Abstract
Many behaviors are learned through trial and error by matching performance to internal goals. Yet neural mechanisms of performance evaluation remain poorly understood. We recorded basal ganglia projecting dopamine neurons in singing zebra finches as we controlled perceived song quality with distorted auditory feedback. Dopamine activity was phasically suppressed after distorted syllables, consistent with a worse-than-predicted outcome, and was phasically activated at the precise moment of the song when a predicted distortion did not occur, consistent with a better-than-predicted outcome. Error response magnitude depended on distortion probability. Thus dopaminergic error signals can evaluate behaviors that are not learned for reward and are instead learned by matching performance outcomes to internal goals.
When practicing piano how do you know if you struck the right or wrong note? The problem is that there is nothing intrinsically ‘good’ or ‘bad’ about the sound of A-sharp. It entirely depends if that’s the note you wanted to strike at that time-step of the song. Performance evaluation requires sensory feedback to be compared with internal benchmarks that change from moment to moment in a sequence. Performance errors during musical performance (1, 2) and speech production (3) are associated with a frontal error-related negativity in the electroencephalogram that may relate to activity in ventral tegmental area (VTA) dopamine neurons (4). Yet while dopamine neurons are known to encode reward prediction error in tasks where animals seek primary rewards such as food or juice (5–7), it is not known if dopamine activity also encodes error in tasks that are not learned for primary reward and are instead learned by matching sensory feedback to internal performance benchmarks (8, 9).
Songbirds use auditory feedback to learn to sing and have a dopaminergic projection from VTA to Area X, a nucleus required for song learning (10–13). It’s hypothesized that a singing bird evaluates its own song to compute an auditory-error based reinforcement signal that guides learning – i.e. a neural signal that ‘tells’ vocal motor circuits if the recent vocalization was ‘good’ and should be reinforced or ‘bad’ and be eliminated (14, 15) (Fig. 1A). The neural correlates of song evaluation remain unknown (16–18), leading to alternative models of learning that do not require online error signals (19).
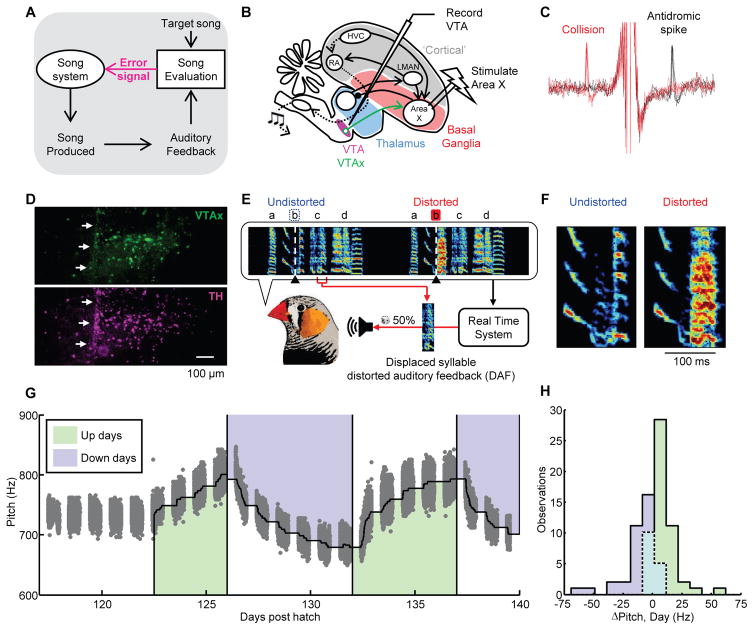
Fig. 1. Experimental test of performance error signals in birdsong.
(A) Evaluation of auditory feedback during singing is hypothesized to result in ‘error’ signals that reach the song system. (B) Strategy for antidromic identification of VTAx dopamine neurons. (C) Antidromic spikes (black) and spike collisions (red) of a VTAx neuron. (D) VTAx neurons labeled by injection of retrograde tracer into Area X (green, top) and co-labeled dopamine neurons stained with anti-tyrosine hydroxylase (TH) antibody (purple, bottom). White arrows point to the visible path of the electrode that recorded the VTAx unit shown in Fig. 2A (Scale, 100 μm; anterior-right, dorsal-top). (E) Example of displaced-syllable DAF. A snippet of syllable ‘c’ was played back during production of the target syllable ‘b’ (Target time, black triangles and white dashed lines). Randomly interleaved target renditions were left undistorted (undistorted trials, blue dashed line). (F) Expanded view of the target syllable. (G) Pitch-contingent displaced-syllable DAF drives learning. Grey dots denote mean pitch of 49716 target syllable renditions sung over 23 days for one bird. Shading demarcates distorted renditions; green, low pitch variants distorted (up days); blue, high pitch variants distorted (down days). (H) Histogram of pitch changes learned during each day (n=4 birds).
To test if dopamine activity encodes performance error, we recorded songbird VTA neurons while controlling perceived song quality with distorted auditory feedback (DAF) (18, 20–24) (Fig. 1B to F). Beginning days prior to recordings, a specific song syllable was either distorted with DAF or, on randomly interleaved trials, left undistorted altogether (distortion rate 44 ± 8%, n = 26 birds, Fig. 1, E and F). DAF was a 50 millisecond snippet of sound with the same amplitude and spectral content as normal zebra finch song (see supplementary text). The snippet was either a segment of one of the bird’s own syllables displaced in time (displaced-syllable DAF, n = 10 birds, Fig. 1E) or a synthesized sound designed to mimic broadband portions of the bird’s own song (broadband DAF, n = 16 birds) (20, 24). Operant broadband DAF drives dopamine and Area X-dependent reinforcement of undistorted syllable variants (13, 23). Displaced-syllable DAF, when operantly delivered contingent on the pitch of a harmonic target syllable, resulted in similar learning (Fig 1G, H) (20).
To test for online error responses, we compared the activity between randomly interleaved renditions of distorted and undistorted songs. We computed the z-scored difference between target onset-aligned distorted and undistorted rate histograms (Fig. 2, A to D, target onset defined as the median DAF onset time relative to distorted syllable onset, n = 125 neurons in 26 birds) (24). We defined the error response as the average z-scored difference in firing in a 50–125 millisecond interval following target onset (24). We plotted the distribution of error responses across the 125 VTA neurons and observed two distinct groups: one that did not exhibit significant error responses (n = 108 neurons, error response: 0.1 ± 0.9) and a group of error-responding neurons (n = 17 neurons, error response 3.3 ± 0.5, Fig. 2, E and F) that formed a distinct cluster (P < 0.001, bootstrap) (24). These two groups, defined as VTAerror (n = 17) and VTAother (n = 108), were spatially intermingled (fig. S1).
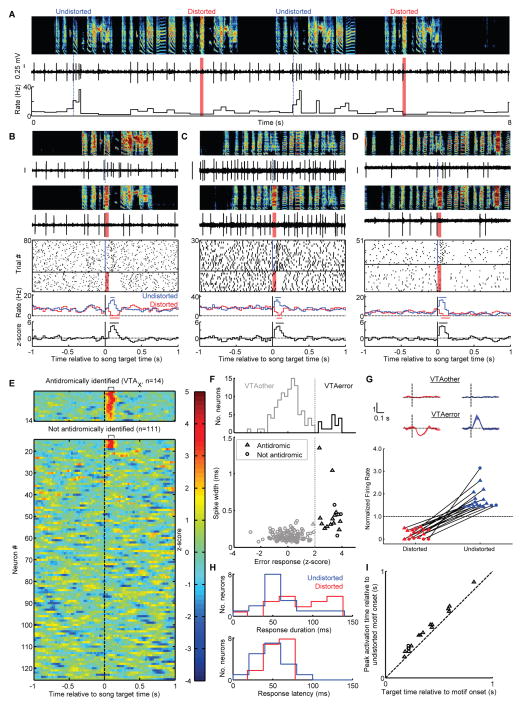
Fig. 2. VTA neurons encode performance error during singing.
(A) Spectrogram, voltage trace and the instantaneous firing rate of a VTAx neuron (DAF, red shading; undistorted targets, blue lines). (B) Top to bottom: spectrograms, spiking activity during undistorted and distorted trials, corresponding spike raster plots and rate histograms, and z-scored difference between undistorted and distorted rate histograms (plots aligned to target onset). Horizontal bars in histograms indicate significant deviations from baseline (P < 0.05, z-test) (24). (C) and (D) Two additional VTAerror neurons as in (B). (E) Each row plots the z-scored difference between undistorted and distorted target-aligned rate histograms. VTAx neurons (top, n=14) and non-antidromic neurons (bottom, n=111) are independently sorted by maximal z-score. (F) Top, distribution of error responses(24). Bottom, spikewidth versus error response (triangles: antidromic, circles: non-antidromic neurons). (G) Normalized response to distorted and undistorted targets (mean ± SEM) for VTAother (top) and VTAerror neurons (middle). Bottom, scatterplot of normalized rate in the 50–125 milliseconds following distorted and undistorted trials (solid fills indicate P < 0.05, bootstrap). (H) Distributions of phasic response durations (top) and latencies (bottom). (I) For each VTAerror neuron, the time of maximal firing rate relative to motif onset is plotted against target time.
All VTAerror neurons were phasically suppressed by DAF during singing (Fig. 2, A to D, G, P < 0.05 in 17/17 VTAerror neurons, bootstrap). Suppressions followed DAF onset with a latency of 58 ± 13 ms, lasted 86 ± 35 ms and resulted on average in a 75% reduction in firing rate (range: 45–100%; (24, 25)). DAF-induced suppressions during singing were highly reliable, occurring on an average of 94% of distorted trials (range: 82–100%). VTAerror neurons also exhibited phasic activations following the precise time-step of undistorted songs where DAF would have occurred but did not occur (Fig. 2, A to D, G, and I, P < 0.05 in the same 17 neurons that exhibited suppressions on distorted trials, bootstrap). Phasic activations mirrored the phasic suppressions: they followed target onsets with a latency of 51 ± 20 ms, lasted 62 ± 27 ms and resulted on average in a 77% (range: 42–214%) increase in firing rate (24) (Fig. 2H).
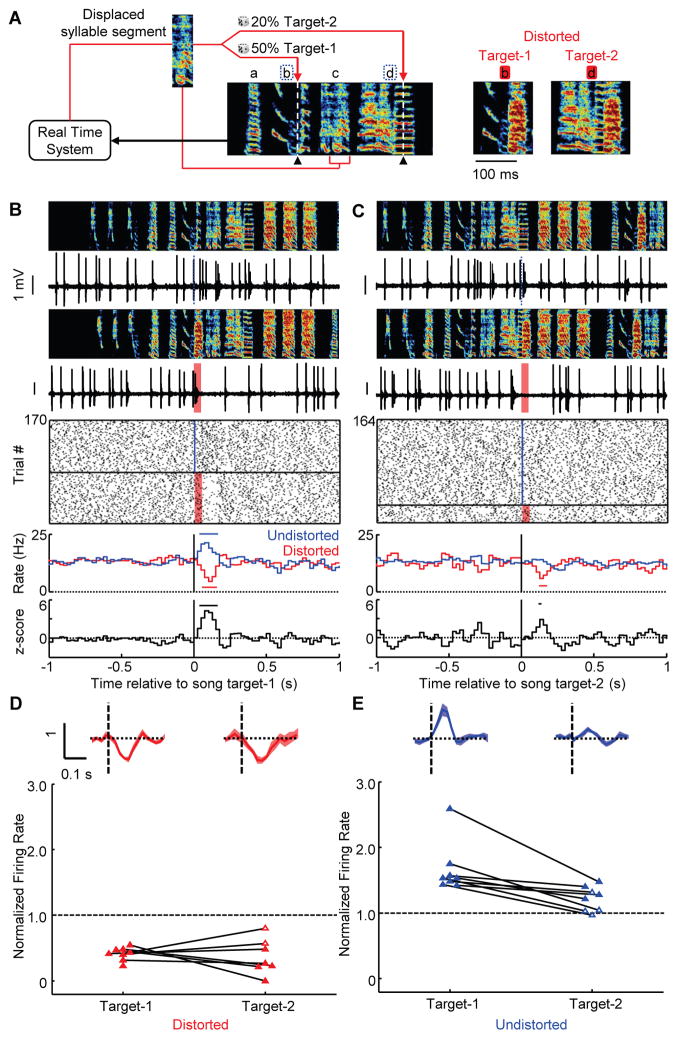
These precisely timed phasic activations suggest that undistorted target syllables are signaled as better than predicted, as if they are evaluated against an estimate of syllable quality that is diminished by a memory of errors (i.e. a flexible performance benchmark, see Supplementary text). To test if error signals are scaled by error history, we trained 10 birds in a two-target paradigm in which one syllable was distorted with a high probability (target 1, 49 ± 4%) and a second syllable with low probability (target 2, 20 ± 4%) (Fig. 3A to C) (24). The magnitude and reliability of phasic suppressions did not depend on error probability (% suppression: target-1: 59%, range: 45–77%; target-2: 63%, range: 20–100%, reliability: target-1: 90%, range: 82–100%; target-2: 86%, range: 71–100%, P > 0.4, rank sum tests, Fig. 3D), consistent with weak scaling of dopaminergic negative reward prediction error responses (6, 7). In contrast, phasic activations were significantly larger following (the more surprising) undistorted renditions of the high probability target (increase in firing rate, target-1: 67%, range: 42–159%; target-2: 22%, range: −3–48%, P < 0.001, rank sum test; Fig. 3E). Error responses to target 2 did not depend on whether or not the preceding target 1 was distorted and vice versa, indicating that song time-steps are independently evaluated against temporally aligned performance benchmarks (P > 0.05, rank sum tests and fig. S2).
Fig. 3. VTAerror responses depend on error probability.
(A) Displaced-syllable DAF scheme with 2 targets per motif (syllable b: target-1, distortion rate: 50%; syllable d, target-2, distortion rate: 20%, target times marked with dashed white line and black triangle). The distorted versions of the two target syllables are shown at right (color scheme as in Fig. 1E). (B) Target-1 and (C) target-2 error responses for the same neuron. Top to bottom: spectrograms, spiking activity during undistorted and distorted trials, corresponding spike raster plots and rate histograms, and z-scored difference between undistorted and distorted rate histograms (all plots aligned to target onset). Horizontal bars in histograms indicate significant deviations from baseline (P < 0.05, z-test) (24). (D) Top, normalized responses to distorted targets (mean ± SEM) for VTAerror neurons. Bottom, scatterplot of normalized rate in the 50–125 milliseconds following target time (solid fills indicate P < 0.05, bootstrap). (E) Same as (D) but for undistorted targets.
Over 95% of Area X projecting VTA neurons are dopaminergic (11). Fourteen of 125 VTA neurons were antidromically identified as projecting to Area X (Fig. 1B to D), and 13/14 VTAx neurons encoded performance error (Fig. 2E and F). VTAerror neurons discharged like mammalian dopamine neurons (see supplementary text, figs. S3 to S5).
Dopamine activity correlates with movement (26, 27). We quantified movement with microdrive-mounted accelerometers (fig. S6 and Movie S1). The activity of many VTA neurons was modulated by movement, which was in turn correlated with singing. But movement patterns during singing were not affected by DAF and error responses were not affected by movement (n = 26/26 birds, P > 0.05, bootstrapped d′ analysis, see supplementary text, Table S1 and S2, and figs. S6 to S10).
VTAerror neurons might not encode performance error but simply the presence or absence of DAF as if it were an aversive stimulus (see supplementary text). An aversive response should persist in birds during non-singing periods whereas performance error should be restricted to singing. During non-singing periods VTAerror neurons did not differentially respond to playback of distorted and undistorted renditions of the bird’s own song (normalized firing rate, distorted: 1.0 ± 0.2, undistorted: 1.1 ± 0.1, P > 0.3, unpaired t-test) (Fig. 4) and did not exhibit pauses in response to DAF (fig. S11). Confinement of VTAerror responses to singing is consistent with performance error.
Fig. 4. Response of VTAerror neurons to birdsong during non-singing.
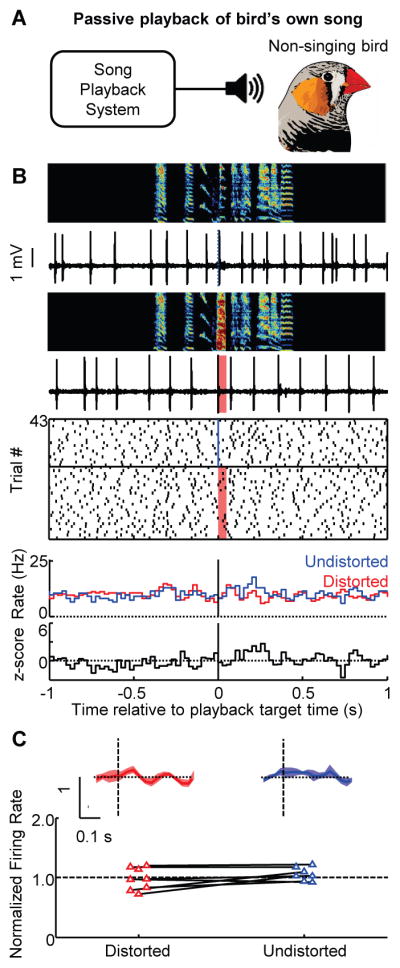
(A) Distorted and undistorted renditions of the bird’s own song was played back during non-singing periods. (B) Top to bottom: spectrograms, spiking activity of the VTAx neuron shown in Fig. 3 during playback of undistorted and distorted songs, corresponding spike raster plots and rate histograms, and z-scored difference between undistorted and distorted rate histograms (all plots aligned to target onset). (C) Normalized responses to distorted and undistorted targets (mean ± SEM) for VTAerror neurons during passive playback (top). Bottom, scatterplot of normalized rate in the 50–125 milliseconds following target time (empty fills indicate no significant response, P > 0.05, bootstrap) (24)).
Performance error signals during singing are similar to prediction error signals during reward seeking (5). Suppression of VTAerror activity after distorted syllables resembles the dopamine response to worse-than-predicted reward outcomes. Activation of VTAerror neurons after undistorted syllables resembles the dopamine response to better-than-predicted reward outcomes. The scaling of positive VTAerror responses according to error history suggests that song is evaluated against flexible performance benchmarks. Positive reward prediction error signals are also scaled by reward prediction (6, 7). Finally, performance and reward prediction error signals could underlie similar learning mechanisms. Dopamine-modulated corticostriatal plasticity links external stimuli to reward-maximizing responses (14). Dopamine-modulated corticostriatal plasticity also exists inside Area X (28) and could similarly link each time-step in the song to the specific vocalization that produces a favorable outcome when produced at that time-step (supplementary text and fig. S12). Such a mechanism would explain the reinforcement of undistorted syllable variants in operant DAF paradigms (Fig. 1G and H) (18, 20, 21, 23) and could contribute to natural song learning (14).
Yet unlike reward prediction error, performance error during singing is not derived from sensory feedback of intrinsic reward or reward-predicting value. The absence of error responses in birds passively hearing distorted or undistorted syllables suggests that there is nothing intrinsically ‘good’ or ‘bad’ about these sounds according to the performance monitoring system. Performance error might instead derive from evaluation of auditory feedback against internal performance benchmarks that require, at each time-step of the song sequence, information about the desired outcome, the actual outcome, and also the predicted probability of achieving the desired outcome. It remains unknown how upstream circuits construct the VTAerror signal. Multiple auditory cortical areas, including one that projects to VTA, respond to DAF specifically during singing (22, 25), providing a candidate pathway for auditory mismatch signals to reach VTA. A newly identified Area X – basal forebrain – VTA pathway (29) might additionally provide a temporally precise and syllable-specific memory of errors required to compute a benchmark against which mismatch error signals are scaled.
Supplementary Material
Acknowledgments
We thank Joe Fetcho, Melissa Warden, Michael Long, Aaron Andalman, and Dmitriy Aronov for comments on the manuscript; Jeremiah Cohen for mouse VTA recording data; Tejapratap Bollu and Don Murdoch for technical support; Jieying Wu and Kamal Maher for histology; Andrew Treska for art. Funding support to JHG by NIH (grant # R01NS094667), Pew Charitable Trusts and Klingenstein Neuroscience Foundation and to VG by Simons Foundation. VG and JHG designed the research, analyzed the data and wrote the paper. VG, PAP, RC, ARF, EB and JHG performed experiments. The authors declare no competing financial interest. Data can be accessed at http://www.nbb.cornell.edu/goldberg/
Footnotes
Materials and Methods
References
- 1.Maidhof C, Vavatzanidis N, Prinz W, Rieger M, Koelsch S. J Cogn Neurosci. 2010 Oct;22:2401. doi: 10.1162/jocn.2009.21332. [DOI] [PubMed] [Google Scholar]
- 2.Katahira K, Abla D, Masuda S, Okanoya K. Neurosci Res. 2008 May;61:120. doi: 10.1016/j.neures.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Trewartha KM, Phillips NA. Front Hum Neurosci. 2013;7:763. doi: 10.3389/fnhum.2013.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holroyd CB, Coles MG. Psychol Rev. 2002 Oct;109:679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 5.Schultz W, Dayan P, Montague PR. Science. 1997 Mar 14;275:1593. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 6.Fiorillo CD, Tobler PN, Schultz W. Science. 2003 Mar 21;299:1898. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 7.Bayer HM, Glimcher PW. Neuron. 2005 Jul 7;47:129. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolpert DM, Diedrichsen J, Flanagan JR. Nat Rev Neurosci. 2011 Dec;12:739. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Lewis R, Barto A, Sorg J. IEEE TRANSACTIONS ON AUTONOMOUS MENTAL DEVELOPMENT. 2010;2:70. [Google Scholar]
- 10.Konishi M. Z Tierpsychol. 1965 Dec;22:770. [PubMed] [Google Scholar]
- 11.Person AL, Gale SD, Farries MA, Perkel DJ. J Comp Neurol. 2008 Jun 10;508:840. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- 12.Scharff C, Nottebohm F. J Neurosci. 1991 Sep;11:2896. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann LA, Saravanan V, Wood AN, He L, Sober SJ. J Neurosci. 2016 Feb 17;36:2176. doi: 10.1523/JNEUROSCI.3883-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fee MS, Goldberg JH. Neuroscience. 2011 Dec 15;198:152. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doya K, Sejnowski T. Advances in Neural Information Processing Systems. 1995;7:101. [PubMed] [Google Scholar]
- 16.Leonardo A. Proc Natl Acad Sci U S A. 2004 Nov 30;101:16935. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozhevnikov AA, Fee MS. J Neurophysiol. 2007 Jun;97:4271. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hamaguchi K, Tschida KA, Yoon I, Donald BR, Mooney R. Elife. 2014;3:e01833. doi: 10.7554/eLife.01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahnloser R, Ganguli S. In: Principles of Neural Coding. Panzeri S, Quiroga P, editors. CRC Taylor and Francis; Boca Raton, FL: 2013. pp. 547–564. [Google Scholar]
- 20.Andalman AS, Fee MS. Proc Natl Acad Sci U S A. 2009 Jul 28;106:12518. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumer EC, Brainard MS. Nature. 2007 Dec 20;450:1240. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 22.Keller GB, Hahnloser RH. Nature. 2009 Jan 8;457:187. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- 23.Ali F, et al. Neuron. 2013 Oct 16;80:494. doi: 10.1016/j.neuron.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Materials and methods are available as supplementary materials on Science Online.
- 25.Mandelblat-Cerf Y, Las L, Denisenko N, Fee MS. Elife. 2014:3. doi: 10.7554/eLife.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X, Costa RM. Nature. 2010 Jul 22;466:457. doi: 10.1038/nature09263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howe MW, Dombeck DA. Nature. 2016 Jul 11; doi: 10.1038/nature18942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding L, Perkel DJ. J Neurosci. 2004 Jan 14;24:488. doi: 10.1523/JNEUROSCI.4358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gale SD, Perkel DJ. The Journal of neuroscience. 2010 Jan 20;30:1027. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg JH, Fee MS. Journal of Neurophysiology. 2010 Apr;103:2002. doi: 10.1152/jn.01038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayer HM, Lau B, Glimcher PW. J Neurophysiol. 2007 Sep;98:1428. doi: 10.1152/jn.01140.2006. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg JH, Fee MS. Nature Neuroscience. 2012 Apr;15:620. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canopoli A, Herbst JA, Hahnloser RH. J Neurosci. 2014 May 14;34:7018. doi: 10.1523/JNEUROSCI.0266-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlesworth JD, Warren TL, Brainard MS. Nature. 2012 Jun 14;486:251. doi: 10.1038/nature11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cynx J. J Comp Psychol. 1990 Mar;104:3. doi: 10.1037/0735-7036.104.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Horita H, Wada K, Jarvis ED. Eur J Neurosci. 2008 Dec;28:2519. doi: 10.1111/j.1460-9568.2008.06535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akutagawa E, Konishi M. J Comp Neurol. 2010 Aug 1;518:3086. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli S, Hahnloser R. paper presented at the CoSyne; Salt Lake City, UT. February 27 2011. [Google Scholar]
- 39.Blakemore SJ, Frith CD, Wolpert DM. J Cogn Neurosci. 1999 Sep;11:551. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- 40.West MJ, King AP. Nature. 1988 Jul 21;334:244. doi: 10.1038/334244a0. [DOI] [PubMed] [Google Scholar]
- 41.Zann RA. The zebra finch: a synthesis of field and laboratory studies. Vol. 5 Oxford University Press; 1996. [Google Scholar]
- 42.Benichov JI, et al. Curr Biol. 2016 Feb 8;26:309. doi: 10.1016/j.cub.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riters LV. Front Neuroendocrinol. 2012 Apr;33:194. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marler P. J Neurobiol. 1997 Nov;33:501. [PubMed] [Google Scholar]
- 45.Aronov D, Fee MS. PLoS One. 2012;7:e47856. doi: 10.1371/journal.pone.0047856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg JH, Adler A, Bergman H, Fee MS. J Neurosci. 2010 May 19;30:7088. doi: 10.1523/JNEUROSCI.0168-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gale SD, Perkel DJ. J Neurophysiol. 2006 Nov;96:2295. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- 48.Marinelli M, McCutcheon JE. Neuroscience. 2014 Dec 12;282:176. doi: 10.1016/j.neuroscience.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobb CJ, Jaeger D. Neurobiol Dis. 2015 Mar;75:177. doi: 10.1016/j.nbd.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niv Y, Daw ND, Joel D, Dayan P. Psychopharmacology (Berl) 2007 Apr;191:507. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 51.Cohen JY, Amoroso MW, Uchida N. Elife. 2015;4 doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanagihara S, Hessler NA. Eur J Neurosci. 2006 Dec;24:3619. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- 53.Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. Biol Psychol. 2013 Jul;93:377. doi: 10.1016/j.biopsycho.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Simons RF. Psychophysiology. 2010 Jan 1;47:1. doi: 10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 55.Barter JW, et al. Front Integr Neurosci. 2015;9:39. doi: 10.3389/fnint.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romo R, Schultz W. J Neurophysiol. 1990 Mar;63:592. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- 57.Sutton RS, Barto AG. Reinforcement learning: an introduction. MIT Press; Cambridge, MA: 1998. p. 322. [Google Scholar]
- 58.Hong S, Hikosaka O. Front Behav Neurosci. 2011;5:15. doi: 10.3389/fnbeh.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kao MH, Doupe AJ, Brainard MS. Nature. 2005 Feb 10;433:638. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- 60.Olveczky BP, Andalman AS, Fee MS. PLoS Biol. 2005 May;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoover JE, Strick PL. Science. 1993 Feb 5;259:819. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- 62.Elemans CP. Curr Opin Neurobiol. 2014 Oct;28:172. doi: 10.1016/j.conb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Luo M, Ding L, Perkel DJ. J Neurosci. 2001 Sep 1;21:6836. doi: 10.1523/JNEUROSCI.21-17-06836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gale SD, Person AL, Perkel DJ. J Comp Neurol. 2008 Jun 10;508:824. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- 65.Nottebohm F, Kelley DB, Paton JA. J Comp Neurol. 1982 Jun 1;207:344. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- 66.Redondo RL, Morris RG. Nature reviews Neuroscience. 2011 Jan;12:17. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 67.Yagishita S, et al. Science. 2014 Sep 26;345:1616. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fee MS. Front Neural Circuits. 2012;6:38. doi: 10.3389/fncir.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.