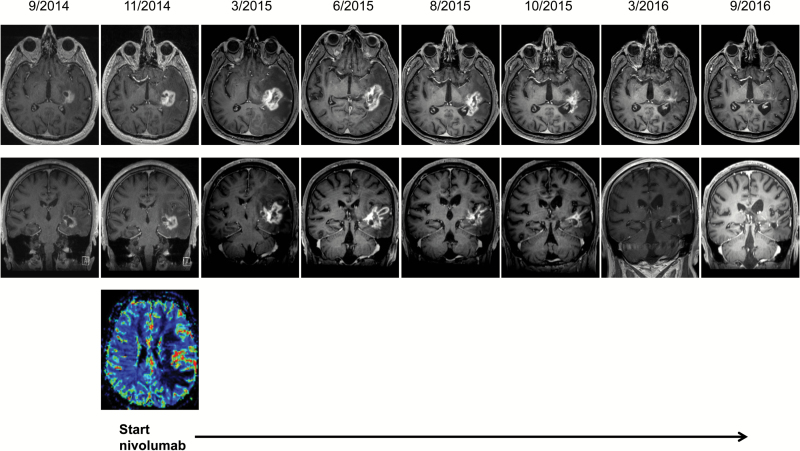
Immunotherapy has long been considered a promising treatment approach without, however, making significant overall progress over several decades. Only with the introduction of immune checkpoint inhibitors and advanced vaccines have these drugs gained widespread use in oncology. The role of immune checkpoint inhibitors such as antibodies blocking programmed cell death (PD)-1 signaling against primary brain tumors remains to be determined, since data from randomized trials are lacking so far.1,2 Still, it has already been recognized that various immunotherapeutic strategies may result in imaging changes which represent a challenge in differentiating true tumor progression from immune-related pseudoprogression. We report on a 60-year-old patient in whom glioblastoma was diagnosed in 2014. Molecular profiling revealed no isocitrate dehydrogenase mutation and an unmethylated O6-methylguanine DNA methyltransferase promoter status. The patient underwent standard treatment consisting of radiotherapy with concomitant temozolomide chemotherapy followed by 6 cycles of maintenance temozolomide. MRI after completion of 6 cycles of temozolomide demonstrated increased contrast enhancement indicating tumor progression, which was further corroborated by perfusion imaging. A decision was made to initiate treatment with the PD-1 inhibitor nivolumab within a clinical trial. Clinically, the patient presented in overall good performance status but had episodes with pronounced aphasia that required transient treatment with steroids. MRI after 3 months of PD-1 inhibitor therapy demonstrated increased edema and contrast enhancement (Fig. 1). Because of the clinically stable situation, a decision was made to continue nivolumab therapy. Subsequent MR imaging revealed a continuous shrinking of the tumor, and the patient has now been on nivolumab treatment for almost 2 years (Fig. 1). Treatment with nivolumab was tolerated without relevant toxicity and the patient has been clinically stable without needing further steroid medication.
Fig. 1.
T1-weighted contrast-enhanced MRI showing stable disease during (9/2014) and tumor progression after 6 cycles of maintenance temozolomide (11/2014). Tumor progression was confirmed by MR perfusion (bottom). The MRI scan 3 months after initiation of nivolumab treatment (3/2015) demonstrates increased contrast enhancement and pronounced edema. Subsequent MRI scans during ongoing nivolumab treatment indicate partial remission and durable tumor control.
The clinical and imaging course of this patient highlights the potential therapeutic activity, but also the challenges associated with the use of PD-1 inhibitors in glioblastoma patients. First, it must be assumed that the therapeutic effects following PD-1 inhibition may only occur after several months of treatment, most likely because of the delayed reinvigoration of the immune system. Furthermore, antitumor immune responses may be associated with increased edema and contrast enhancement due to immune cell infiltration and inflammation. The corresponding MR findings need to be interpreted with caution, since the differentiation of immune-related pseudoprogression and true progression can be challenging. This has been addressed by the recently developed immunotherapy Response Assessment for Neuro-Oncology (iRANO) criteria.3 Hence, premature cessation of PD-1 inhibitor therapy should be avoided in order to allow these drugs to fully exert their therapeutic potential.
In summary, this is one of the first reports supporting the idea that PD-1 inhibition can exert strong therapeutic activity against glioblastoma. Furthermore, the clinical course over almost 2 years as well as the MRI findings suggest that PD-1 inhibition may result in long-lasting tumor control following initial pseudoprogression.
Funding
This work was supported by a grant from the Canton of Zurich (HSM-2).
Conflict of interest statement. Patrick Roth has received honoraria from MSD, Roche, Novartis, and Molecular Partners for advisory board participation or lectures. Antonios Valavanis reports no disclosures. Michael Weller has received research grants from Acceleron, Actelion, Bayer, Isarna, MSD, Merck & Co, Novocure, PIQUR, and Roche and honoraria for lectures or advisory board participation or consulting from Celldex, Immunocellular Therapeutics, Isarna, Magforce, MSD, Merck & Co, Northwest Biotherapeutics, Novocure, Pfizer, Roche, and Teva.
References
- 1. Preusser M, Lim M, Hafler DA, et al. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11(9):504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss T, Weller M, Roth P. Immunotherapy for glioblastoma: concepts and challenges. Curr Opin Neurol. 2015;28(6):639–646. [DOI] [PubMed] [Google Scholar]
- 3. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]