Abstract
Background.
Molecular markers provide important biological and clinical information related to the classification of brain tumors, and the integration of relevant molecular parameters into brain tumor classification systems has been a widely discussed topic in neuro-oncology over the past decade. With recent advances in the development of clinically relevant molecular signatures and the 2016 World Health Organization (WHO) update, the views of the neuro-oncology community on such changes would be informative for implementing this process.
Methods.
A survey with 8 questions regarding molecular markers in tumor classification was sent to an email list of Society for Neuro-Oncology members and attendees of prior meetings (n=5065). There were 403 respondents. Analysis was performed using whole group response, based on self-reported subspecialty.
Results.
The survey results show overall strong support for incorporating molecular knowledge into the classification and clinical management of brain tumors. Across all 7 subspecialty groups, ≥70% of respondents agreed to this integration. Interestingly, some variability is seen among subspecialties, notably with lowest support from neuropathologists, which may reflect their roles in implementing such diagnostic technologies.
Conclusion.
Based on a survey provided to the neuro-oncology community, we report strong support for the integration of molecular markers into the WHO classification of brain tumors, as well as for using an integrated “layered” diagnostic format. While membership from each specialty showed support, there was variation by specialty in enthusiasm regarding proposed changes.
The initial results of this survey influenced the deliberations underlying the 2016 WHO classification of tumors of the central nervous system.
Keywords: classification, molecular markers, pathology.
Brain tumors were, until recently, classified according to the 2007 World Health Organization (WHO) Classification of Tumours of the Central Nervous System (CNS WHO), which principally relied on histological features for classification and grading.1,2 Recent advances in the development of clinically relevant molecular signatures over the past decade have, however, strongly suggested that appropriate molecular markers should be added to histological features in any updated WHO classification.3,4 Currently a number of molecular markers are recognized to be of clinical value and consequently have been incorporated into routine practice in many centers, although strictly speaking, they are not required components in the current WHO classification. The status of 1p/19q codeletion is used at many centers as a classification marker in gliomas and is often but not invariably associated with oligodendroglioma histology.5–8 While codeletion of 1p and 19q is highly correlated with oligodendroglioma, 1p/19q codeletion may also be a predictive marker for chemotherapy and patient outcome, and therefore it has been used as a key for therapeutic decision making.9–13 Another key recently identified molecular marker is the presence of isocitrate dehydrogenase (IDH) mutation, where IDH mutation (mut) is being recognized as correlating with a distinct biology that is most clinically relevant to a favorable prognosis, compared with IDH wild-type (wt) tumors, in diffuse gliomas of similar grade, despite histological similarities.14 Similarly, in glioblastoma multiforme (GBM) the majority of tumors (90%–95%) are IDH-wt, but those that are IDH-mut have an improved prognosis, which could raise the question as to whether IDH-mut and -wt GBM should be classified together and given a similar malignancy grade.15 Conversely, while most lower-grade gliomas (WHO grades II and III) are IDH-mut, many of those that are IDH-wt have biological characteristics and prognosis that approach that of GBM, raising the question as to the relationship of grading of IDH-wt anaplastic astrocytoma (AA) relative to IDH-mut AA.16,17 Presently the use of the mutant-specific IDH1 R132H immunohistochemical marker has evolved as an expected diagnostic test in management of gliomas, and therefore consideration to formally integrate this molecular test into WHO classification is timely. As an additional important and emerging concept, pediatric gliomas of similar histological appearance are known to have distinct molecular genetic changes compared with adult glioma, raising the question as to whether they should be “lumped together” with adult gliomas.18,19
In advance of the WHO Consensus and Editorial Meeting in 2015, we reasoned that knowing the views of the neuro-oncology community about such changes would be informative for advising the WHO updating process. We therefore undertook a survey of the neuro-oncology community, spanning all major disciplines of the field and utilizing the resources of the Society for Neuro-Oncology (SNO). We therein conducted a survey through SNO in anticipation of the 2016 updated CNS WHO. We believe that the results of this survey would inform the application of the classification as the neuro-oncology community begins to implement the changes in the 2016 CNS WHO.
The goal of this survey was to assess the views of the neuro-oncology community in relation to the introduction of molecular markers as a component of the classification of brain tumors. As the classification is updated and revised, we hope that the impact of this survey will in part reflect the opinions of the neuro-oncology community, which will bear on future iterations of the WHO classification moving forward.
Methods
Materials and Procedure
The survey was developed according to the framework initiated as a result of the update for the WHO classification of CNS tumors, with a particular focus on the integration of molecular information into tumor classification. A link to a survey of 8 questions (Supplementary Table S1) regarding the role of molecular markers in tumor classification was sent to an email list following the 2014 19th Annual SNO Scientific Meeting. All members of SNO received the survey via the registered emails. All responses were anonymous. Tests to compare results between subspecialties were performed using either chi-square or Fisher’s exact test (both 2-tailed). Numerical values of the survey responses are available in Supplementary Table S2.
Participants
The survey was distributed to an email list (n=5065) that included SNO members and previous SNO Scientific Meeting attendees. The email message requested participation in the study, ensuring anonymity and providing a hyperlink to SurveyMonkey. Following an initial email request, a second reminder request was sent 2 weeks later. Data were collected 2 weeks following this second request. There were 403 respondents to the survey. Respondents were asked to report their subspecialty. Self-reported subspecialties of the respondents consisted of neuro-oncology (n=139), neurosurgery (n=106), research (n=37), neuropathology (n=36), radiation oncology (n=34), medical oncology (n=27), nursing/allied health (n=5), neuro-radiology (n=2), and other (“please specify”) (n=17). Based on this distribution, we combined the 5 nursing/allied health respondents and 2 neuro-radiology respondents into the “other” category for analytic purposes.
Self-reported degree status was recorded (MD vs PhD vs MD/PhD vs Other). There were 224 respondents reporting a degree status of “MD or Equivalent,” 119 reporting a degree status of “MD/PhD,” 45 reporting a degree status of “PhD,” and 14 reporting “Other.” A description of the respondents is shown in Table 1.
Table 1.
A description of the survey respondents
| Total | ||
|---|---|---|
| Degree | MD or equivalent | 224 |
| MD/PhD or equivalent dual degree | 119 | |
| PhD | 45 | |
| Other | 14 | |
| Not reported | 1 | |
| Subspecialty | Neuro-oncology | 139 |
| Neurosurgery | 106 | |
| Research | 37 | |
| Neuropathology | 36 | |
| Radiation oncology | 34 | |
| Medical oncology | 27 | |
| Other | 24 |
Results
The results of the survey are presented here based on the responses received for each individual question.
Is our knowledge of molecular classification of brain tumors at a level where it can be integrated into the WHO classification system?
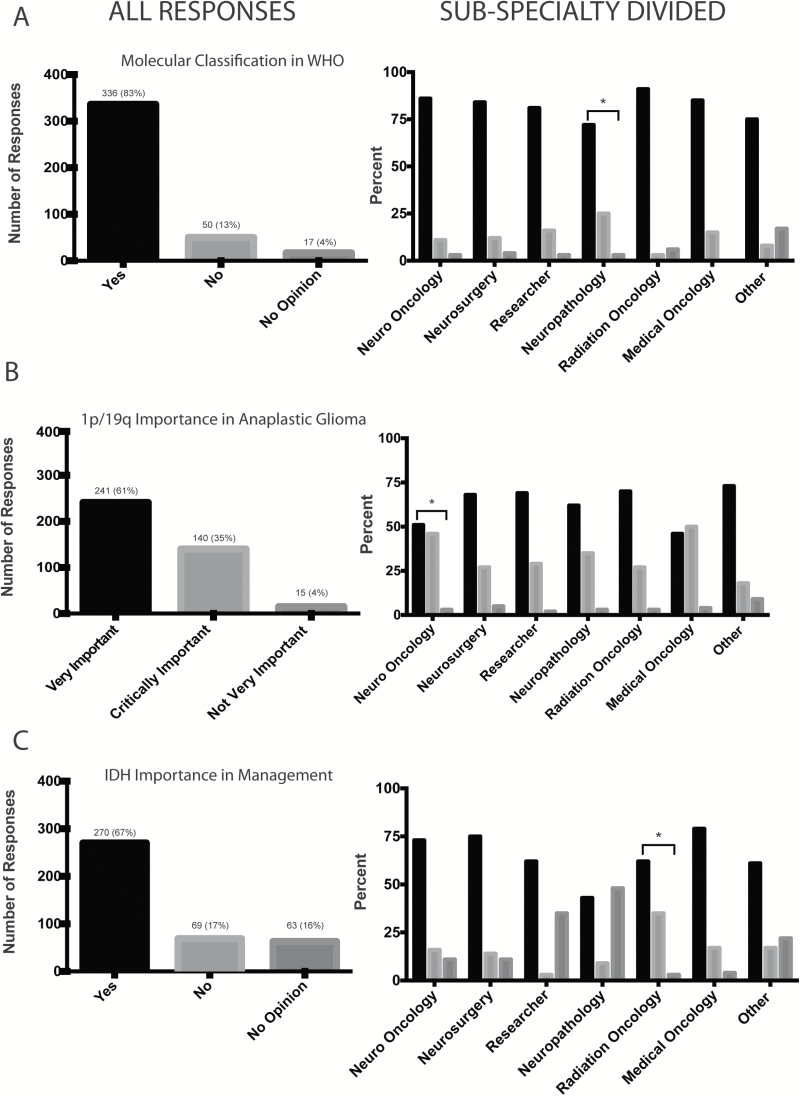
The majority of respondents agreed (n=336/403, 83%) with the integration of molecular markers as a component of the WHO classification (Fig. 1A). There was overall consistency among subspecialty groups, with approximately >70% of respondents in favor of WHO considering inclusion of a molecular based classification model. The variability among subspecialties is shown in Fig. 1A. Respondents who self-identified as neuropathologists showed a slightly lower rate of agreement (72%) for the integration of molecular markers compared with the remainder of respondents (84%) among the other subspecialties.
Fig. 1.
Responses to the survey divided into whole group response (left) and response according to self-identified subspecialty (right). Responses within each subspecialty were compared with responses of the remainder of the respondents and noted with an asterix if significantly different (P<.05 by either chi-square or Fisher’s exact test). (A) Responses to whether knowledge of molecular classification of brain tumors is at a level where it can be integrated into the WHO classification system (n=403). (B) Responses to address how important the incorporation of 1p/19q testing into the classification system is for patient management of grade III gliomas (n=396). (C) Responses to whether IDH mutation testing is as important in determining treatment and/or management decisions for patients with diffuse glioma (n=402).
If the result of 1p/19q testing were incorporated into the classification system, how important would this information be for patient management for grade III glioma?
Respondents were asked to select among agreeing that this was “Critically important: patient management would be driven by the 1p/19q result, and this result would ‘trump’ histologic classification”; “Very important: management would be based on both histologic diagnosis and 1p/19q status”; or “Not very important: the patient would be treated according to the histologic diagnosis.” Ninety-six percent of respondents (n=381/396) answered that 1p/19q testing was either critically or very important for the management of patients diagnosed with grade III gliomas. Of those in favor of 1p/19q testing, 35% (n=140/396) voted that 1p/19q testing should supersede histological classification, while 61% (n=241/396) voted that patient management should be based on both this molecular marker and histological classification of all subspecialties (Fig. 1B). Neuro-oncologists and medical oncologists showed the highest rate of response to this question as “critically important” compared with other subspecialties, and the responses from neuro-oncologists were significantly different (P<.05) than the remainder of the respondents’. Only 4% of the total respondents (15/396) said that 1p/19q testing is “not very important” in clinical decision making.
Do you consider the results of IDH mutation testing important in determining treatment and/or management decisions for patients with diffuse glioma?
Respondents were asked to state whether they agreed, disagreed, or had no opinion. Two hundred seventy out of the 402 respondents (67%) selected yes, indicating their belief that IDH status is important for determining treatment and/or management for patients with diffuse gliomas. The remainder of the respondents voted equally between having no opinion (n=63/402, 16%) on the importance of IDH-mut testing compared with 17% (69/402) disagreeing with the statement that IDH-mut testing is an important factor in determining treatment and/or management (Fig. 1C). Neuropathologists and radiation oncologists answered this question “yes” at a lower rate than respondents in other specialties.
If IDH-wt and IDH-mut AA are considered the same grade (grade III) by WHO, would you still change treatment and overall management and approach based on the IDH result?
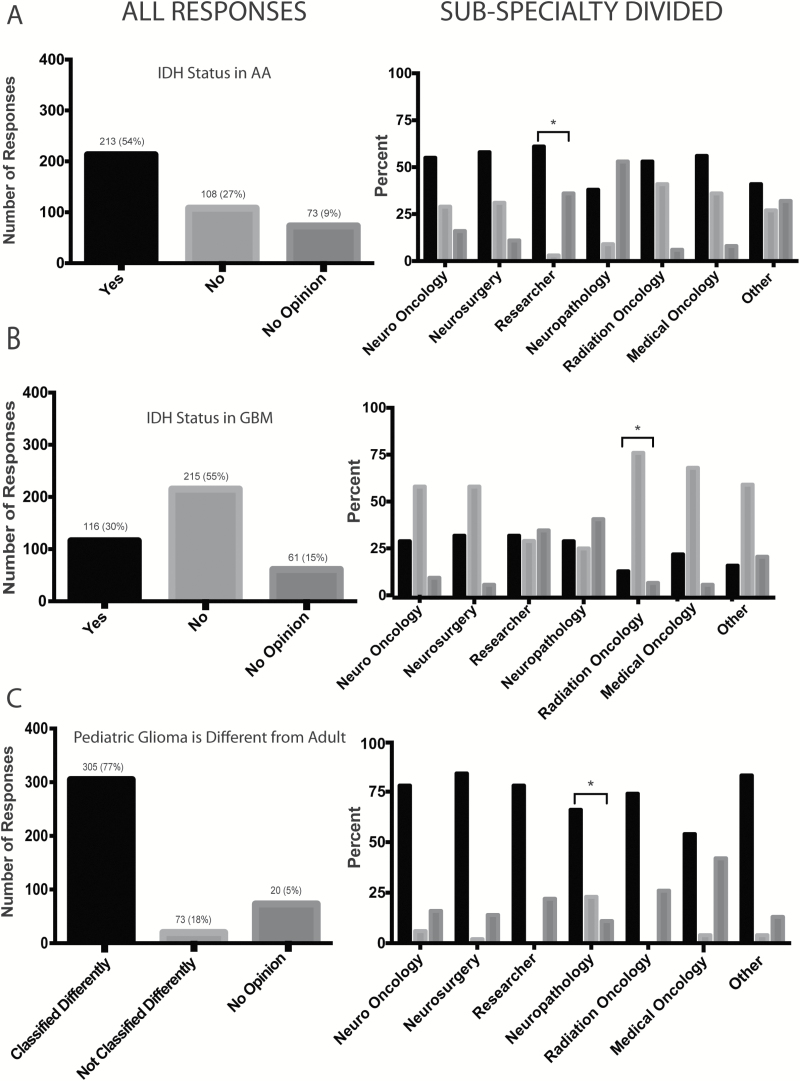
Slightly over half of the respondents (n=213/394, 54%) agreed that they would change patient treatment and overall management based on IDH status (Fig. 2A). One hundred and eight (27%) indicated “no” to this question, with 19% of the overall votes (n=73/394) expressing no opinion. Respondents who self-identified as researchers showed a slightly higher rate of “yes” responses than the remainder of respondents.
Fig. 2.
(A) Responses to whether treatment should change the overall management and approach of grade III AA IDH-wt vs IDH-mut tumors (n=394). (B) Responses addressing whether the treatment and overall management of GBM should be affected based on IDH mutation status (n=392). (C) Responses to whether pediatric gliomas should be classified differently than their histologically similar adult counterparts due to their distinct molecular profile (n=398).
If IDH-wt and IDH-mut GBM are considered the same grade (grade IV) by WHO, would you still change treatment and overall management and approach based on the IDH result?
Less than one-third of respondents (116/392, 30%) indicated that they would alter management for GBM based on the IDH result. Slightly over half of the respondents (n=215/392, 55%) stated that they would not change treatment and overall management based on the IDH result, and the remainder (61/392, 15%) expressed no opinion (Fig. 2B). Radiation oncologists tended to respond “no” more often than the remainder of respondents.
Diffuse gliomas in the pediatric population do not show molecular findings seen in histologically similar adult counterparts. Based on these considerations, would you classify pediatric gliomas differently from histologically similar adult counterparts?
The majority of survey respondents, 77% (305/398), were in favor of classifying pediatric gliomas differently from adult gliomas according to WHO criteria, given the differences in molecular profiles (Fig. 2C).
If the results of MGMT testing were included in the classification of GBM, would this affect the approach and management of elderly patients with GBM?
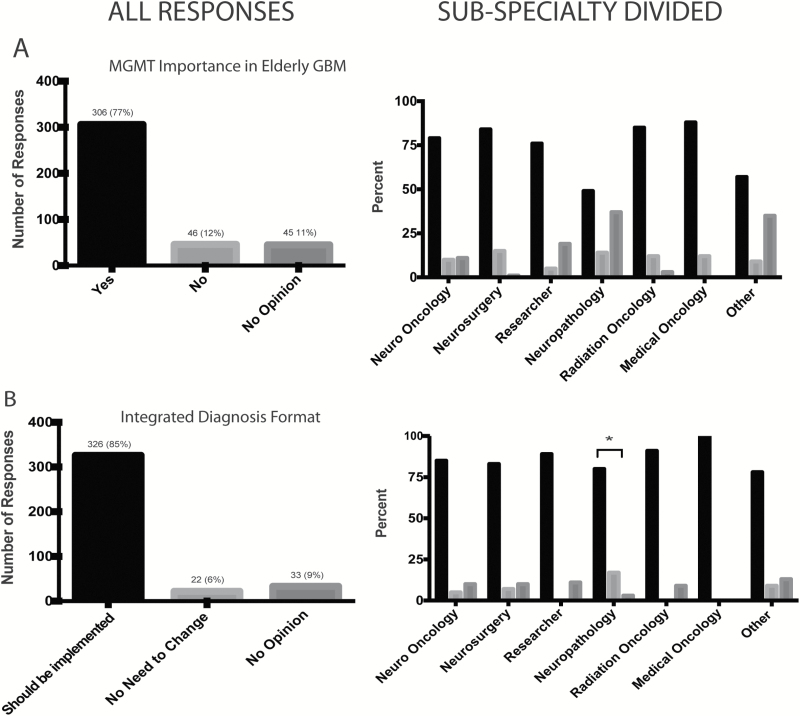
The majority, 77% (n=306/397), of respondents agreed that O6-DNA methylguanine-methyltransferase (MGMT) methylation status would affect approach and management for elderly patients with GBM (Fig. 3A). This was in contrast to the 12% (n=46/397) of respondents who disagreed; 11% (n=45/397) gave no opinion.
Fig. 3.
(A) Responses to whether approach and management of elderly patients should change patients if the results of MGMT testing were included in the classification of GBM (n=397). (B) Responses to what the neuro-oncology community thinks of the proposed integrated diagnosis and the summary description of the recent meeting to plan the next update of the WHO classification (n=381).
Based on the summary description of the recent meeting to plan the next update of the WHO classification, along with the proposed integrated diagnosis format as published in the white paper (respondents provided examples of a “layered” diagnosis format as presented in the consensus paper reference), what do you think? (Respondents are asked their opinion of the “integrated diagnosis” format.)
The final question addressed whether a proposed “layered” diagnosis format, as suggested in a recent meeting report that integrates histological and molecular findings, would be an improvement over existing reporting practices.20 Three hundred and twenty-six respondents of a total 381 who responded (85%) agreed that such an integrated diagnosis format, which incorporated both histological and molecular findings into the “diagnostic line” of the report, would be an improvement (Fig. 3B). Patterns related to incorporation of molecular markers by subspecialty. Since this was perhaps one of the more practical questions in the survey, we examined the responses by subspecialty. A total of 348 respondents answered this question either “yes” or “no.” Among self-identified neuropathologists, 6/34 (18%) answered this question “no,” whereas among the remainder who did not self-identify as a neuropathologist, only 16/314 (5%) responded “no.” This difference in response was statistically significant (P<.05) and suggests some variation among subspecialty in the neuro-oncology community.
Respondents were also offered the opportunity to enter opinions or comments as free text. Some of the responses expressed opinions that related to the availability of molecular tests at various health care institutions across the world, which could pose a limitation in introducing molecular tests worldwide; the need for specific guidelines as to how tests are conducted; and the fact that some molecular tests, in practice, are not available within the clinical time frame needed to affect management decisions. Other responses indicated the need to go deeper than was addressed in the survey—for example, molecular subtypes of medulloblastoma and the use of ATRX and TERT promoter mutations in diffuse glioma. Points were indicated regarding the role of molecular testing in reducing interobserver variability in histopathological interpretation, as well as the need to prioritize molecular distinctions only when alternative molecularly targeted therapies would be available. A sample of free-text responses is shown in Supplementary Table S3.
Discussion
Prior editions of the WHO Classification of Tumours of the Central Nervous System (in 1979, 1993, 2000, and 2007) relied on histological features (including special analyses such as immunohistochemistry and electron microscopy) to classify and grade brain tumors.1 However, the advances in the development of clinically relevant molecular signatures over the past decade has resulted in an increasing desire to incorporate appropriate molecular markers into CNS WHO updates.21 This survey shows generally strong support (>70% of respondents) for integrating molecular markers into CNS WHO updates, and reflects the desire of neuro-oncology to adopt a more personalized medicine approach to the management of brain tumors. (Fig. 1A).
The basic results of this survey were available in early June 2015, in advance of the WHO Consensus and Editorial Meeting that took place in later June and that was attended by 2 of the authors (K.A., D.N.L.), who had the opportunity to share the results with the meeting participants. The sense of strong approval by the widely representative neuro-oncology community provided additional support for the participants to develop consensus over the incorporation of key molecular parameters in the CNS WHO update. Notably, WHO classifications do not endorse particular reporting formats, and therefore, while the participants agreed that layered, integrated reports were highly practical ways to include both histological and molecular data into final diagnoses, the meeting did not discuss this issue in detail, relying on the prior endorsement via the International Society of Neuropathology–Haarlem guidelines.20
With regard to 1p/19q testing, the survey results overwhelmingly confirm the importance and the already widespread use of this test in clinical neuropathology and neuro-oncology practices. The survey response on this point indicated that 96% believe that 1p/19q testing was “critically” (35%) or “very” (61%) important. The overwhelming endorsement for using 1p/19q testing most likely reflects the fact that this was the first genetic marker shown to have potential importance in estimating prognosis and guiding therapy in brain tumor patients22 and that the field has therefore had over 15 years of experience using such testing both for clinical management and clinical trials. Including 1p/19q testing as an integral component of classification, when results are available, was thus clearly viewed as an important step forward.
IDH mutation status is increasingly established as a marker that distinguishes biological subtypes of glioma, and also as a maker of prognosis among histologically similar tumors, but with information on this marker going back less than 7 years. Approximately two-thirds of respondents (67%) indicated that IDH status is important for clinical decision making for management of patients with diffuse gliomas. Somewhat surprisingly, one-third of the respondents indicated that IDH status was not important in guiding clinical decision making for AA, with 17% responding “no” and 16% responding “no opinion” (Fig. 2A). The relevance of IDH status in GBM clinical decision making resulted in lower support, with only 30% indicating that they would alter management for GBM based on the IDH result, while 16% were undecided and 15% had no opinion (Fig. 2B). Notably, based on these results, it appears that the value for inclusion of IDH status remains controversial when it comes to guiding treatment decisions for GBM populations. We note that the survey question was oriented around treatment decisions, not around subtyping of gliomas for diagnostic and/or prognostic reasons, which forms the basis of the WHO classification. While the survey questions did not touch upon the specifics of IDH testing, it is important to note that a common method to assess IDH mutation involves the use of the R132H mutant-specific antibody. While useful, a negative R132H immunohistochemical result is not sufficient to indicate an absence of IDH mutation, since 10%–15% of all IDH mutations in glioma are noncanonical. Examination and testing for noncanonical IDH mutations (especially in the setting of R132H-negative cases) is warranted and necessary in these cases, as has been explicitly stated in the 2016 WHO classification.
Within adult glioblastoma, MGMT promoter methylation appears to be both a prognostic marker overall and a predictive marker, particularly for chemotherapy in elderly (age >65) patients with glioblastoma.23–25 Specifically, elderly GBM patients whose tumors are MGMT methylated had an improved outcome following temozolomide compared with patients with MGMT-unmethylated tumors, while the same was not seen for patients receiving radiation alone. The majority (77%) of respondents agreed that MGMT promoter methylation status could guide management for elderly patients with glioblastoma, suggesting that an integrated diagnostic report as highlighted in the International Society of Neuropathology–Haarlem guidelines should include MGMT promoter methylation status as a reported feature. However, because MGMT promoter methylation status is not known to be a diagnostic criterion for subtyping glioblastoma, it would less likely be a candidate for incorporation into diagnostic nomenclature.
Another important consideration inherent in a histology-based classification has arisen based on the conceptual advance that, as a group, pediatric diffuse gliomas do not share similar genomic signatures compared with adult tumors. For example, the presence of IDH mutations is not commonly seen in pediatric glioma, and in contrast, pediatric gliomas are characterized by histone H3 gene mutations26,27 and ACVR1 mutations, which are not characteristic of gliomas in adults.28–31 The majority of survey respondents were in favor of classifying pediatric gliomas differently from adult gliomas according to WHO criteria, given the differences in driver molecular alterations. One consideration is that in future iterations of the WHO classification, a dedicated section for specific pediatric brain tumor entities would be incorporated.
While the survey was designed for opinions across the neuro-oncology community and not for attitudes across relevant subspecialties, some observations of responses among subspecialties are nonetheless of interest. The community of individuals most directly responsible for patient management of brain tumors (neuro-oncologists, medical oncologists, radiation oncologists, and neurosurgeons) was highly supportive of many of the concepts related to incorporation of molecular markers. Interestingly, while neuropathologists were also supportive overall, the rate of positive responses to some of the questions was lower in this discipline. This may reflect the concern of neuropathologists in the practical challenges of implementing the methods needed to undertake molecular analyses—challenges that are technological, regulatory, and financial. Neuropathologists face these challenges routinely with the development of all new testing modalities and may therefore be more cognizant (and wary) of these practical hurdles compared with other disciplines in neuro-oncology.
The limitations of this work are those associated with any survey-based study, including possible interpretation issues with questions as well as possible selection bias of the respondent group due to a low response rate (8%). Moreover, there might be bias toward a more engaged group of individuals interested in this topic, and due to the method used to select respondents, there may also be a bias toward respondents from developed countries. Additionally, the survey did not address a broad range of issues or tumor types for which molecular information might be available, instead focusing on a subset of markers relevant to diffuse gliomas. Given the recent publication of the WHO 2016 update,32 we note inclusion of key molecular markers (for example, IDH mutation and 1p/19q status) in the definition of diffuse glioma entities, which is broadly consistent with the overall results of this survey.
Nonetheless, the overall conclusions are that the neuro-oncology community sees great benefit to patients and standardizing practice for a combined histological-molecular approach to glioma classification and grading. The WHO 2007 edition did not include molecular criteria in the definition of tumor entities. The WHO 2016 edition has included some molecular criteria, which is a promising step forward. This survey shows that there is broad support to integrate molecular data into WHO classification, and knowing the survey results may be of help as the community works toward implementing the 2016 CNS WHO.32,33
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
This work was supported by the Princess Margaret Cancer Centre Foundation.
Conflict of interest statement. The authors have no conflicts of interest.
Supplementary Material
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN OH, Wiestler OD, Cavenee WK. World Health Organization Histological Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 3. Wiestler B, Capper D, Sill M, et al. Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. [DOI] [PubMed] [Google Scholar]
- 4. Labussiere M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. [DOI] [PubMed] [Google Scholar]
- 5. Dubbink HJ, Atmodimedjo PN, Kros JM, et al. Molecular classification of anaplastic oligodendroglioma using next-generation sequencing: a report of the prospective randomized EORTC Brain Tumor Group 26951 phase III trial. Neuro Oncol. 2016;18(3):388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hinrichs BH, Newman S, Appin CL, et al. Farewell to GBM-O: genomic and transcriptomic profiling of glioblastoma with oligodendroglioma component reveals distinct molecular subgroups. Acta Neuropathol Commun. 2016;4(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain MC, Born D. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. J Neurooncol. 2015;125(2):249–251. [DOI] [PubMed] [Google Scholar]
- 8. Leeper HE, Caron AA, Decker PA, et al. IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget. 2015;6(30):30295–30305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taal W, van der Rijt CC, Dinjens WN, et al. Treatment of large low-grade oligodendroglial tumors with upfront procarbazine, lomustine, and vincristine chemotherapy with long follow-up: a retrospective cohort study with growth kinetics. J Neurooncol. 2015;121(2):365–372. [DOI] [PubMed] [Google Scholar]
- 10. Intergroup Radiation Therapy Oncology Group Trial , Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. [DOI] [PubMed] [Google Scholar]
- 11. van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. [DOI] [PubMed] [Google Scholar]
- 12. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 13. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorlia T, Delattre JY, Brandes AA, et al. New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of European Organisation for Research and Treatment of Cancer Brain Tumour Group Study 26951. Eur J Cancer. 2013;49(16):3477–3485. [DOI] [PubMed] [Google Scholar]
- 15. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cancer Genome Atlas Research Network , Brat DJ, Verhaak RG, et al. comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buczkowicz P, Hawkins C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front Oncol. 2015;5:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chamdine O, Gajjar A. Molecular characteristics of pediatric high-grade gliomas. CNS Oncol. 2014;3(6):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology‒Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014;24(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Louis DN. The next step in brain tumor classification: “Let us now praise famous men” . . . or molecules? Acta Neuropathol. 2012;124(6):761–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 23. Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 24. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 25. Mur P, Rodriguez de Lope A, Diaz-Crespo FJ, et al. Impact on prognosis of the regional distribution of MGMT methylation with respect to the CpG island methylator phenotype and age in glioma patients. J Neurooncol. 2015;122(3):441–450. [DOI] [PubMed] [Google Scholar]
- 26. Pathak P, Jha P, Purkait S, et al. Altered global histone-trimethylation code and H3F3A-ATRX mutation in pediatric GBM. J Neurooncol. 2015;121(3):489–497. [DOI] [PubMed] [Google Scholar]
- 27. Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–678. [DOI] [PubMed] [Google Scholar]
- 28. Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. 2014;46(5):462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46(5):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Louis DN, Wiestler OD, Cavenee WK. (eds.) World Health Organization Histological Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 33. Louis DN, Reifenberger G, von Deimling A, et al. The 2016 WHO classification of tumours of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.