Martin G. R. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochemistry. 1983 Oct 11;22(21):4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Peltonen J., Akiyama S. K., Yamada S. S., Gralnick H. R., Uitto J., Yamada K. M. Novel function for beta 1 integrins in keratinocyte cell-cell interactions. J Cell Biol. 1990 Mar;110(3):803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M. R., Finkelstein J. L., Staiano-Coico L., Goodwin C. W., Shires G. T., Nolan E. E., Hefton J. M. Grafting of cultured allogeneic epidermis on second- and third-degree burn wounds on 26 patients. J Trauma. 1986 Nov;26(11):955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E., Sundelin J., Lind P., Peterson P. A. The cell attachment domain of fibronectin. Determination of the primary structure. J Biol Chem. 1982 Aug 25;257(16):9593–9597. [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Rettig W. J., Murty V. V., Mattes M. J., Chaganti R. S., Old L. J. Extracellular matrix-modulated expression of human cell surface glycoproteins A42 and J143. Intrinsic and extrinsic signals determine antigenic phenotype. J Exp Med. 1986 Nov 1;164(5):1581–1599. doi: 10.1084/jem.164.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Daams H., Van der Valk M. A., Hilkens J., Hilgers J. Development of mouse mammary gland: identification of stages in differentiation of luminal and myoepithelial cells using monoclonal antibodies and polyvalent antiserum against keratin. J Histochem Cytochem. 1986 Aug;34(8):1037–1046. doi: 10.1177/34.8.2426332. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Hogervorst F., Osterop A., Veltman F. E. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988 Oct 5;263(28):14030–14038. [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Teepe R. G., Kreis R. W., Koebrugge E. J., Kempenaar J. A., Vloemans A. F., Hermans R. P., Boxma H., Dokter J., Hermans J., Ponec M. The use of cultured autologous epidermis in the treatment of extensive burn wounds. J Trauma. 1990 Mar;30(3):269–275. doi: 10.1097/00005373-199003000-00004. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]