Abstract
With the median survival of 14.6 months following best available standard of care, malignant gliomas (MGs) remain one of the biggest therapeutic challenges of the modern time. Although the last several decades have witnessed tremendous advancement in our understanding of MG and evolution of many successful preclinical therapeutic strategies, even the most successful preclinical therapeutic strategies often fail to cross the phase I/II clinical trial threshold. One of the significant, but less commonly discussed, barriers in developing effective glioma therapy is the lack of a robust preclinical model. For the last 30 years, rodent orthotopic xenograft models have been extensively used in the preclinical setting. Although they provide a good basic model for understanding tumor biology, their value in successfully translating preclinical therapeutic triumph into clinical success is extremely poor. Companion dogs, which share the same environmental stress as their human counterparts, also spontaneously develop MGs. Dog gliomas that develop spontaneously in an immunocompetent host are very similar to human gliomas and potentially provide a stronger platform for validating the efficacy of therapeutic strategies proven successful in preclinical mouse models. Integrating this model can accelerate development of effective therapeutic options that will benefit both human subjects and pet dogs.
Keywords: canine glioma, comparative oncology, glioblastoma, malignant glioma, spontaneous glioma
Malignant gliomas (MGs) are high-grade brain tumors of glial origin that universally carry a poor prognosis. Several recent early-phase clinical studies have shown improved survival in selective groups of patients1,2; however, very few studies progressed beyond phase I/II clinical trials, and no novel interventions have even approached the threshold for challenging the current standard of care.
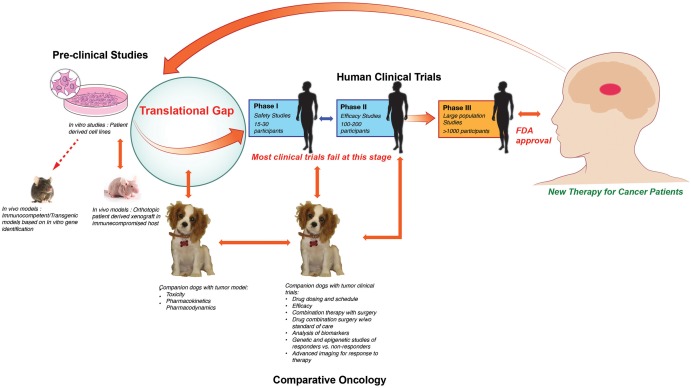
MGs are inherently heterogeneous, within patients as well as among patients, and poorly modeled by homogeneous xenografts in standardized hosts. There is a large “translational gap” between highly artificial, orthotopic xenograft glioma models in generally immunodeficient rodents of clonal origin versus spontaneously developing, highly heterogeneous, and constantly evolving natural human glioma. Moreover, the small size of these rodents limits imaging and is prohibitive to testing novel therapeutic strategies in conjunction with surgical resection, a key component of current standard of care.
Pet dogs share the same environment as their human counterparts and are also susceptible to various spontaneous malignancies that affect the human population, including MGs.3 These naturally occurring tumors in pet dogs are very similar to their human counterparts regarding their clinical presentation and pathophysiology. They carry similar natural history and prognosis condensed into the approximately 7 times shorter overall lifespan of dogs. In the US, cancer is diagnosed in almost 1 million dogs per year, and the incidence is rising.4 Dedicated pet owners strive to provide the highest level of health care to their companion dogs and actively seek out novel treatment options in the form of experimental therapies and clinical trials when available. These factors provide a unique opportunity to advance the care and understanding of cancer in both man and companion dogs. Out of this symbiotic relationship developed the discipline of comparative oncology, which integrates the study of naturally occurring tumors in animals into studies of human cancer biology and therapeutics (Fig. 1).4 Treatment of many tumor types, such as osteosarcoma, lymphoma, melanoma, etc,4 have directly benefited from this approach; however, it is not fully utilized in the field of brain tumors.
Fig. 1.
Translational gap. Most preclinical studies that progress to human clinical trial fail at phase I/II stage. This failure is most likely due to the large translational gap between a orthotopic xenograft murine model and a complex immunocompetent human host. Comparative oncology provides an ideal platform to bridge this gap.
Comparative Histology
On the basis of histological features, the World Health Organization (WHO) classifies astrocytomas into 4 grades: grade I (pilocytic astrocytoma), grade II (diffuse astrocytoma), grade III (anaplastic astrocytoma), and grade IV (glioblastoma [GBM]). Grade III and grade IV are considered high-grade gliomas or MGs.5 Human MGs are histologically characterized by higher cellularity, a greater degree of pleomorphism, and increased proliferation compared with lower-grade gliomas. Pseudopalisading necrosis and vascular proliferation are the hallmark characteristics of GBM.
Although there are no studies correlating glioma histology with survival in canine brain tumors to parallel the human grading system, to facilitate translational studies, it is standard veterinary practice to use the current human WHO grading system6 to grade canine gliomas.7 Similar to the human grading system, within the veterinary WHO system8 a low-grade astrocytoma is a diffusely infiltrating glioma composed of well-differentiated astrocytes without any signs of anaplasia, and low to moderate cellularity, whereas a high-grade astrocytoma is highly anaplastic, with high cellularity and heterogeneous histological appearance. As in humans, high-grade gliomas are overall more frequent than low-grade gliomas in dogs.7 Canine GBM shares many gross, microscopic, and immunohistochemical pathological features with human GBM,9,10 such as predominantly cerebral or thalamic location, with gross hemorrhage, necrosis, and cyst formation. Histological findings such as pleomorphic cells and coagulative necrosis, glomeruloid vascular proliferation, and pseudopalisading of neoplastic cells around necrotic areas are key features and are found to be less pronounced or absent in rodent xenograft models.9,10
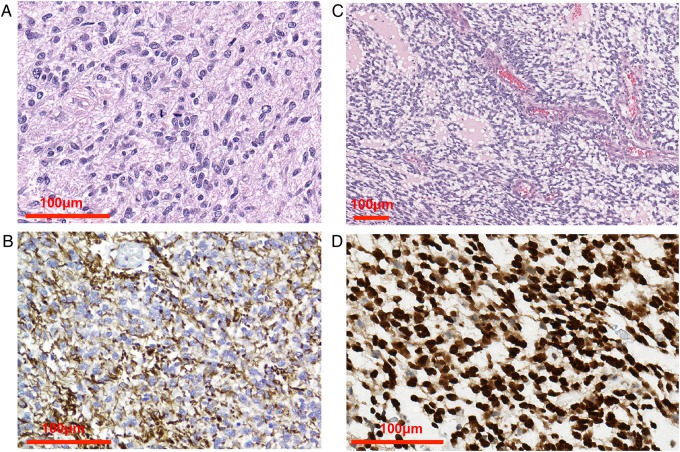
While grade IV astrocytoma is the most common human glioma, in dogs oligodendrogliomas (or oligoastrocytomas) occur roughly equally to astrocytomas,3,7,11,12 and GBM typically accounts for a minority of the astrocytoma cases.3,11 Canine astrocytoma occurs relatively evenly in each of grades II–IV, whereas most oligodendrogliomas are high grade (grade III).13,14 Extensive recurrent genomic losses of regions corresponding to human chromosome 1p/19q were not seen in any case of canine oligodendroglioma, indicating either a lack of evolutionary conservation or that 1p/19q translocation may occur without incurring genomic imbalance.7 As in humans, the differentiation of astrocytoma and oligodendroglioma can be assisted with glial fibrillary acidic protein (GFAP) and Olig2 immunohistochemistry (Fig. 2). Human and canine GBM show consistent expression of GFAP, which is more variable in rodent xenograft models.9 Most canine gliomas are already high grade when tissue analysis is first performed, and since reoperation for recurrence is not a common practice, there is not yet any evidence that GBM progresses from benign astrocytoma.15 Invasion of the normal brain is a key feature of canine and human GBM but is variable in rodent xenografts.9 Tumors grafted into rodents often form discrete masses at the injection site and have been criticized for compressing rather than invading the surrounding healthy brain.16
Fig. 2.
Canine glioma histology. (A) Anaplastic astrocytoma with pleomorphism, anisokaryosis, nuclear atypia, and mitotic activity. (B) GFAP staining of anaplastic astrocytoma. (C) High-grade oligodendroglioma composed of ovoid to fusiform cells and endothelial vascular proliferation. (D) Olig2 immunohistochemistry demonstrates strong nuclear positivity of more than 90% of neoplastic cells. Images courtesy of Dr Miller, Purdue Veterinary Medicine.
In order to fully integrate canine glioma models into neuro-oncological studies, canine histology grading has to be further advanced and refined to match the advances made in the context of human gliomas. The National Institutes of Health has recently launched a Comparative Brain Tumor Consortium, one of the first goals of which is to create an updated canine glioma grading and classification system for canine gliomas based on the current WHO human system, to improve comparison between human and canine glioma.17
Epidemiology of Brain Tumors in Humans and Dogs
According to the 2015 annual report of the Central Brain Tumor Registry of the United States, the incidence rate of MG is 7.23 per 100 000 people, accounting for a total of 117 023 patients, and an estimated 24 790 new cases of MG are expected to be diagnosed in 2016. The median age of diagnosis is 64 years, with peak incidence rates between 75 to 84 years. Thus in the US, with a growing aging population, the incidence rate is projected to increase.18 The MG incidence rate is 1.6 times higher in men compared with women and is also higher in Caucasians compared with other ethnic groups.
The only firmly established risk factor for MG is prior therapeutic high-dose radiation.19 One of the most interesting findings emerging over the past decade from epidemiological data is the statistically significant inverse association between prior history of allergies, chicken pox, presence of anti-varicella-zoster immunoglobulin (Ig)G or increased serum IgE and adult glioma.20 This indicates that MG develops on the backdrop of immune dysregulation and that the immune system plays a critical role in gliomagenesis and progression. This also highlights the most important deficiency of the commonly used preclinical model, the immunodeficient murine orthotopic xenograft model, and the possible reason why preclinical findings fail to predict clinical outcome.
Canine brain tumors, spontaneously originating in an immunocompetent host, have an estimated incidence rate of 20 cases/100 000 dogs/year.21 As in humans, dogs' nervous system cancers are thought to be the cause of 1%–3% of deaths,7 and brain tumors account for about 1 in 6 dogs presenting with intracranial disease.22 In dogs, gliomas represent over one-third of all primary brain tumors,11 second only to meningiomas. In addition, metastatic tumors are almost as common as primary brain tumors, with metastatic hemangiosarcoma and lymphoma being most common.3 As in people, glioma occurs in middle-age dogs continuing through into the geriatric population, with the peak age of diagnosis at 7–8 years.3 In the canine population, the major identifiable risk factor thus far is breed, with short-nosed (brachycephalic) breeds harboring the highest incidence. The most commonly presented breed is the Boxer dog, while Boston Terriers develop gliomas almost to the complete exclusion of other primary brain tumors.3,7,11 Although there is significant variation among breeds, within each dog breed there is significant inbreeding and genetic homogeneity; this genetic bottleneck within each breed could have limitations in the generalizability to humans. However, any 2 groups of dogs (even closely related breeds such as the Boxer and the Boston Terrier) exhibit considerably more phenotypic variation than any 2 groups of purpose-bred laboratory rodents.
Breed also appears to be connected to the glioma type. In the Boxer and French Bulldog, oligodendrogliomas are more common than astrocytomas, whereas the Boston Terrier has a statistically significant increased risk of both astrocytoma and oligodendroglioma.3,23 Male and female dogs are affected roughly equally.11,14,23
Currently, there is no standard-of-care treatment of brain tumors in dogs, and treatment modalities range from symptom management to surgery, radiation, and chemotherapy. Traditionally, most cases have been diagnosed presumptively on advanced imaging without histological confirmation and treated with symptomatic therapy or radiation therapy.24
The average survival of dogs receiving only symptomatic treatment (glucocorticoids and anticonvulsants) for primary brain tumors is around 2 months.25,26 Median survival with radiation is around 9–14 months for presumed gliomas and other intra-axial masses.14,27 A more recent study of stereotactic radiosurgery revealed a median survival of 15 months for 3 dogs with presumed or histologically confirmed glioma.28 However, dogs with glioma treated with CyberKnife may have a poorer prognosis compared with other tumor types.29 Although the median survival of dogs with glioma is higher than the median survival of around 17–48 days for rodents implanted with glioma,9,16 the former still represents a more economically attractive model when accounting for the cost associated with failed human clinical trials based on mouse preclinical data.
Comparison of Frequently Altered Pathways in Glioma
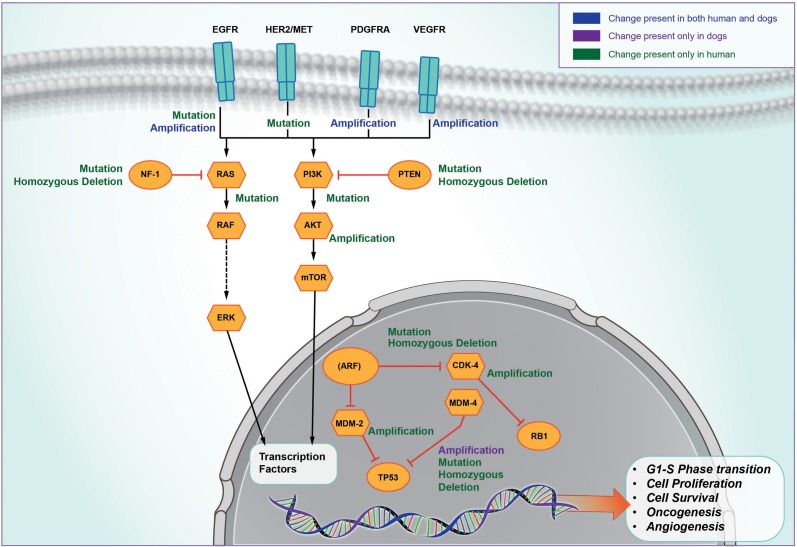
Systematic analysis of human GBM by The Cancer Genome Atlas network revealed that 3 critical pathways—p53, retinoblastoma signaling, and epidermal growth factor receptor (EGFR)/receptor tyrosine kinase /Ras/phosphatidylinositol-3 kinase—play a central role in human gliomagenesis.30 Shared molecular biology has been seen in many aspects of canine and human glioma (Fig. 3), often with a similar relationship with tumor grade.
Fig. 3.
Common signaling pathways. Spontaneously occurring canine glioma share several molecular abnormalities with human glioma. PDGFRA, platelet derived growth factor receptor alpha.
The p53 Pathway
Known as the “guardian of the genome,” the p53 pathway acts as the cellular gatekeeper in response to various stress-induced signaling, including DNA activation, hypoxia, and oncogenic activation; orchestrates biological counterresponses such as DNA repair, cell cycle arrest, and cellular senescence; and initiates apoptosis cascades in damaged cells.31 Because of its potent tumor suppressor activity, the majority of human cancers acquire mutations that abrogate the p53 function. In human GBM, a single nucleotide polymorphism–based analysis revealed that chromosome 17p containing p53 is the most significant region on loss of heterozygosity.32 About 70% of the 206 tested human samples harbored various kinds of somatic alteration in the p53 pathway. The p53 gene product in humans is 393 amino acids with an 87% sequence homology compared with 381 amino acids in the canine p53 gene product.33 In humans, about 90% of p53 mutations are located in the DNA binding domain (exon 4–8).34 The rates of overexpression of p53 show overlap between human and canine gliomas, whereas actual p53 mutation is more prevalent in human astrocytoma, especially in secondary GBM progressing from lower-grade tumors.33,35 There is overexpression of p53 based on immunohistochemistry in 35% of canine astrocytomas36 compared with around 60% of human astrocytomas depending upon the grade.37,38 However, mutations of p53 were found in just 2 cases of GBM out of a total of 30 canine astrocytomas analyzed across 2 studies.36,39 Mutations in p53 are present in 20%–40% of human astrocytomas32,37 and occur with increasing grade.38 However, these p53 mutations are found in only 10% of primary de novo human GBM, compared with around 65% of secondary GBM.38 A study by York and colleagues showed that only about 3.4% of canine samples had p53 exonic mutations compared with about 26% of human brain tumors.34,39 Twenty-five percent of all mutations in human brain tumor occur at the genomic hotspot within the critical DNA binding domain of the p53 protein, specifically human codons 273, 248, 175, and 245 in descending order of frequency. The codon 233 mutation that corresponds to codon mutation 245 in humans is the only similar hotspot mutation detected in the canine brain tumor.39 Additional mechanisms of p53 pathway inactivation identified in human GBM may also be responsible for an aberrant p53 pathway in canine GBM. DNA damaging agents such as doxorubicin induced expression of the p53 gene product and 3 other p53 family proteins in canine tumors, indicating that the function of p53 may be conserved between human and dog.40,41 Based on this, it is conceivable that additional mechanisms of p53 pathway inactivation besides those identified in human GBM may also be responsible for the aberrant p53 pathway in canine GBM.
Epidermal Growth Factor Receptor
Aberrant EGFR signaling is critical for many human cancers and has been extensively investigated in brain tumors. EGFR gene amplification and overexpression are associated with GBM.42 Hyperactive EGFR signaling can activate downstream Shc-Grb2-Ras signaling as well as phosphatidylinositol-3 kinase signaling cascades and regulate various critical cellular responses, such as apoptosis, angiogenesis, and aberrant cell proliferation.43 About 30%–40% of human GBM carries EGFR amplification, and 10% of GBM overexpress EGFR at the protein level without any gene amplification.44 A dominant EGFR mutation in human GBM is known as EGFR variant (v)III, which contains an in-frame deletion of 267 amino acids from the extracellular domain of wild-type EGFR and leads to abolishing the ligand binding capacity as well as constitutive activation of downstream signaling. Approximately 31% of human GBM overexpresses both wild-type EGFR and EGFRvIII.45 At the protein level, tissue microarray analysis in dogs revealed that about 57% of GBM, 40% of grade III astrocytomas, and 28% of grade II astrocytomas expressed an elevated level of EGFR.46 The canine orthologue of EGFR, located on chromosome 18, is increased in genomic copy number7; however, a canine counterpart of EGFRvIII has yet to be identified.
Vascular Endothelial Growth Factor
The vascular endothelial growth factor (VEGF) family of growth factors and associated receptors are considered critical for promoting angiogenesis in GBM. In human GBM, VEGF expression at the mRNA level not only is unregulated, but its expression is also directly correlated with the vascularity in the glioma.47 VEGF upregulation in human GBM is mediated by various signaling events associated with oncogenic transformation such as p53 or EGFR mutations.47 The human VEGF gene consists of 8 exons that produce 9 different VEGF isoforms via alternative splicing.48 VEGF165 and VEGF121 are the predominant isoform presence in human brain tumors.49 VEGF also correlates with canine glioma grade; an overlap exists between GBM and high-grade oligodendroglioma, with expression being significantly higher in MG compared with low-grade glioma or meningioma.50,51 Plasma levels are higher in astrocytoma compared with oligodendroglioma or meningioma, and highest in GBM.51 The expression of VEGF mRNA is significantly elevated in canine GBM compared with grade II.50 Expression of VEGF receptors 1 and 2 are also highest in GBM and high-grade oligodendroglioma.13 The canine counterpart of VEGF165, VEGF164 (one amino acid shorter), is the dominant isoform present in CNS tumors. Even though the VEGF mRNA level in canine GBM is significantly elevated, its expression at the protein level has yet to be investigated.
Immunology
Immunotherapy in the form of antiglioma vaccine, antibodies targeting immune checkpoints, or adoptive T-cell transfer is emerging as one of the most promising novel antiglioma treatment strategies.52 Immunocompetent spontaneous canine gliomas provide the ideal opportunity for further optimizing the promising outcomes of antiglioma immunotherapy.
Similarities between the canine and human immune systems and their involvement in cancer have been documented; although, much of this work has been done outside of brain tumors. For example, the EGFR family members ErbB1 and ErbB2 are molecules of homology between dogs and humans, and antibody targeting of these molecules results in the same signaling and biological effects.53 Similar conservations have been found in carcinoembryonic antigen receptor (99% sequence identity) and subtypes of transforming growth factor (TGF)–β and TGF-β receptors (>87% sequence identity). In both human and canine cancers, TGF-β is generally derived from Foxp3+ regulatory T cells (Tregs), and the appearance of Tregs negatively correlated with prognosis in dogs, as observed in human glioma patients.53,54 In the tumor microenvironment, TGF-β promotes tumorigenesis, by mediating proliferation, migration, invasion, and metastasis. In this respect, dogs are much closer to humans than are mice.53
Programmed death 1 (PD-1) is an immunoinhibitory receptor with shared biology between human and canine cancer.55 Together with its ligand (PD-L1), it can induce T-cell “exhaustion” and immune evasion by tumor cells. Analysis of the PD-L1 phylogenetic tree reveals that dogs diverged from humans more recently than rodents.55 Blockade of PD-1 to PD-L1 binding enhances interferon (IFN)-γ, and anti–PD-L1 antibody was reported to restore antitumor immunity and cause regression in a subset of human tumors.56 Similar results were seen in a canine cancer model, where anti–PD-L1 antibody blockade in mononuclear cell culture leads to enhanced IFN-γ production. PD-L1 was expressed on the same canine cancers as in human cancers.55 Studies are under way to assess whether canine glioma also shares PD-1 and PD-L1 expression patterns with human glioma.
Infiltration of macrophages and T cells occurs in canine GBM, as with human GBM.9 Both species express interleukin (IL)-13RA2 in varying degrees.57 In dogs and humans, expression was significantly higher in high-grade oligodendrogliomas and GBM compared with other brain tumor types but was virtually negative in normal brain. IL-13RA2 was identified as a treatment target, and monoclonal antibodies directed against it are currently being evaluated in a canine glioma clinical trial.58
Comparative Radiology
MRI plays a central role in the clinical diagnosis, grading, surgical planning, response to therapy, and tumor recurrence in MG. Standard T1-weighted sequences with and without contrast, fluid attenuated inversion recovery, and T2-weighted sequences are mainstays of glioma diagnosis and monitoring.59 MRI is critical to assessing tumor response to therapy, and several response criteria have been proposed that measure the change in the maximal area of contrast-enhancing tumor over time.60,61 One of the biggest challenges in neuro-oncology clinical practice today is the differentiation of treatment-related changes (particularly radiation-related) and true tumor progression, both of which result in an increase in contrast enhancement. In addition, recent development of anti-VEGF therapy that directly decreases tumor vascular permeability by targeting angiogenesis renders the monitoring criteria based on contrast enhancement alone ineffective. To keep up with the emerging novel treatment strategies for MG, several new imaging modalities are being developed and validated with the goal of effectively and noninvasively monitoring tumor response to therapy.59 Imaging studies for orthotopic xenograft glioma models are highly limited to basic tumor visualization and are completely ineffective for studying recurrence and pseudoprogression.
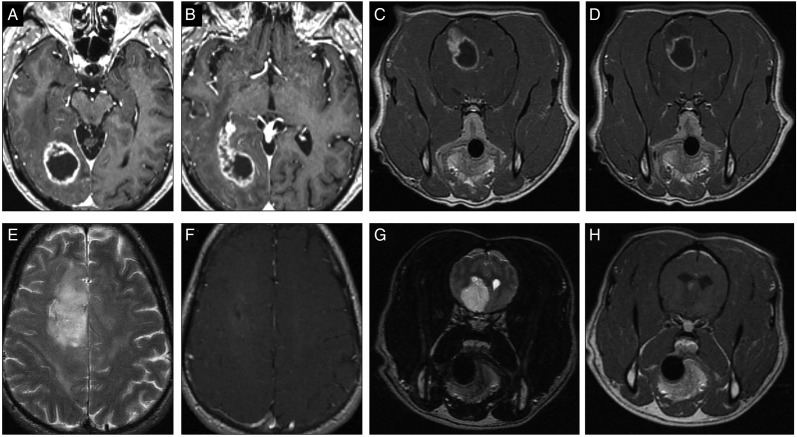
The MRI features of canine gliomas have been well studied.10,62,63 Several guiding criteria are available for differentiating brain tumors from cerebrovascular accidents and inflammatory syndromes.64,65 Common features of most canine gliomas include T2-hyperintensity and T1-isointensity to hypointensity12,63,66,67 (Fig. 4). Heterogeneous signal intensities are common and useful in predicting neoplasia over other similar MRI lesions.68 Most often, they have a clear intra-axial origin, but they may appear intraventricular or even extra-axial.69,70 Case-to-case variation is also seen in other characteristics, including variable intensity and pattern of contrast enhancement, the presence of gradient echo (T2* weighted) signal voids and cystic regions, the degree of peritumoral edema, the regularity of margins, and the degree of mass effect.12,63
Fig. 4.
Human and canine glioma on MRI imaging. (A, B) Classical human high-grade glioma with the hallmark ring-enhancing lesion. (C, D) Canine high-grade gliomas that can also show similar ring-enhancing pattern on contrast T1 sequence MRI. (E, F) Human oligodendroglioma with minimal to no contrast enhancement and T2 signal changes. (G, H) Canine oligodendroglioma with T2 signal changes and minimal contrast enhancement.
A canine glioma study demonstrated that MRI is more sensitive and correlated more effectively with microscopic findings than CT, which tends to underrepresent tumors.71 Similar to humans, the strongest and most reproducible predictor of high-grade glioma in dogs by MRI is the presence of contrast enhancement.12,63 Ring-like enhancement with a central nonenhancing core is seen in high-grade glioma but can also occur in low-grade glioma,10,12 mimicking the situation in humans.72 As in people, the detection of cystic regions and possible necrosis may also be of use in predicting a higher grade. Differentiating between astrocytoma and oligodendroglioma is less complete, but T1-hypointensity and ventricular distortion are more common in oligodendrogliomas, whereas peritumoral edema is more pronounced in astrocytoma.12 There is much overlap in the appearance of high-grade astrocytoma and high-grade oligodendroglioma.70 MR spectroscopy of dogs with brain tumors also mirrors results from human patients.73
Pseudoprogression on MRI has not been reported in dogs, although radiation-induced necrosis has74 (Fig. 5). At this time, there are no studies concerning the differentiation of progression and pseudoprogression for canine models. Early postoperative MRI findings have recently been reported,64 largely describing the same features seen in human patients,75 including thin borders of contrast enhancement at the edges of resection cavities and adjacent regions of restricted diffusion. Perfusion imaging (eg, dynamic contrast-enhanced MRI) has been used as a noninvasive method of differentiating brain tumor types65,74 and could prove useful in distinguishing between progression and pseudoprogression (in humans, perfusion imaging aids differentiation of treatment-related necrosis from recurrent glioma49). While pseudoprogression has not yet been reported in dogs, pseudoresponse associated with bevacizumab (reduction in contrast enhancement) has been observed, matching the MRI response seen in human studies.74 The first generation of guidelines for neuro-imaging in the assessment of treatment response is available for canine brain tumors, developed with translational clinical trials in mind.74
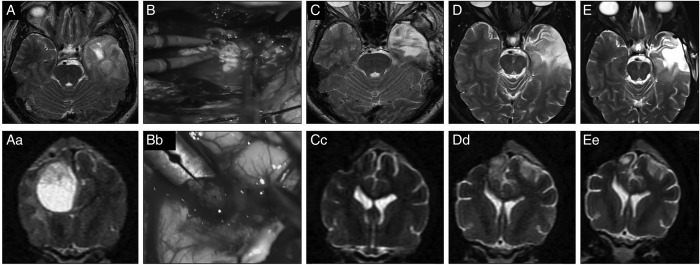
Fig. 5.
Effect of treatment on human and canine glioma on MRI imaging. Human and canine high-grade oligodendroglioma. (A, Ac) T2-weighted image, a hyperintense and heterogeneous mass is present. (B, Bc) Surgical photo during craniectomy and gross total resection. (C, Cc) Three-month postoperative MRI, complete remission. (D, Dc) Nine-month postoperative MRI, early recurrence. Stereotactic radiosurgery was performed. (E, Ec) Fifteen-month postoperative MRI (4 months after radiosurgery), a small, highly T2-hyperintense area is suggestive of encephalomalacia rather than persistent tumor.
Advantages and Limitations
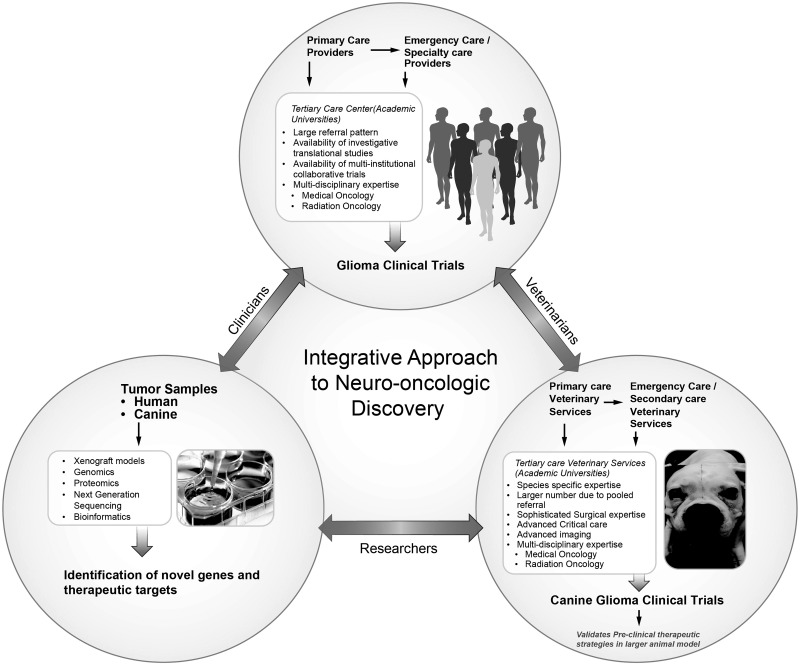
It has been proposed that veterinary clinical trials should take a position between preclinical research and human clinical trials76 (Fig. 6). A major proposed benefit is decreased failure rate in human clinical trials. Since there is no standard of care for treatment of cancer in dogs, this population provides a relatively less pretreated cohort compared with the human cancer patient population for testing novel therapeutic agents.
Fig. 6.
Model of integrative approach to neuro-oncology. Strategic collaboration between the basic science researchers, veterinarians, clinicians, and clinician scientists can provide a robust model for neuro-oncological discoveries and advancement that will benefit both human and canine populations.
Even with several limitations, the rodent xenograft model remains an effective and economical preclinical model for performing initial molecular studies. Murine models can also be used for dog glioma xenograft models to parallel the studies of human xenografts and delineate the molecular biology, including role of specific genes in regulating canine glioma. However, toxicities initially encountered in human phase I/II trials are not often initially identified in rodent studies, thus utilizing pet dogs may facilitate early detection of such side effects and save the larger cost associated with failed human clinical trials.77 Although canine glioma studies are more expensive and time-consuming compared with rodent models, they are within the cost range of other large animal toxicity studies necessitated for investigational new drug applications and may be more cost-effective if the cost of failed human clinical trials due to weak preclinical data is taken into consideration.4 One of the limitations of spontaneous canine glioma is that unlike the transgenic mouse model, it is not useful for studying the effect of single gene mutations and has limited experimental manipulation potential.
Another limitation of using a canine model for regular neuro-oncology studies is the availability of an adequate number of dogs with glioma for the studies on a regular basis. Studies suggest that there are 12 000 spontaneous canine brain tumors per year in the United States78 and that a veterinary teaching hospital could accrue 18–20 dogs per year for a GBM clinical trial.9 Thus strategic collaborations and organized referral patterns between veterinarians need to be developed to take full advantage of this model. In the true meaning of bridging the translational gap, the ideal place for the spontaneous canine model will be to validate and further develop the therapeutic strategies proven successful in preclinical mouse models before advancing into human clinical trials.
An ethical advantage of this approach over the experimental rodent model is that treatment studies do not involve induction of a disease state but rather involves treating a spontaneous disease and therefore ameliorating suffering and extending survival. Dogs who are not included in such studies face a median survival of just 2 months until euthanasia/death, when managed with symptomatic therapy due to owners' financial constraints. Pet dogs with primary brain tumors recruited to neuro-oncology trials (Table 1) may experience remissions lasting many months or over a year.58
Table 1.
Current canine clinical trials in malignant glioma
| Clinical Trial | Veterinary School | Enrollment Status |
|---|---|---|
| Surgery and Metronomic Chemotherapy for Brain Tumors in Dogs | Purdue | Open |
| The Treatment of Canine Brain Tumors with Temozolomide Incorporated into Poly Lactic-Co-Glycolic-Acid (PLGA) Microcylinders | University of Georgia | Open |
| The Treatment of Canine Brain Tumors with Cetuximab Administered Using Convection Enhanced Delivery (CED) | University of Georgia | Closed |
| Phase I Clinical Trial of Recombinant Newcastle Disease Virus for Canine Intracranial Meningiomas | Virginia-Maryland College of Veterinary Medicine | Closed |
| Molecular Combinatorial Therapy for Canine Malignant Gliomas | Virginia-Maryland Regional College of Veterinary Medicine | Open |
| Surgery and Chemotherapy, Surgery/Vaccine Therapy, Surgery/Gene Therapy and Combinations of the Three | University of Minnesota | Open |
| Immunotherapy Using Intravenously Administered, Encapsulated Micro-RNA (LUNAR-301) to Inhibit STAT3 Activity in Immune Cells | Texas A & M | Open |
| Convection-Enhanced Delivery of Liposomal CPT-11 with Real-Time MRI in Canine Primary Gliomas | University of California Davis | Closed |
| Toca 511 & Toca FC Gene Transfer with Surgical Resection & Radiation Therapy | University of California Davis | Open |
Conclusion
Spontaneous canine gliomas can effectively bridge the translational gap between preclinical mouse studies and human clinical trials. Developing a systematic and multidisciplinary approach to incorporate this model is likely to result in speedy development of effective antiglioma therapies and improved care for both human and canine glioma patients. Collaborative effort among research scientists, clinician scientists, clinicians, and veterinarians is essential for building multifaceted trials that systematically progress from preclinical stages to canine trials to successful human trials.
Funding
This work was supported by the NIH NRCDP K-12 (to M.D.) and the National Cancer Institute (R00 CA160775 to A.U.A).
Acknowledgments
The authors thank Christopher Brown, MS for his assistance with the image illustrations. The article is dedicated to the loving memory of two wonderful canines, Casper Dey and Magical Dey.
Conflict of interest statement. None declared.
References
- 1. Sampson JH, Heimberger AB, Archer GE et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prins RM, Soto H, Konkankit V et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song RB, Vite CH, Bradley CW et al. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J Vet Intern Med. 2013;27(5):1143–1152. [DOI] [PubMed] [Google Scholar]
- 4. Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8(2):147–156. [DOI] [PubMed] [Google Scholar]
- 5. Louis D, Duc ML, Reix P et al. Partial splenectomy for portal hypertension in cystic fibrosis related liver disease. Pediatr Pulmonol. 2007;42(12):1173–1180. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Ohgaki H, Wiestler OD et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas R, Duke SE, Wang HJ et al. ‘Putting our heads together’: insights into genomic conservation between human and canine intracranial tumors. J Neurooncol. 2009;94(3):333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koestner A, Bilzer T, Fatzer R et al. Histological Classification of Tumors of the Nervous System of Domestic Animals. Washington, DC: Armed Forces Institute of Pathology; 1999; Second Ser. [Google Scholar]
- 9. Candolfi M, Curtin JF, Nichols WS et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipsitz D, Higgins RJ, Kortz GD et al. Glioblastoma multiforme: clinical findings, magnetic resonance imaging, and pathology in five dogs. Vet Pathol. 2003;40(6):659–669. [DOI] [PubMed] [Google Scholar]
- 11. Snyder JM, Shofer FS, Van Winkle TJ et al. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med. 2006;20(3):669–675. [DOI] [PubMed] [Google Scholar]
- 12. Bentley RT, Ober CP, Anderson KL et al. Canine intracranial gliomas: relationship between magnetic resonance imaging criteria and tumor type and grade. Vet J. 2013;198(2):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dickinson PJ, Roberts BN, Higgins RJ et al. Expression of receptor tyrosine kinases VEGFR-1 (FLT-1), VEGFR-2 (KDR), EGFR-1, PDGFRalpha and c-Met in canine primary brain tumours. Vet Comp Oncol. 2006;4(3):132–140. [DOI] [PubMed] [Google Scholar]
- 14. Bley CR, Sumova A, Roos M et al. Irradiation of brain tumors in dogs with neurologic disease. J Vet Intern Med. 2005;19(6):849–854. [DOI] [PubMed] [Google Scholar]
- 15. Johnson GC, Coates JR, Wininger F. Diagnostic immunohistochemistry of canine and feline intracalvarial tumors in the age of brain biopsies. Vet Pathol. 2014;51(1):146–160. [DOI] [PubMed] [Google Scholar]
- 16. Inoue S, Ichikawa T, Kurozumi K et al. Novel animal glioma models that separately exhibit two different invasive and angiogenic phenotypes of human glioblastomas. World Neurosurg. 2012;78(6):670–682. [DOI] [PubMed] [Google Scholar]
- 17. LeBlanc A, Mazcko C, Brown DB et al. Creation of an NCI Comparative Brain Tumor Consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro-Oncol (in press) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thakkar JP, Dolecek TA, Horbinski C et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109(1):93–108. [DOI] [PubMed] [Google Scholar]
- 20. Schwartzbaum J, Ahlbom A, Malmer B et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer Res. 2005;65(14):6459–6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dobson JM, Samuel S, Milstein H et al. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43(6):240–246. [DOI] [PubMed] [Google Scholar]
- 22. Fluehmann G, Doherr MG, Jaggy A. Canine neurological diseases in a referral hospital population between 1989 and 2000 in Switzerland. J Small Anim Pract. 2006;47(10):582–587. [DOI] [PubMed] [Google Scholar]
- 23. Stoica G, Levine J, Wolff J, Murphy K. Canine astrocytic tumors: a comparative review. Vet Pathol. 2011;48(1):266–275. [DOI] [PubMed] [Google Scholar]
- 24. Spugnini EP, Thrall DE, Price GS et al. Primary irradiation of canine intracranial masses. Vet Radiol Ultrasound. 2000;41(4):377–380. [DOI] [PubMed] [Google Scholar]
- 25. Rossmeisl JH Jr, Jones JC, Zimmerman KL et al. Survival time following hospital discharge in dogs with palliatively treated primary brain tumors. J Am Vet Med Assoc. 2013;242(2):193–198. [DOI] [PubMed] [Google Scholar]
- 26. Van Meervenne S, Verhoeven PS, de Vos J et al. Comparison between symptomatic treatment and lomustine supplementation in 71 dogs with intracranial, space-occupying lesions. Vet Comp Oncol. 2014;12(1):67–77. [DOI] [PubMed] [Google Scholar]
- 27. Brearley MJ, Jeffery ND, Phillips SM et al. Hypofractionated radiation therapy of brain masses in dogs: a retrospective analysis of survival of 83 cases (1991–1996). J Vet Intern Med. 1999;13(5):408–412. [DOI] [PubMed] [Google Scholar]
- 28. Mariani CL, Schubert TA, House RA et al. Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol. 2015;13(4):409–423. [DOI] [PubMed] [Google Scholar]
- 29. Charney S, Witten M, Berg J et al. CyberKnife radiosurgery for irradiation of brain tumors in dogs and cats. In: Proceedings of the American College of Veterinary Internal Medicine Forum; 2010. [Google Scholar]
- 30. Shankar GM, Francis JM, Rinne ML et al. Rapid intraoperative molecular characterization of glioma. JAMA Oncol. 2015;1(5):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–331. [DOI] [PubMed] [Google Scholar]
- 32. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Chen X, Kent MS et al. Establishment of a dog model for the p53 family pathway and identification of a novel isoform of p21 cyclin-dependent kinase inhibitor. Mol Cancer Res. 2009;7(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petitjean A, Mathe E, Kato S et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–629. [DOI] [PubMed] [Google Scholar]
- 35. von Deimling A, von Ammon K, Schoenfeld D et al. Subsets of glioblastoma multiforme defined by molecular genetic analysis. Brain Pathol. 1993;3(1):19–26. [DOI] [PubMed] [Google Scholar]
- 36. Stoica G, Kim HT, Hall DG et al. Morphology, immunohistochemistry, and genetic alterations in dog astrocytomas. Vet Pathol. 2004;41(1):10–19. [DOI] [PubMed] [Google Scholar]
- 37. Hwang SL, Hong YR, Sy WD et al. Expression and mutation analysis of the p53 gene in astrocytoma. J Formos Med Assoc. 1999;98(1):31–38. [PubMed] [Google Scholar]
- 38. Lang FF, Miller DC, Koslow M et al. Pathways leading to glioblastoma multiforme: a molecular analysis of genetic alterations in 65 astrocytic tumors. J Neurosurg. 1994;81(3):427–436. [DOI] [PubMed] [Google Scholar]
- 39. York D, Higgins RJ, LeCouteur RA et al. TP53 mutations in canine brain tumors. Vet Pathol. 2012;49(5):796–801. [DOI] [PubMed] [Google Scholar]
- 40. Rungsipipat A, Tateyama S, Yamaguchi R et al. Immunohistochemical analysis of c-yes and c-erbB-2 oncogene products and p53 tumor suppressor protein in canine mammary tumors. J Vet Med Sci. 1999;61(1):27–32. [DOI] [PubMed] [Google Scholar]
- 41. Jaffe MH, Hosgood G, Taylor HW et al. Immunohistochemical and clinical evaluation of p53 in canine cutaneous mast cell tumors. Vet Pathol. 2000;37(1):40–46. [DOI] [PubMed] [Google Scholar]
- 42. Gullick WJ. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991;47(1):87–98. [DOI] [PubMed] [Google Scholar]
- 43. Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. [DOI] [PubMed] [Google Scholar]
- 44. Watanabe K, Tachibana O, Sata K et al. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6(3):217–223. discussion 223–224. [DOI] [PubMed] [Google Scholar]
- 45. Heimberger AB, Hlatky R, Suki D et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–1466. [DOI] [PubMed] [Google Scholar]
- 46. Higgins RJ, Dickinson PJ, LeCouteur RA et al. Spontaneous canine gliomas: overexpression of EGFR, PDGFRalpha and IGFBP2 demonstrated by tissue microarray immunophenotyping. J Neurooncol. 2010;98(1):49–55. [DOI] [PubMed] [Google Scholar]
- 47. Samoto K, Ikezaki K, Ono M et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55(5):1189–1193. [PubMed] [Google Scholar]
- 48. Bates DO, Cui TG, Doughty JM et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62(14):4123–4131. [PubMed] [Google Scholar]
- 49. Berkman RA, Merrill MJ, Reinhold WC et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest. 1993;91(1):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dickinson PJ, Sturges BK, Higgins RJ et al. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol. 2008;45(2):131–139. [DOI] [PubMed] [Google Scholar]
- 51. Rossmeisl JH, Duncan RB, Huckle WR et al. Expression of vascular endothelial growth factor in tumors and plasma from dogs with primary intracranial neoplasms. Am J Vet Res. 2007;68(11):1239–1245. [DOI] [PubMed] [Google Scholar]
- 52. Calinescu AA, Kamran N, Baker G et al. Overview of current immunotherapeutic strategies for glioma. Immunotherapy. 2015;7(10):1073–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jensen-Jarolim E, Fazekas J, Singer J et al. Crosstalk of carcinoembryonic antigen and transforming growth factor-beta via their receptors: comparing human and canine cancer. Cancer Immunol Immunother. 2015;64(5):531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim JH, Hur JH, Lee SM et al. Correlation of Foxp3 positive regulatory T cells with prognostic factors in canine mammary carcinomas. Vet J. 2012;193(1):222–227. [DOI] [PubMed] [Google Scholar]
- 55. Maekawa N, Konnai S, Ikebuchi R et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-gamma production from tumor-infiltrating cells by PD-L1 blockade. PloS One. 2014;9(6):e98415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brahmer JR, Tykodi SS, Chow LQ et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Debinski W, Dickinson P, Rossmeisl JH et al. New agents for targeting of IL-13RA2 expressed in primary human and canine brain tumors. PloS One. 2013;8(10):e77719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rossmeisl JH. New treatment modalities for brain tumors in dogs and cats. Vet Clin North Am Small Anim Pract. 2014;44(6):1013–1038. [DOI] [PubMed] [Google Scholar]
- 59. Kalpathy-Cramer J, Gerstner ER, Emblem KE et al. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res. 2014;74(17):4622–4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 61. Macdonald DR, Cascino TL, Schold SC Jr et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 62. Bentley RT, Burcham GN, Heng HG et al. A comparison of clinical, magnetic resonance imaging and pathological findings in dogs with gliomatosis cerebri, focusing on cases with minimal magnetic resonance imaging changes. Vet Comp Oncol. 2014; doi:10.1111/vco.12106. [DOI] [PubMed] [Google Scholar]
- 63. Young BD, Levine JM, Porter BF et al. Magnetic resonance imaging features of intracranial astrocytomas and oligodendrogliomas in dogs. Vet Radiol Ultrasound. 2011;52(2):132–141. [DOI] [PubMed] [Google Scholar]
- 64. Chow KE, Tyrrell D, Long SN. Early postoperative magnetic resonance imaging findings in five dogs with confirmed and suspected brain tumors. Vet Radiol Ultrasound. 2015;56(5):531–539. [DOI] [PubMed] [Google Scholar]
- 65. Zhao Q, Lee S, Kent M et al. Dynamic contrast-enhanced magnetic resonance imaging of canine brain tumors. Vet Radiol Ultrasound. 2010;51(2):122–129. [DOI] [PubMed] [Google Scholar]
- 66. Wisner ER, Dickinson PJ, Higgins RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound. 2011;52(1 Suppl 1):S52–S61. [DOI] [PubMed] [Google Scholar]
- 67. Kraft SL, Gavin PR, DeHaan C et al. Retrospective review of 50 canine intracranial tumors evaluated by magnetic resonance imaging. J Vet Intern Med. 1997;11(4):218–225. [DOI] [PubMed] [Google Scholar]
- 68. Young BD, Fosgate GT, Holmes SP et al. Evaluation of standard magnetic resonance characteristics used to differentiate neoplastic, inflammatory, and vascular brain lesions in dogs. Vet Radiol Ultrasound. 2014;55(4):399–406. [DOI] [PubMed] [Google Scholar]
- 69. Rissi DR, Levine JM, Eden KB et al. Cerebral oligodendroglioma mimicking intraventricular neoplasia in three dogs. J Vet Diagn Invest. 2015;27(3):396–400. [DOI] [PubMed] [Google Scholar]
- 70. Bentley RT. Magnetic resonance imaging diagnosis of brain tumors in dogs. Vet J. 2015;205(2):204–216. [DOI] [PubMed] [Google Scholar]
- 71. Whelan HT, Clanton JA, Wilson RE et al. Comparison of CT and MRI brain tumor imaging using a canine glioma model. Pediatr Neurol. 1988;4(5):279–283. [DOI] [PubMed] [Google Scholar]
- 72. Jenkinson MD, Du Plessis DG, Walker C et al. Advanced MRI in the management of adult gliomas. Br J Neurosurg. 2007;21(6):550–561. [DOI] [PubMed] [Google Scholar]
- 73. Mikoloski K, March P, Faissler D. Diagnostic value and discriminatory ability of proton magnetic resonance spectroscopy for intracranial neoplasia in dogs. Proc Am Coll Vet Intern Med Forum. 2012;26(3):1111–1117. [Google Scholar]
- 74. Rossmeisl JH Jr, Garcia PA, Daniel GB et al. Invited review—neuroimaging response assessment criteria for brain tumors in veterinary patients. Vet Radiol Ultrasound. 2014;55(2):115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hygino da Cruz LC Jr, Rodriguez I, Domingues RC et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kol A, Arzi B, Athanasiou KA et al. Companion animals: translational scientist's new best friends. Sci Transl Med. 2015;7(308):308ps321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen L, Zhang Y, Yang J et al. Vertebrate animal models of glioma: understanding the mechanisms and developing new therapies. Biochim Biophys Acta. 2013;1836(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer Res. 2007;67(10):4541–4544. [DOI] [PubMed] [Google Scholar]