Abstract
Background
More than 100,000 US troops were potentially exposed to chemical warfare agents sarin (GB) and cyclosarin (GF) when an ammunition dump at Khamisiyah, Iraq was destroyed during the 1991 Gulf War (GW). We previously reported reduced hippocampal volume in GW veterans with suspected GB/GF exposure relative to matched, unexposed GW veterans estimated from 1.5 T magnetic resonance images (MRI). Here we investigate, in a different cohort of GW veterans, whether low-level GB/GF exposure is associated with structural alterations in specific hippocampal subfields, estimated from 4 T MRI.
Methods
The Automatic Segmentation of Hippocampal Subfields (ASHS) technique was used to quantify CA1, CA2, CA3 and dentate gyrus (DG), and subiculum (SUB) subfields volumes from high-resolution T2-weighted images acquired on a 4 T MR scanner in 56 GW veterans with suspected GB/GF exposure and 56 “matched” unexposed GW veterans (mean age 49 ± 7 years).
Results
GB/GF exposed veterans had smaller CA2 (p = 0.003) and CA3/DG (p = 0.01) subfield volumes compared to matched, unexposed GW veterans. There were no group difference in total hippocampal volume, quantified with FreeSurfer, and no dose–response relationship between estimated levels of GB/GF exposure and total hippocampal or subfield volume.
Conclusions
These findings extend our previous report of structural alterations in the hippocampi of GW veterans with suspected GB/GF exposure to volume changes in the CA2, CA3, and DG hippocampal subfields in a different cohort of GW veterans with suspected GB/GF exposure.
Keywords: Magnetic resonance imaging, Hippocampus, Sarin, Cyclosarin, Gulf War veterans
1. Introduction
During the first Gulf War (GW), US combat engineers detonated a munitions storage pit at Khamisiyah, Iraq that was later found to contain stockpiles of sarin (GB; o-isopropyl methylphosphonoflouridate) and cyclosarin (GF; cyclohexyl methylphosphonoflouridate). The destruction of this bunker generated an airborne plume that potentially exposed as many as 100,000 troops in the surrounding area to low-levels of GB/GF. After the Gulf War ended, the Department of Defense (DoD) and the Central Intelligence Agency (CIA) tried to model possible GB/GF exposures over a 4-day period based on simulated meteorological conditions and analyses of likely chemical agent dispersal. Military personnel attached with units located in areas covered by the estimated zone of exposure were notified about their possible exposure (Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness, 1997). In 2002, the exposure plume model was refined and re-analyzed using additional meteorological modeling information, updated estimates of the total number of rockets destroyed, updated information about personnel and unit-level location, and updated exposure thresholds for GB and GF, the combined toxicity aspects of GB/GF, and consideration of agent removal mechanisms. This effort resulted in a second round of notification to service members that reversed some of the previous notifications and placed others into the possible zone of exposure (Assistant Secretary of Defense (Health Affairs) and Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness, and Military Deployments, 2002).
Although two epidemiological studies have failed to find any health outcome differences between groups based on exposure predictions using the revised “plume” model data (Kang and Bullman, 1996; Enserink, 2001; Lindauer et al., 2004), we (Chao et al., 2010, 2011) and others (Proctor et al., 2006; Heaton et al., 2007) have reported neurobehavioral and structural brain changes in GW Veterans with suspected GB/GF exposure compared to unexposed GW veterans. One of the structural brain difference that we found was reduced hippocampal volume quantified from the 1.5 T magnetic resonance images (MRI) of 40 GW GB/GF exposed veterans relative to 40 matched, unexposed GW veterans (Chao et al., 2010). However, we were unable to replicate this hippocampal finding in a follow-up study of 64 different GW veterans with suspected GB/GF exposure and 64 matched controls scanned on a 4 T MR scanner (Chao et al., 2011).
One reason for our discrepant hippocampal results may be related to the different image processing software that we used to estimate hippocampal volume. We used a high dimensional brain mapping tool to estimate hippocampal volume in the 1.5 T dataset. Because of issues related to B1 inhomogeneity in the 4 T dataset, FreeSurfer (Martinos Center for Biomedical Imaging, Harvard-MIT, Boston, USA) (Fischl et al., 2002) was used to estimate hippocampal volume in the 4 T dataset. Compared to the gold standard of manual marking, the high dimensional brain mapping tool that we used on the 1.5 T dataset generates smaller hippocampal volumes because it excludes the alveus and fimbria (Hsu et al., 2002). In contrast, FreeSurfer generates larger hippocampal volumes than manual marking (Morey et al., 2009; Pardoe et al., 2009; Tae et al., 2008). This suggests that the FreeSurfer hippocampal mask includes neuroanatomical substrates beyond the hippocampus proper. Because animal studies suggest that organophosphate poisoning has selective effects on particular hippocampal subfields (Abdel-Rahman et al., 2002; Pazdernik et al., 2001), it is possible that FreeSurfer hippocampal volumetry is less sensitive to the effects of GB/GF exposure than the high dimensional brain mapping tool that we used to estimate hippocampal volume in the 1.5 T dataset.
The hippocampus consists of two convoluted formations: the dentate gyrus (DG) and the Cornu Ammonis (CA; Duvernoy, 2005), which can be further separated into four subdivisions (CA1–4). In recent years, several groups have tried to measure hippocampal subfields at the macroscopic level. We (Mueller et al., 2007, 2008; Mueller and Weiner, 2009; Mueller et al., 2010) and others (La Joie et al., 2010; Malykhin et al., 2010; Thammaroj et al., 2005; Zeineh et al., 2000, 2003) have utilized a high-resolution T2-weighted MRI sequence that yields sufficient gray matter contrast to visualize the dark band of stratum moleculare and stratum lacunosum to serve as a key landmark for defining the boundary between the CA4/DG region and the other CA subfields and the subiculum (Amaral and Lavenex, 2007; Eriksson et al., 2008). In this study, we used an automatic hippocampal subfield segmentation (ASHS) technique that has shown good accuracy relative to manual segmentation (Yushkevich et al., 2010) to investigate the effect of GB/GF exposure. Based on the rodent studies cited above (Abdel-Rahman et al., 2002; Pazdernik et al., 2001), we hypothesize that GW veterans with suspected GB/GF exposure have smaller CA1 and CA3/DG hippocampal subfields than unexposed GW veterans.
2. Methods
2.1. Participants
All participants were GW veterans who took part in a 4 T imaging study on the effects of Gulf War Illness on the brain, which was conducted at the San Francisco Veterans Affairs Medical Center between 2005 and 2010. A detailed description of predicted exposure and exposure dosage estimates has been described previously (Chao et al., 2010, 2011). The current analysis focused on 56 GB/GF exposed GW veterans who had high-resolution T2-weighted MRIs. All 56 GB/GF exposed veterans in the current analysis were part of the sample described in Chao et al. (2011). Furthermore, there is no overlap with the sample of GW veterans in whom we previously reported reduced hippocampal volume from 1.5 T MRI (Chao et al., 2010). Fifty-six unexposed GW veterans were selected from a group of 127 GW veterans with high-resolution T2-weighted images to match the GB/GF-exposed veterans for age, sex, level of education, and diagnoses of chronic multisymptom illness (CMI, Fukuda et al., 1998), current PTSD according to the Clinician-Administered PTSD Scale (CAPS, Blake et al., 1995), and current major depressive disorder, according to the Structured Clinical Interview for DSM-IV (First et al., 1995). Thirty-four these 56 unexposed GW veterans were part of the sample described in (Chao et al., 2011).
The Institutional Review Boards of the University of California, San Francisco, the San Francisco Veterans Affairs Medical Center (VAMC), and the Department of Defense Human Research Protection Office approved both studies. Informed consent was obtained from all participants.
2.2. Image acquisition
All subjects were scanned at the San Francisco VAMC on a Bruker MedSpec 4 T MRI system equipped with a USA instruments eight-channel array head coil. The MRI scan protocol included a high-resolution T2-weighted fast spin echo sequence (repetition time, 3500 ms; echo time, 19 ms) with a train of 15 spin-echoes per k-space segment, 160° refocusing pulses, and 100% oversampling in the phase-encoding direction to avoid aliasing, yielding a nominal in-plane resolution of 0.4 mm × 0.4 mm. Twenty-four contiguous slices, each 2-mm thick, were acquired in interleaved fashion. The coronal oblique slices were angulated perpendicular to the long axis of the hippocampal formation to achieve consistent images of hippo-campal subfields from subject to subject (Mueller et al., 2007). A volumetric T1-weighted magnetization prepared gradient echo (MPRAGE) sequence (repetition time, 2300 ms; time following inversion pulse, 950 ms; echo time, 4 ms; 7° excitation pulses; 1 mm × 1 mm × 1 mm resolution) was also acquired.
2.3. Determination of total hippocampal volume
An automated, non-biased atlas-based Bayesian segmentation procedure, applied in Freesurfer v.4.5 (http://surfer.nmr.mgh.harvard.edu/), was used to derive quantitative estimates of total hippocampal volume from the volumetric T1-weighted MPRAGE (Dale et al., 1999; Desikan et al., 2006; Fischl et al., 1999).
2.4. Automatic Segmentation of Hippocampal Subfields
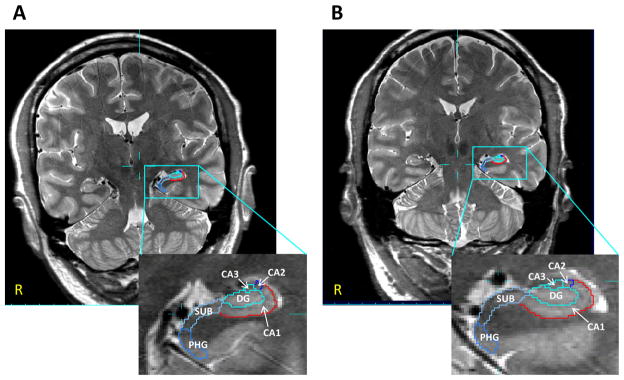
Segmentations of the hippocampal formation were generated using the Automatic Segmentation of Hippocampal Subfields (ASHS; Yushkevich et al., 2010). ASHS uses a combination of multi-atlas segmentation, similarity-weighted voting, and a learning-based bias correction technique to segment hippocampal sub-fields. Briefly, each subject’s T2-weighted image was registered to a set of atlases (i.e., T2-weighted images of 32 subjects (mean age: 64.8 ± 11.8 years) with manual segmentations) and the candidate segmentations provided by the different registered atlases are combined into a single consensus segmentation based on a voting scheme that is weighted locally by the image intensity similarity. The segmentation of each voxel was corrected by a classifier trained to recognize systematic errors made by the above approach. Subfield definitions were based on the method described by Mueller et al. (2007) but were expanded to cover the whole hippocampus, similar to La Joie et al. (2010) and Shing et al. (2011). The following subfields were defined: CA1, CA2, CA3, CA4, dentate gyrus (DG), subiculum (SUB), and head and tail of the hippocampus. Because there are no reliable marks to distinguish between the CA3, CA4, and DG subfields on the T2-weighted image, these subfields were combined into one region, referred to as CA3/DG, as described in Mueller et al. (2007) (see Fig. 1 for an example of the segmented hippocampal subfields in a GB/GF exposed and an unexposed GW veteran). Because the head and tail of the hippocampus are macroscopic definitions of those hippocampal regions that include more than one subfield, the volumes from these regions were not included in the current analysis.
Fig. 1.
Coronal slices of a T2-weighted image (0.4 mm × 0.4 mm in-plane resolution) in a 43-year old GB/GF-exposed GW veteran (A) and a 43-year old unexposed GW veteran (B) showing segmentation of the left hippocampus. DG, dentate gyrus; SUB, subiculum; PHG, parahippocampal gyrus.
2.5. Gray and white matter tissue segmentation
We used the default unified segmentation algorithm in SPM8 (Statistical Parametric Mapping; Institute of Neurology, London, United Kingdom) to segment the volumetric T1 images into gray matter (GM), white matter (WM) and cerebral spinal fluid (CSF). In order to calculate the volumes of the separate tissue compartments, we determined the number of voxels belonging to the GM, WM, and CSF partitions. Each volume count was then multiplied with the voxel dimensions (1 mm × 1 mm × 1 mm) and converted into cubic centimeters. Every single voxel in the compartmental images contains a certain probability value, ranging from zero to one, identifying the likelihood that this voxel belongs to a particular compartment. Hence we also multiplied the number of voxels with the probability values of each voxel. After determining volumes of the GM, WM and CSF compartments, total intracranial volume (TIV) was estimated by summing the compartmental volumes (i.e., GM + WM + CSF).
3. Data analysis
3.1. Subfield size estimate
To avoid having the volume of the hippocampal subfields be proportional to the anterior–posterior extent of the hippocampal body, which is not reflective of the overall size of the hippocampus but rather the extent of the region where the differentiation between subfields was deemed feasible and reliable, we normalized the volume of each subfield by the number of slices that spanned the segmentation of that subfield, as described in Pluta et al. (2012). This normalization method also takes into account slice angulation variability, inconsistencies from image to image to image in the position of the uncal apex, which determines the boundary between head and body slices (due to anatomical variability and low out-of-plane resolution), and ensures that the variance in subfield volume is not dominated by the number of slices. The normalized subfield volumes were computed according to the following formula: NVs = RVs/(Ns × T), where NVs is the normalized subfield volume, RVs is the raw volume of the voxels assigned to a subfield, Ns is the number of slices spanned by the subfield, and T is slice thickness.
3.2. Statistical analyses
Statistical analyses of the demographic, clinical, neuropsycho-logical, and volumetric measures were conducted using IBM SPSS Statistics, version 21. Demographic and descriptive characteristics were compared across the two exposure groups with Student’s t tests for continuous variables and chi-square tests for categorical variables. Because we previously found significant effects of GB/GF exposure on total GM and WM volumes, total GM and WM volume and TIV were included as covariates in a multivariate analysis of covariance (MANCOVA) of the volumetric imaging data in the two exposure groups. Individual analyses of covariance (ANCOVAs) were used to examine group differences in total hippocampal and individual subfield volumes. We used Spearman’s rank correlations to assess the relationship between estimated GB/GF exposure dosages and total hippocampal and hippocampal subfield volumes. Normal probability plots and the Shaprio–Wilks test indicated that the cumulative exposure level estimates were not normally distributed. Therefore the cumulative exposure level estimates were log-transformed. Because some individuals were not identified as having been with units that were in the hazard area until after the last modeling effort that determined the cumulative GB/GF exposure level estimates in 2002, cumulative exposure level estimates were available only for 53 of the 56 GB/GF exposed GW veterans in the current analysis.
3.3. Post-hoc analyses
Although the two exposure groups were matched for incidence of CMI, because we previously found evidence of an interaction between CMI and GB/GF exposure on total GM and total WM volume (Chao et al., 2011), CMI was included as a covariate along with total GM, total WM, and TIV in post-hoc analyses. Because there were slightly more unexposed GW veterans taking psychotropic medication than GB/GF exposed veterans and because there is evidence that antidepressant treatment increases hippocampal volume (Vermetten et al., 2003), use of psychotropic medication was also included as a covariate along with total GM, total WM, and ICV in post-hoc analyses. Finally, because we previously found an effect of GB/GF exposure on the number of omission errors and response times on the Continuous Performance Test (CPT), a measure of sustained and selective attention (Chao et al., 2011), bivariate correlation analyses (i.e., Spearman’s rank correlations) were used to explored the relationship between hippocampal subfield volumes and CPT performance in post-hoc analyses.
4. Results
Table 1 summarizes the demographic, military, and clinical data of the GB/GF exposed and unexposed veterans. Although the two groups were matched for age, gender, years of education, and diagnoses of current PTSD, current MDD, and CMI based on criteria previously described by Fukuda et al. (1998), there were differences in ethnicity (χ2 = 20.67, df = 3, p < 0.001) and military status during the Gulf War (χ2 = 8.30, df = 2, p = 0.02). There were more Caucasians among the unexposed GW veterans and more Latinos and National Guard personnel among the GB/GF exposed veterans (Table 1).
Table 1.
Demographic, military, and clinical characteristics GB/GF exposed and unexposed GW veterans.
| Exposed | Unexposed | |
|---|---|---|
| N | 56 | 56 |
| Age (years) | 49.0 (7.2) | 49.6 (7.6) |
| No. females (%) | 5 (9%) | 5 (9%) |
| Ethnicitya | ||
| African American (%) | 6 (11%) | 8 (14%) |
| Caucasian (%) | 24 (43%) | 43 (77%) |
| Latino (%) | 20 (36%) | 5 (9%) |
| Other (%) | 6 (11%) | 0 (0%) |
| Education (years) | 15.3 (2.3) | 15.7 (2.3) |
| Military status during Gulf Wara | ||
| Active duty (%) | 42 (75%) | 49 (88%) |
| National guard (%) | 10 (18%) | 1 (2%) |
| Reserves (%) | 4 (7%) | 6 (11%) |
| No. with service-connected disability | 22 (39%) | 30 (54%) |
| Average % disability (range) | 13 (5–90) | 18 (5–100) |
| No. with CMI (%) | 28 (50%) | 28 (50%) |
| No. with current PTSD diagnosis (%) | 3 (5%) | 3 (5%) |
| Current CAPS score | 8.3 (16.0) | 4.8 (10.5) |
| No. with current MDD diagnosis (%) | 4 (7%) | 3 (5%) |
| HAM-D score | 4.3 (4.4) | 3.8 (3.7) |
| No. on psychotropic medication | 10 (18%) | 15 (27%) |
| No. with history of alcohol abuse/dependence | 20 (36%) | 26 (46%) |
| Current average no. drinks/month | 20.6 (19.7) | 18.4 (21.6) |
| No. with history of drug abuse/dependence | 6 (11%) | 6 (11%) |
Means (SD) or N (%) reported. CMI, chronic multisymptom illness; PTSD, posttraumatic stress disorder; CAPS, clinician administered PTSD scale; MDD, major depressive disorder; HAM-D, Hamilton depressive scale
Significantly different between the two groups, p <0.05.
4.1. Binary comparisons between the exposed and unexposed GW veterans
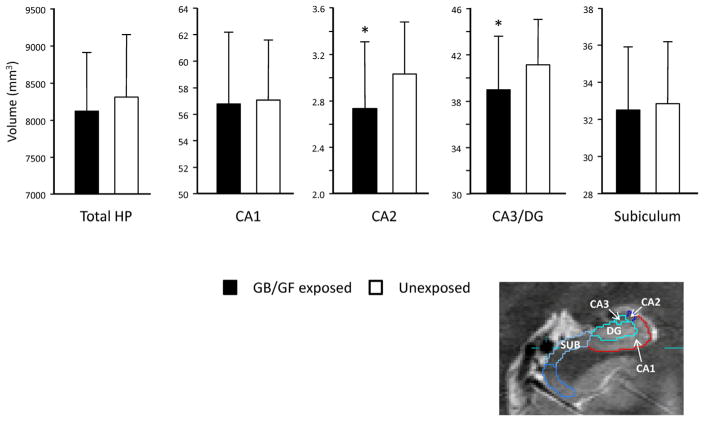
Table 2 lists the volumes of the total hippocampus and hippocampal subfield in the GB/GF exposed veterans and the matched controls. Fig. 2 shows a graphic representation of the subfield volumes the two exposure groups. The overall MANCOVA revealed a significant main effect of group (Wilks Lambda = 0.88, Pillais approximated F5,103 = 2.83, p = 0.02). The individual ANCO-VAs revealed significant group effects in CA2 (F1,107 = 10.10, p = 0.002) and CA3/DG (F1,107 = 7.06, p = 0.009) subfields. Including CMI as a covariate along with total brain GM, WM, and TIV in post-hoc analysis did not alter the significance of the MANCOVA or the individual ANCOVAS. Because there was slightly more unexposed GW veterans taking psychotropic medication than GB/GF exposed veterans and because there is evidence that antidepressant treatment increases hippocampal volume (Vermetten et al., 2003), psychotropic medication use was included as a covariate along with total GM, total WM, and TIV in post-hoc analysis. However, this also did not alter the significance of the MANCOVA or the individual ANCOVAS.
Table 2.
Least Square Meana Volumes of the total Hippocampus and Subfields.
| Exposed | Unexposed | F1,107 | |
|---|---|---|---|
| Total HP | 8130.27 ± 780.07 | 8311.59 ± 840.74 | 2.18 |
| CA1b | 56.76 ± 5.45 | 57.07 ± 4.55 | 0.11 |
| CA2b | 2.73 ± 0.58 | 3.03 ± 0.45 | 10.10c |
| CA3/DGb | 38.95 ± 4.63 | 41.08 ± 3.94 | 7.06c |
| SUBb | 32.48 ± 3.41 | 32.82 ± 3.36 | 0.31 |
Values (mm3) are mean ± SD.
Accounting for total GM, total WM volume and TIV.
Volumes normalized for number of slices in segmentation.
p <0.01.
Fig. 2.
Graphical representation of subfield volumetric differences between the groups.
4.2. Correlation between estimated GB/GF exposure dosages and total hippocampal and subfield volumes
There was no significant correlation between the log-transformed estimated exposure dosage and total hippocampal volume or any of the hippocampal subfield volumes.
4.3. Correlation between hippocampal subfield volumes and Continuous Performance Test
Because we previously found an effect of GB/GF exposure on errors of omission and response time on the CPT (Chao et al., 2011), we explored the relationship between hippocampal subfield volumes and CPT performance in post-hoc analyses. CA2 volume correlated negatively with the number of CPT omission errors in the entire sample of GW veterans (r = −0.31, p = 0.002). When we analyzed the two exposure groups separately, there was a negative correlation between CA2 volume and the number of CPT omissions in the unexposed (r = −32, p = 0.03) but not the GB/GF exposed group (r = −0.24, p = 0.10).
5. Discussion
Despite uncertainty in the assumptions of GB/GF exposure based on the plume model analyses (Enserink, 2001; Kang and Bullman, 1996; Lindauer et al., 2004), decrements in manual dexterity and visuospatial functions, reduced total brain white matter volume, and increased lateral ventricle volume have been reported in GW veterans with higher estimated levels of presumed GB/GF exposure based on the refined models of the Khamisiyah hazard (Proctor et al., 2006; Heaton et al., 2007). Subsequently, we reported evidence of reduced total brain gray matter and hippocampal volumes in an independent cohort of 40 GW veterans with suspected GB/GF exposure relative to 40 matched, unexposed GW veterans scanned on a 1.5 T MRI (Chao et al., 2010). We later replicated the findings of reduced total brain gray and white matter volumes in a third cohort of GW veterans with suspected GB/GF exposure scanned at 4 T MRI (Chao et al., 2011). Here we extend our 1.5 T findings of hippocampal volume change in GW veterans with suspected GB/GF exposure to reduced volume in the CA2 and CA3/DG hippocampal subfields in a different group of 56 GB/GF exposed veterans scanned on 4 T. Together, these findings are consistent with research suggesting that low-level exposure to anticholinesterase compounds can result in neurotoxic consequences to the mammalian hippocampus (Veronesi et al., 1990), with the reports of smaller than normal hippocampal volumes in victims of the 1995 Tokyo subway GB attack (Yamasue et al., 2007), and with animal studies suggesting organophosphate poisoning has selective effects on specific hippocampal subfields (Abdel-Rahman et al., 2002; Pazdernik et al., 2001). Our group has previously found reduced CA3/DG subfield volumes in veterans with PTSD (Wang et al., 2010). However it is unlikely that the reduced CA3/DG subfield volume that we observed in veterans with GB/GF exposure is related to PTSD because the GB/GF exposed and unexposed veterans were matched for PTSD and because there were no significant group differences in current PTSD symptom severity (i.e., CAPS).
After the end of the 1991 Gulf War, military personnel who served in that conflict began to report health problems that were not adequately explained by established medical or psychiatric diagnoses (Research Advisory Committee on Gulf War Veterans’ Illness, 2008; Kang et al., 2009; Institute of Medicine, 2010). Through factor analysis of self-report data, Robert Haley and his colleagues published one of the first attempts to systematically characterize the illness experienced by many GW veterans, identifying six clusters of symptoms (Haley et al., 1997a). Using this classification scheme, Haley et al. (1997b) later reported significant differences in neurological function, as measured by the Halstead impairment index, and decreased N-acetylaspartate (NAA) to creatine (Cr) ratio in the pons of ill GW veterans with syndrome 2 relative to ill GW veterans with other syndromes and non-symptomatic GW veterans (Haley et al., 2000). More recently, Haley’s group reported different patterns of cerebral blood flow in response to a physostigmine infusion-challenge (Li et al., 2011; Liu et al., 2011) and different patterns of functional brain activity in the prefrontal cortex during a high-demand working memory task (Hubbard et al., 2014) in GW veterans with Syndromes 1, 2, and 3 compared to control GW veterans.
Although Haley’s six syndromes represent one of the first attempts to systematically characterize Gulf War Illness, the case definition of Gulf War Illness (or chronic multisymptom illness, CMI) established by the Centers for Disease Control and Prevention (CDC, Fukuda et al., 1998) is more commonly used. The CDC case definition specifies two out of three complaints of fatigue, neurocognitive deficits, and musculoskeletal pain experienced for 6 months or longer. Using this definition, Rayhan et al. (2013) reported higher axial diffusivity, a diffusion tensor imaging measure that is sensitive to axonal degeneration (Song et al., 2002), in right inferior frontal-occipital fasciculus of 31 GW veterans with CMI and Chronic Fatigue Syndrome relative to 20 healthy GW veterans and civilian controls. We previously found evidence of an interaction between CMI and GB/GF exposure on total GM and total WM volume (Chao et al., 2011). However, the GW veterans with and without suspected GB/GF exposure were matched for CMI in the current study. Furthermore, including CMI as a covariate in post-hoc analysis did not alter the significant effect of GB/GF exposure on hippocampal subfield volume.
We suggested in the introduction that that the reason why we were unable to replicate our 1.5 T finding of hippocampal atrophy in GW veterans with suspected GB/GF exposure in the 4 T dataset (Chao et al., 2011) is because FreeSurfer hippocampal volumetry lacks sensitivity to the effects of GB/GF exposure. In the current study, we found a significant effect of GB/GF exposure on CA2 and CA3/DG volumes, estimated from the T2-weighted images processed with ASHS. However, we did not find a significant effect of GB/GF exposure on total hippocampal volume estimated from T1-weighted images processed with FreeSurfer. This finding not only supports our theory, but it is consistent with rodent studies that suggest organophosphate poisoning has selective effects on specific hippocampal subfields (Abdel-Rahman et al., 2002; Pazdernik et al., 2001). The CA2 hippocampal subfield where we found an effect of GB/GF exposure was originally designated as the CA1–2 transition zone in the manual hippocampal subfield segmentation method described by Mueller et al. (2007) because of its overlap with the dorsomedial aspects of CA1 and because it is influenced by the width of the dorsal CA1. Therefore it may be possible that CA2 volume reduction that we observed in the GB/GF exposed veterans is actually reflective of changes in CA1 volume, which would be consistent with the findings reported in rats by Abdel-Rahman et al. (2002).
There is suggestive evidence that the reduced CA2 volume that we found in GB/GF exposed veterans may have functional significance. Post-hoc analyses revealed an inverse relationship between CA2 subfield volume and the number of omission errors on the CPT, a test of sustained and selective attention, in the combined sample of GB/GF exposed and non-exposed veterans and in non-exposed GW veterans alone. Related to this, a study of children and adolescents with attention deficit/hyperactivity disorder has linked morphometric abnormalities in the CA1 and CA2 subfields with deficits in attention (Plessen et al., 2006).
There is evidence from other morphometric MRI studies that different pathological conditions exert selective effects on different hippocampal subfields. For example, numerous studies have found abnormalities in the CA1 region of the hippocampus early in the course of Alzheimer’s disease (AD, Chetelat et al., 2008; Csernansky et al., 2000, 2005; Mueller et al., 2010; Pluta et al., 2012; Qiu et al., 2008; Wang et al., 2006). We previously reported CA3/DG structural deficits in non-demented individuals who are at increased risk for AD because they carry the e4 allele variant of the apolipoprotein E (ApoE) gene (Mueller et al., 2008; Mueller and Weiner, 2009). This suggests that risk for AD may also be associated with structural changes in the CA2 and CA3 hippocampal subfields. Recently, Hanseeuw et al. (2011) reported atrophy in the CA2–3 hippocampal subfield of patients with amnestic mild cognitive impairment (aMCI), a diagnostic entity considered to be a preclinical phase of AD (Petersen et al., 1999). Yassa et al. (2010) have similarly found shape and volume changes in the CA3/DG region of aMCI patients. Thus the smaller CA2 and CA3/DG volumes that we observed in GB/GF exposed veterans may be an indication that these veterans are at greater risk for developing AD. Future longitudinal studies will be needed to determine if this is indeed the case.
Several limitations should be considered in the interpretation of the current findings. First, we did not have information about the participants’ ApoE genotype, which plays a role in the maintenance and repair of neurons (Mahley et al., 2006) and has been associated with smaller CA3/DG volumes in normal aging and in patients with AD (Mueller et al., 2008). Second, there were more unexposed veterans who were taking psychotropic medication than GB/GF exposed veterans. Because there is evidence that antidepressant treatment increases the size of the hippocampus (Vermetten et al., 2003), there is a possibility that the GB/GF effect we observed may be related to the fact that more GB/GF exposed veterans were using psychotropic medication than unexposed GW veterans. However, accounting for psychotropic medication use in post-hoc analysis did not alter the significance of the main findings. The third limitation is that we did not have information about the severity of the symptoms associated with CMI, smoking status, or history of head injury (although history of head injury associated with prolonged loss of consciousness was exclusionary for the study). It is possible that military personnel more likely to be in the higher GB/GF exposure areas are different from those in low exposure areas. However, we are not able to address this question in the current study. Fourth, we did not have cumulative estimated GB/GF exposure levels for all GW veterans whose units were deemed to be located within the modeled plume areas. Fifth, the cumulative GB/GF exposure level estimates that we did have were calculated at the unit rather than individual level. Therefore, there is no definitive way of knowing if an individual soldier was with his or her unit on the four target dates the model. The modeled exposure estimates may also be subject to misclassification (Government Accounting Office, 2004). However, general misclassification errors are likely to be random, thus would have limited influence on the present results. These limitations notwithstanding, the current study suggests that GW veterans with suspected low-level GB/GF exposure have smaller CA2 and CA3/DG hippocampal subfield volumes than their unexposed peers. Future longitudinal, follow-up studies will be needed to determine if these subfield changes render GB/GF exposed GW veterans more vulnerable to risk for AD.
Acknowledgments
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Army, Department of Defense, or Department of Veterans Affairs. This study also supported by Department of Defense grant DAMD17-01-1-0764 entitled ‘Magnetic Resonance and Spectroscopy of the Human Brain in Gulf War Illness’, awarded to the Northern California Institute for Research and Education from the Department of Defense Gulf War Illnesses Research Program, US Army Medical Research and Materiel Command and a Department of Veterans Affairs grant VA GWI No. B3776 entitled ‘Effects of Gulf War Illness on Brain Structure Function and Metabolism: MRI/MRS at 4 T’, and VA grant No. I01BX007080 entitled ‘Longitudinal Assessment of Gulf War Veterans with Suspected Sarin Exposure’ awarded to Dr. Michael Weiner. This material is the result of work supported with resources and the use of facilities at the San Francisco Veterans Affairs Medical Center. The authors would like to thank Dr. Michael Weiner for his support and the Gulf War veterans who participated in these studies.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
Disclosure statement
The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or reflecting the views of the Army, Department of Defense, or Department of Veterans Affairs. Dr. Chao receives grant support from the Department of Veteran’s Affairs.
References
- Abdel-Rahman A, Shetty AK, Abou-Donia MB. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: dose–response relationships. Neuroscience. 2002;113:721–41. doi: 10.1016/s0306-4522(02)00176-8. [DOI] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The hippocampus book. New York: Oxford University Press; 2007. pp. 37–129. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinican-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Abadjian L, Hlavin J, Meyerhoff DJ, Weiner MW. Effects of low-level sarin and cyclosarin exposure and Gulf War Illness on brain structure and function: a study at 4 T. Neurotoxicology. 2011;32:814–22. doi: 10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Fouquet M, Kalpouzos G, Denghien I, Dela Sayette V, Viader F, et al. Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia. 2008;46:1721–31. doi: 10.1016/j.neuropsychologia.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology. 2000;55:1636–43. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, et al. Preclinical detection of Alzheimer’s disease: hippocampal shape and volume predict dementia onset in the elderly. Neuroimage. 2005;25:783–92. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blackdr D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Potential exposure to sarin from the demolitions at Khamisiyah, Iraq on March 10, 1991. Falls Church, VA: Aug, 1997. [accessed 16.01.98]. Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for GulfWar Illness Medical Readiness, and Military Deployments. http://www.gulflink.osd.mil. [Google Scholar]
- Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for GulfWar Illness Medical Readiness and Military Deployments. [accessed 12.04.05];U.S. demolition operations at the Khamisiyah ammunition point (case narrative) 2002 Apr; http://www.gulflink.osd.mil/khamisiyahiii.
- Duvernoy HM. Functional anatomy, vascularization and serial sections with MRI. Berlin: Springer Verlag; 2005. [Google Scholar]
- Enserink M. Congress explores the scientific fringe. Science. 2001;291:814. doi: 10.1126/science.291.5505.814. [DOI] [PubMed] [Google Scholar]
- Eriksson SH, Thom M, Bartlett PA, Symms MR, McEvoy AW, Sisodiya SM, et al. Propeller MRI visualizes detailed pathology of hippocampal scelrosis. Epilepsia. 2008;49:33–9. doi: 10.1111/j.1528-1167.2007.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gobbon M, Williams J. Structured clinical interview for DMS-IV. New York: New Work State Psychiatric Institute Biometrics Research Department; 1995. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Niesenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–8. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J. Is there a Gulf War syndrome? JAMA. 1997a;277:215–22. [PubMed] [Google Scholar]
- Haley RW, Hom J, Roland PS, Bryan WW, Van Ness PC, Bonte FJ, et al. Evaluation of neurologic function in Gulf War veterans. A blinded case–control study. JAMA. 1997b;277:223–30. [PubMed] [Google Scholar]
- Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL. Brain abnormalities in Gulf War syndrome: evaluation with 1H MR spectroscopy. Radiology. 2000;215:807–17. doi: 10.1148/radiology.215.3.r00jn48807. [DOI] [PubMed] [Google Scholar]
- Hanseeuw B, Van Leemput K, Kavec M, Grandin C, Seron X, Ivanoiu A. Mild cognitive impairment: differential atrophy in the hippocampal subfields. Am J Neuroradiol. 2011;32:1658–61. doi: 10.3174/ajnr.A2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007;28:761–9. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Weiner MW. Comparison of automated and manual MRI of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, et al. Central executive dysfunction and deferred prefrontal processing in veterans with Gulf War Illness. Clin Psychol Sci. 2014;2:319–27. doi: 10.1177/2167702613506580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Gulf War and health: health effects of serving in the Gulf War. Vol. 8. Washington, DC: Institute of Medicine; 2010. [Google Scholar]
- Kang HK, Bullman TA. Mortality among US veterans of the Persian Gulf War. N Engl J Med. 1996;335:1498–504. doi: 10.1056/NEJM199611143352006. [DOI] [PubMed] [Google Scholar]
- Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf War: a follow-up survey in 10 years. J Occup Environ Med. 2009;51:401–10. doi: 10.1097/JOM.0b013e3181a2feeb. [DOI] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mezenge F, Landeau B, Villain N, Mevel K, et al. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage. 2010;53:506–14. doi: 10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Li X, Spence JS, Buhner DM, Hart J, Jr, Cullum CM, Biggs MM, et al. Hippocampal dysfunction in Gulf War veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology. 2011;261:218–25. doi: 10.1148/radiol.11101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, et al. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–63. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Liu P, Aslan S, Li X, Buhner DM, Spence JS, BRiggs RW, et al. Perfusion deficit to cholinergic challenge in veterans with Gulf War Illness. Neurotoxicology. 2011;32:242–6. doi: 10.1016/j.neuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;106:5644–51. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin NV, Lebel RM, Coupland NJ, Wilman AH, Carter R. In vivo quantification of hippocampal subfields using 4. 7 T fast spin echo imaging. Neuroimage. 2010;49:1224–30. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Morey RA, Pettey CM, Xu Y, Hayes PJ, Wagner RH, II, Lewis DV, et al. A comparison of automated segmented and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2010;31:1339–47. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer’s disease using high resolution MRI at 4 T. Neuroimage. 2008;42:42–8. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19:558–64. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Stables L, Du AT, Schuff N, Truran D, Cashdollar N, et al. Measurements of hippocampal subfields and age related changes with high resolution MRI at 4 T. Neurobiol Aging. 2007;28:719–26. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Pell GS, Abbott DF, Jackson GD. Hippocampal volume assessment in temporal lobe epilepsy: how good is automated segmentation? Epilepisa. 2009;50:2586–92. doi: 10.1111/j.1528-1167.2009.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdernik TL, Emerson MR, Cross R, Nelson SR, Samson FE. Soman-induced seizures: limbic activity, oxidative stress, and neuroprotective proteins. J Appl Toxicol. 2001;21:S87–94. doi: 10.1002/jat.818. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta J, Yushkevich P, Das S, Wolk D. In vivo analysis of hippocampal subfield atrophy in mild cognitive impairment via semi-automatic segmentation of T2-weighted MRI. J Alzheimers Dis. 2012;31:85–99. doi: 10.3233/JAD-2012-111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor SP, Heaton KJ, Heeren T, White RF. Effects of chemical warfare agent exposure on central nervous system functioning in US army veterans of the 1991 Gulf War. Neurotoxicology. 2006;27:931–9. doi: 10.1016/j.neuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Qiu A, Younes L, Miller MI, Csernansky JG. Parallel transport in diffeomorphisms distinguishes the time-dependent pattern of hippocampal surface deformation due to healthy aging and the dementia of the Alzheimer’s type. Neuroimage. 2008;40:68–76. doi: 10.1016/j.neuroimage.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Timbol CR, Adewuyi O, Walitt B, VanMeter JW, et al. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War Illness. PLOS ONE. 2013;8:e58493. doi: 10.1371/journal.pone.0058493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Advisory Committee on Gulf War Veterans? Illnesses, Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. U.S. Government Printing Office; Washington, DC: 2008. [Google Scholar]
- Shing YL, Rodrigue KM, Kennedy KM, Fandakova Y, Bodammer N, Werkle-Bergner M, et al. Hippocampal subfield volumes: age, vascular risk, and correlation with associative memory. Front Aging Neurosci. 2011;3:1–8. doi: 10.3389/fnagi.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–81. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Thammaroj J, Santosh C, Bhattacharya J. The hippocampus: modern imaging of its anatomy and pathology. Pract Neurol. 2005;5:150–9. [Google Scholar]
- United States General Accounting Office. DOD’s conclusions about U.S. troops’ exposure cannot be adequately supported. Washington, DC: United States General Accounting Office; 2004. [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi B, Jones K, Pope C. Electrophysiological and biochemical effects of single and multiple doses of the organophosphate diazinon in the mouse. Toxicol Appl Pharmacol. 1990;104:440–56. [Google Scholar]
- Wang L, Miller JP, Gado MH, McKeel DW, Rothermich M, Miler MI, et al. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuroimage. 2006;30:52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Kasai K, Suga M, Iwanami A, Yamada H, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010;51:1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Wang H, Pluta J, Das SR, Craige C, Avants BB, et al. Nearly automatic segmentation of hippocampal subfields in in vivo focal T2 weighted MRI. Neuroimage. 2010;53:1208–24. doi: 10.1016/j.neuroimage.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11:668–83. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–80. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]