Abstract
Background.
Chordomas are rare, locally aggressive bony tumors associated with poor outcomes. Recently, the single nucleotide polymorphism (SNP) rs2305089 in the T (brachyury) gene was strongly associated with sporadic chordoma development, but its clinical utility is undetermined.
Methods.
In 333 patients with spinal chordomas, we identified prognostic factors for local recurrence-free survival (LRFS) and overall survival and assessed the prognostic significance of the rs2305089 SNP.
Results.
The median LRFS was 5.2 years from the time of surgery (95% CI: 3.8–6.0); greater tumor volume (≥100cm3) (hazard ratio [HR] = 1.99, 95% CI: 1.26–3.15, P = .003) and Enneking inappropriate resections (HR = 2.35, 95% CI: 1.37–4.03, P = .002) were independent predictors of LRFS. The median overall survival was 7.0 years (95% CI: 5.8–8.4), and was associated with older age at surgery (HR = 1.11 per 5-year increase, 95% CI: 1.02–1.21, P = .012) and previous surgical resection (HR = 1.73, 95% CI: 1.03–2.89, P = .038). One hundred two of 109 patients (93.6%) with available pathologic specimens harbored the A variant at rs2305089; these patients had significantly improved survival compared with those lacking the variant (P = .001), but there was no association between SNP status and LRFS (P = .876).
Conclusions.
The ability to achieve a wide en bloc resection at the time of the primary surgery is a critical preoperative consideration, as subtotal resections likely complicate later management. This is the first time the rs2305089 SNP has been implicated in the prognosis of individuals with chordoma, suggesting that screening all patients may be instructive for risk stratification.
Keywords: brachyury, chordoma, rs2305089, SNP, spine, T gene.
Chordomas are rare, locally aggressive bony lesions of the axial skeleton comprising 1% to 4% of all primary bone tumors.1 From population-based studies with Surveillance, Epidemiology, and End Results (SEER) data, their incidence has been estimated to be 0.8 per 100 000 individuals in the general population and are most often observed in men in their fifth and sixth decades of life.2 Despite their indolent course, their locally aggressive nature and large size at diagnosis often complicate management. Wide margin, en bloc surgical resection is the evidence-based treatment, but this comes with morbidity challenges due to critical neurological and vascular anatomy. Radiation therapy is now favored as an adjuvant particularly when true oncologic margins are not achievable.3,4
Almost all chordomas demonstrate overexpression of the T (brachyury) gene, a transcription factor of the T-box family critical for notochord development. Germline duplications of a region containing only the T gene, 6q27, have been found in patients with familial chordoma.5,6 Of sporadic chordomas, several single nucleotide polymorphisms (SNPs) have been implicated in chordoma susceptibility, the most prominent of which is rs2305089, where the variant A allele results in a Gly177Asp alteration and has been associated with a sixfold increase in the risk of developing chordoma.7,8 The low incidence of chordomas and absence of correlational clinical–histologic data have made identifying other clinical factors with prognostic significance and assessing the value of the rs2305089 SNP difficult. The present study, which included 333 patients from 13 international spine oncology institutions, was conducted with the largest contemporary cohort of patients with chordomas of the spine.9 The aims were to (i) identify prognostic factors for local recurrence-free survival (LRFS) and overall survival (OS) of patients with surgically treated spinal chordomas and (ii) assess the prognostic significance of the rs2305089 SNP.
Materials and Methods
Design
Study design was a retrospective review of primarily prospectively collected data with cross-sectional survival. An initial cohort of 333 patients with chordomas treated at 13 centers within Europe, North America, and Australia between December 1985 and January 2013, through the AOSpine Knowledge Forum Tumor (AOSKFT) primary tumor database was reviewed. Patients were included if they were admitted to one of the participating centers and received surgical treatment for their spinal column chordoma. Ethics approval was applied for and received individually at each center prior to accessing patient data. The AOSKFT model and a subset of patients included in this study have been described in previous publications.10,11 A secure web-based application (REDCap, Vanderbilt University) was used to gather demographic, clinical, diagnostic, therapeutic, local recurrence, perioperative morbidity, and survival data. For this study, approximately 70% of all data were collected prospectively, with the remaining 30% of data collected through retrospective chart review. Information regarding patient mortality was acquired cross-sectionally. Archived paraffin embedded pathologic specimens were available for 109 patients from 5 of the 13 centers.
Definitions and Staging
The overall neurologic status of patients was classified based on the American Spinal Injury Association (ASIA) impairment scale (AIS) and the Frankel classification system. Patients were all evaluated with CT and MRI of the spine. Tumor volume was estimated using tumor measurements in 3 dimensions in an ellipsoid model.12 Histologic classification and tumor grading were performed by a musculoskeletal tumor pathologist at each individual center.
For guidance and standardization of treatment, the validated Enneking classification for determination of appropriate surgical margins of musculoskeletal tumors (intralesional, marginal, or wide) was used. A subset of patients when indicated received preoperative embolization and/or adjuvant treatment in the form of preoperative/postoperative chemotherapy and/or radiation therapy. The final surgical margin was based on the pathological assessment, and if wide or marginal margins were achieved, the specimen was classified as Enneking appropriate (EA) resection, while if the specimen was deemed intralesional, the tumor was considered to have undergone Enneking inappropriate (EI) resection.
SNP rs2305089 Genotyping
Chordoma samples were acquired from patients with appropriate consent and institutional approval. DNA was extracted from formalin-fixed paraffin-embedded (FFPE) slides using the DNA FFPE Tissue Kit (Qiagen, catalog # 56404). Genotyping was performed using Sanger Sequencing of the brachyury gene using the following primers: (forward) 5ʹ-CAGGAAACAGCTATGACCACCGCTATGAACTGGGTCT-3ʹ and (reverse) 5ʹ-TAATACGACTCACTATAGGGCTTTCAGTGCCACCAATCCT-3ʹ.
Statistical Analysis
Descriptive statistics regarding demographics, treatment, recurrence, and mortality were generated. Data are presented as mean±SD, number with (%), or median with interquartile range (IQR). For differences between cohorts, the chi-square test (Pearson’s or Fisher’s exact) and Student’s t-tests or Wilcoxon-Mann-Whitney tests were used to establish significance. Kaplan–Meier survival analyses were performed over a 10-year period postoperative and the Mantel–Cox log-rank test was used to assess factors associated with LRFS and OS. Cox proportional hazards regression was used for multivariate analyses. Factors with at least a marginal effect on LRFS and OS (P ≤ .1) according to univariate analysis were selected for the multivariate analysis. Due to multicollinearity, some variables, although significant at the univariate level, were not finally included in the multivariate models. Data are presented with 95% confidence intervals (CIs). Statistical significance was set at P < .05. All statistical analyses were performed using STATA (version 12.0).
Results
Patient Population and Baseline Characteristics
A total of 333 patients with spinal column chordomas treated with surgery were included (Table 1). The majority of patients, 209 (62.8%), were male. Patients had a mean age of 58±14 years at the time of surgery. At the time of presentation, 294 of 318 (92.5%) with available data had pain, 37 of 303 (12.2%) had myelopathy, and 51 of 301 (16.9%) presented with cauda equina syndrome. Most patients did not exhibit significant preoperative neurologic impairment based on their AIS and Frankel scores (Table 1). Sixty-five of 332 patients (19.6%) had previously undergone surgical resection; in 2 of 50 cases (4.0%) wide resection was achieved, 5 (10.0%) marginal resections, and 43 (86.0%) intralesional resections. Upon imaging, tumors were found to be located mostly in the lumbosacral spine, with 78 of 219 patients (35.6%) with lumbar and 63 (28.8%) sacral lesions. Classified as Enneking stage I were 224 of 312 patients (71.8%), and 88 (28.2%) as stage II. Additional tumor parameters are provided in Supplementary Table 1.
Table 1.
Demographics, baseline, and chordoma characteristics for the total sample of 333 patients
| Variable | |
|---|---|
| Gender (n = 333) | |
| Male | 209 (62.8) |
| Female | 124 (37.2) |
| Age at surgery, years (n = 333) | 58±14 |
| Presentation | |
| Pain (n = 318) | 294 (92.5) |
| Myelopathy (n = 303) | 37 (12.2) |
| Cauda equina (n = 301) | 51 (16.9) |
| Preoperative function | |
| Frankel classification (n = 257) | |
| A | 3 (1.2) |
| B | 1 (0.4) |
| C | 10 (3.9) |
| D | 62 (24.1) |
| E | 181 (70.4) |
| AIS (n = 217) | |
| A | 3 (1.4) |
| B | 1 (0.5) |
| C | 8 (3.7) |
| D | 31 (14.3) |
| E | 174 (80.1) |
| Previous surgical resection (n = 332) | 65 (19.6) |
| Type of previous surgical resection (n = 50) | |
| Intralesional | 43 (86.0) |
| Marginal | 5 (10.0) |
| Wide | 2 (4.0) |
| Chordoma characteristics | |
| Spinal level (n = 333) | |
| Mobile | 166 (49.8) |
| Fixed | 167 (50.2) |
| Level involved (n = 219) | |
| Cervical | 54 (24.7) |
| Thoracic | 24 (11.0) |
| Lumbar | 78 (35.6) |
| Sacral | 63 (28.8) |
| Number of contiguous levels involved (n = 330) | |
| 1 | 110 (33.3) |
| 2–3 | 110 (33.3) |
| ≥4 | 110 (33.3) |
| Tumor diameter, cm | |
| Anterior-posterior | 5.5 (3.5, 9.5) |
| Left-right | 5.0 (3.3, 8.0) |
| Superior-inferior | 5.0 (3.3, 8.0) |
| Volume, cm3 (n = 265) | |
| <100 | 151 (57.0) |
| ≥100 | 114 (43.0) |
| Pathological fracture (n = 320) | 36 (11.3) |
| Diagnostic biopsy (n = 333) | |
| Open | 50 (15.0) |
| Trocar CT-guided | 165 (49.5) |
| Intraoperative | 61 (18.3) |
| Other | 57 (17.1) |
| Enneking grade (n = 312) | |
| I | 224 (71.8) |
| II | 88 (28.2) |
Values are given as number with (%), mean±SD, or as median (p25, p75).
One hundred nine patients had pathologic specimens available for study and a comparable mean age at surgery (59±14 y) and gender distribution (n = 67, 61.5% male) to the complete study cohort (Supplementary Table 1). Thirty-four (31.2%) had lesions of the mobile spine, while 75 (68.8%) were of the fixed spine. The histologic subtype was known in 36 patients, with 26 (72.2%) with classic chordomas, 3 (8.3%) with chondroid, and 2 (5.6%) with dedifferentiated tumors; 102 (93.6%) harbored the A variant at rs2305089, with 66 patients (60.6%) with an AA genotype, 36 (33.0%) GA, and 7 (6.4%) GG. No statistically significant associations were observed between patient SNP status and gender (P = .426), ethnicity (P = 1.000), age at surgery (P = .149), spinal level (P = .675), tumor grade (P = .432), and chordoma subtype (P = 1.000).
Treatment
All patients underwent surgical resection of their chordomas. The treatment data are summarized in Table 2. Overall, surgery was classified as EA in 179 out of 300 patients (59.7%) and EI in 121 (40.3%). Several factors were significantly associated with EA resections in a univariate model, including younger age at surgery (P = .017), no previous surgical resection (P < .001), trocar CT-guided biopsy compared with open and intraoperative biopsy (P < .001), lack of neurologic impairment as assessed by the Frankel classification and AIS (P = .007), tumors of the fixed spine (P < .001), greater number of vertebral levels spanned by the tumor (P = .029), nerve root sacrifice (P < .001), and lack of adjuvant therapy (P < .001) (Supplementary Table 2). The extent of resection was comparable in patients with and without the A variant, with 62 patients (62.6%) harboring the A variant and 3 of 7 (42.9%) without the A variant undergoing EA resections (P = .426). Of 326 patients, 117 (35.9%) received adjuvant therapy in the form of radiation therapy or chemotherapy (Table 2). Significantly fewer patients harboring the A variant underwent radiation therapy compared with those without [21 (20.6%), 3 (42.9%), respectively; P = .048].
Table 2.
Summary of chordoma treatment for the total sample of 333 patients
| Variable | |
|---|---|
| Preoperative embolization (n = 319) | 40 (12.5) |
| Surgical approach (n = 322) | |
| Anterior | 16 (5.0) |
| Posterior | 169 (52.5) |
| Combined | 137 (42.5) |
| Fixation (n = 329) | 179 (54.4) |
| Sacrifice of neurologic elements | |
| Spinal cord (n = 307) | 1 (0.3) |
| Cauda equina (n = 307) | 10 (3.3) |
| Nerve roots (n = 306) | 183 (59.8) |
| Number of levels (n = 7)a | 3 (2, 4) |
| Side (n = 129)a | |
| Unilateral | 20 (15.5) |
| Bilateral | 109 (84.5) |
| Level (n = 183)a, b | |
| Cervical | 12 (6.6) |
| Thoracic | 21 (11.5) |
| Lumbar | 37 (20.2) |
| Sacral | 125 (68.3) |
| Coccygeal | 34 (18.6) |
| Surgical margins (n = 299) | |
| Wide or marginal | 201 (67.2) |
| Intralesional | 98 (32.8) |
| Enneking appropriateness (n = 300) | |
| EA | 179 (59.7) |
| EI | 121 (40.3) |
| Adjuvant therapy (n = 326) | 117 (35.9) |
| Chemotherapy (n = 330) | |
| Preoperative | 10 (3.0) |
| Postoperative | 12 (3.6) |
| Both | 2 (0.6) |
| None | 306 (92.7) |
| Radiation therapy (n = 326) | |
| Preoperative | 40 (12.3) |
| Postoperative | 65 (19.9) |
| Both | 6 (1.8) |
| None | 215 (66.0) |
| Type of radiation therapy (n = 97) | |
| Conventional | 41 (42.3) |
| IMRT | 22 (22.7) |
| Proton beam | 26 (26.8) |
| SRS | 8 (8.2) |
Values are given as number with (%) or as median (p25, p75).
EA: Enneking appropriate, EI: Enneking inappropriate, IMRT: intensity-modulated radiation therapy, SRS: stereotactic radiosurgery.
aRepresents the patients with nerve root sacrifice.
bPercentages given out of 183; patients with tumors spanning multiple levels were counted multiple times.
Patient Outcomes
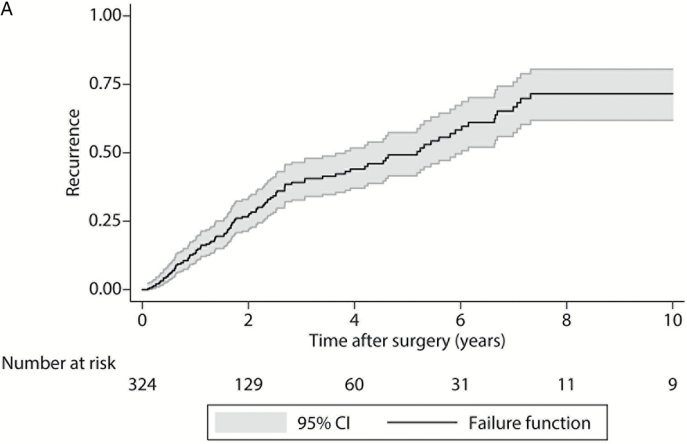
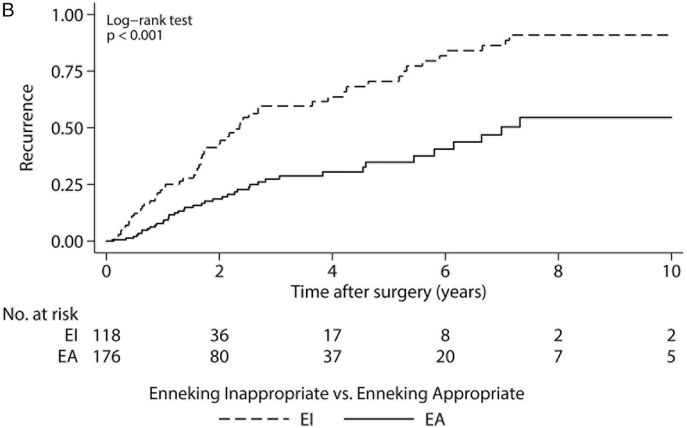
Following treatment, 110 of 324 patients (34.0%) experienced local recurrence within 10 years of surgical resection, and 104 of 333 (31.2%) had died (Supplementary Table 3). The median LRFS was 5.2 years from the time of surgery (95% CI: 3.8–6.0) (Fig. 1A). In our univariate model, the development of local recurrence was significantly associated with previous surgical resection (P < .001), intraoperative biopsy compared with open and trocar CT-guided biopsy (P < .001), Frankel classification or AIS grade E at presentation compared with grades A through D (P = .021), greater tumor volume (≥100cm3) (P = .010), Enneking stage I disease (P = .045), sacrifice of nerve roots (P = .019), intralesional compared with wide or marginal margins at resection (P < .001), EI resection compared with EA (Fig. 1B, P < .001), and use of adjuvant therapy (P = .009). In the multivariate model, greater tumor volume (≥100cm3) (hazard ration [HR] = 1.99, 95% CI: 1.26–3.15, P = .003) and Enneking appropriateness (EI resection) (HR = 2.35, 95% CI: 1.37–4.03, P = .002) were both independent predictors of LRFS (Supplementary Table 4).
Fig. 1.
Kaplan–Meier local recurrence-free survival curves. (A) For 324 patients; (B) 294 patients by Enneking appropriateness. P value from log-rank test. Gray shaded regions represent 95% CIs. EA: Enneking appropriate; EI: Enneking inappropriate.
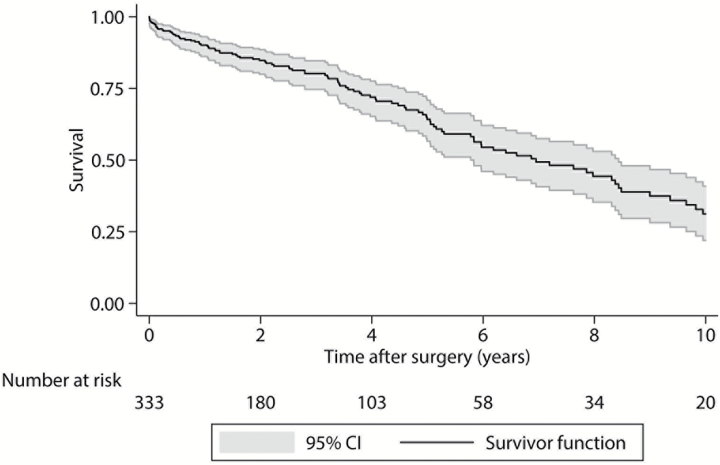
The median OS following surgery was 7.0 years (95% CI 5.8–8.4) (Fig. 2). Decreased survival was significantly associated with older age at the time of surgery (≥65 y) (P < .001), previous surgical resection (P = .002), open and intraoperative biopsy compared with trocar CT-guided biopsy (P = .035), Frankel classification or AIS grades A through D at presentation compared with grade E (P < .001), and use of adjuvant therapy (P = .036). Degree of resection and Enneking appropriateness were not significantly associated with OS (P = .182 and P = .113, respectively). In our final multivariate model, older age at surgery (HR = 1.11 per 5-y increase, 95% CI: 1.02–1.21, P = .012) and previous resection (HR = 1.73, 95% CI: 1.03–2.89, P = .038) were associated with decreased survival (Supplementary Table 4).
Fig. 2.
Kaplan–Meier overall survival curve. Gray shaded regions represent 95% CIs.
We repeated the multivariate Cox regression analysis for local recurrence and survival over a 10-year period following surgical resection in the subgroup of 109 patients who had available genotyping data. Regarding local recurrence, adjustment for SNP genotype attenuated somehow the harmful effect of both EI resection and high volume. Nevertheless, there was no evidence for a significant association between local recurrence and SNP genotype. After adjusting for SNP genotype in the multivariate Cox regression model evaluating survival, the association of survival with age at surgery and previous surgical resection, identified in the overall sample size, remained almost unchanged. We revealed significantly higher mortality risk for patients with GA or GG genotype compared with patients with AA (Table 3).
Table 3.
Results of the multivariate Cox regression analysis for local recurrence and survival over a 10-year period following surgical resection
| Variables | Category | Local Recurrence | |
|---|---|---|---|
| Hazard Ratio (95% CI) | P value | ||
| Age at surgery | 5 y more | 1.04 (0.90, 1.20) | 0.606 |
| Volume (cm3) | < 100 | Ref. | 0.193 |
| ≥ 100 | 1.75 (0.75, 4.05) | ||
| Enneking appropriateness | EA | Ref. | 0.118 |
| EI | 2.02 (0.84, 4.86) | ||
| SNP genotype | AA | Ref. | |
| GA | 0.86 (0.36, 2.07) | 0.735 | |
| GG | 1.20 (0.26, 5.57) | 0.814 | |
| Variables | Category | Survival | |
| Hazard Ratio (95% CI) | P value | ||
| Age at surgery | 5 y more | 1.17 (1.02, 1.34) | 0.024 |
| SNP genotype | AA | Ref. | |
| GA | 2.80 (1.15, 6.85) | 0.024 | |
| GG | 6.85 (2.33, 20.17) | < 0.001 | |
| Previous surgical resection | No | Ref. | 0.026 |
| Yes | 2.91 (1.14, 7.45) | ||
EA: Enneking appropriate, EI: Enneking inappropriate.
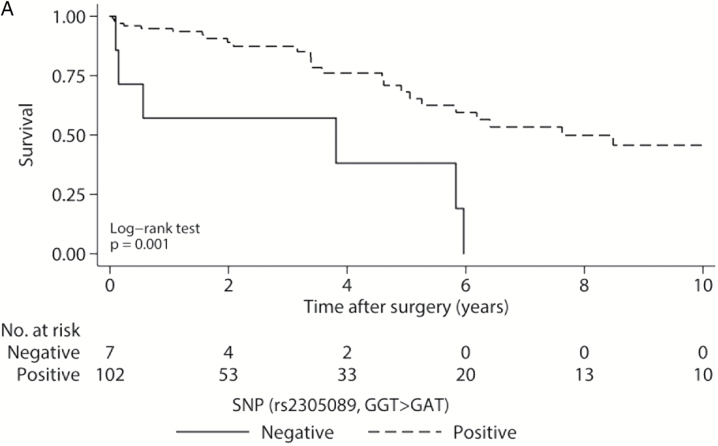
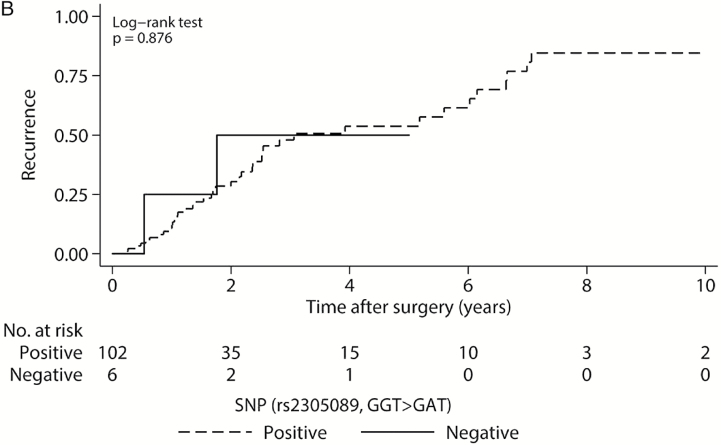
Of the genotyped patients, 42 of 108 (38.9%) experienced local recurrence within 10 years of surgical resection, and 31 of 109 (28.4%) had died (Supplementary Table 3). Patients harboring the A variant were found to have significantly improved survival, with a median OS of 7.6 years compared with those lacking the variant, at 3.8 years (Fig. 3A, P = .001). However, the median LRFS for patients with the A variant was 3.1 years, comparable to 1.8 years in those without (Fig. 3B, P = .876).
Fig. 3.
Kaplan–Meier survival curves for genotyped patients. (A) Overall survival for all 109 genotyped patients by SNP status. (B) Local recurrence-free survival for genotyped patients by SNP status. P values from log-rank tests. Gray shaded regions represent 95% CIs.
Discussion
The rarity of chordomas has limited previous studies, as most of the evidence guiding management is single-center level IV or V.9 Three hundred thirty-three patients were included in this study from multiple centers, the largest contemporary surgical cohort of chordomas of the mobile spine and sacrum assembled. Overall, the median LRFS was 5.2 years and median OS 7.0 years, comparable to a 2013 study using SEER administrative data up to 2009, where the median OS was 7.7 years.13 In our study, greater tumor volume and Enneking appropriateness (EI resection) were both independent predictors of LRFS, and older age and previous surgical resection were independent predictors of OS. In several single-center small studies of chordomas of the mobile spine and sacrum from the 1990s to early 2000s, the degree of surgical resection was the only factor consistently identified as predictive of LRFS and OS.14–18 Two studies using registry data by Mukherjee et al and Lee et al included over 400 patients, and metastatic disease, tumor outside of the periosteum and tumor size greater than 5.0cm, use of chemotherapy, and surgical margins were found to be predictive of OS.19,20 While adequately powered, these administrative databases lack detailed information regarding patient management and can contain inaccuracies.
Of the more recent studies, that of Stacchiotti and colleagues,21 in a series of 138 patients with chordomas of the mobile spine and sacrum, also identified tumor size and surgical margins to be predictive of LRFS in a multivariate model. In contrast to our study, the only factor they found to be an independent predictor of OS was tumor size. Aggressive approaches have been demonstrated in numerous studies to be associated with lower rates of local recurrence and improved disease-free survival.9,11,21–23 Violation of the tumor capsule during resection and subtotal resection have both been associated with early local recurrence.18,24 However, the ultimate goal of treatment is maximal safe aggressive resection and preservation of neurologic function. Previous studies have found that wide en bloc resection is achievable in up to 50% of lesions of the sacrum, but at significantly lower rates for spinal and skull base chordomas.18,25,26
Interestingly, in our multivariate analysis of prognostic factors for OS, younger age at surgery and lack of previous surgical resection were the only factors independently associated with improved survival, and Enneking appropriateness was not predictive of survival. The majority of previous studies did not include prior surgery in their analyses of prognostic factors, and of the studies which considered this factor, poorer outcomes were seen in patients who had undergone previous resections. In a study by Boriani and colleagues,10 16 of 18 patients (89%) who had undergone prior resections experienced disease recurrence. However, it is important to also recognize the relationship between previous tumor resection and later surgical outcomes. In our cohort, 48 of 50 patients (98.0%) were known to have previous subtotal resections, which likely made achieving an Enneking appropriate resection during reoperation considerably more difficult. These results suggest that the ability to achieve a wide en bloc resection at the time of the primary surgery is a critical preoperative consideration, as intralesional resections may complicate later treatment options for recurrent disease and ultimately have a negative impact on outcomes. Therefore, consideration should be made to refer these patients to centers that have expertise in the multidisciplinary surgical management of these rare tumors.
The benefit of adjuvant therapy in treating spinal chordomas has still not been definitively demonstrated. Chemotherapy has been used after subtotal resections, but no chemotherapeutic agents are currently approved for chordoma. With respect to radiation therapy, reported rates of local recurrence remain high, with LRFS around 50% to 60% at 5 years.3,4 Stacchiotti and colleagues found no significant effect of radiotherapy on OS and LRFS but noted that low doses were used and newer techniques were not employed.21 Indeed, most studies of chordoma incorporate patients treated in the 1960s to 1980s, and radiotherapy regimens in these older studies are heterogeneous. Within our cohort, adjuvant therapy was not a significant predictor of LRFS in the multivariate model, which may be due to the significant association between Enneking appropriateness and adjuvant therapy. However, newer radiotherapy regimens have shown promise in recent clinical trials; in a phase II trial of high dose photo/proton radiotherapy for spinal chordomas and other primary spinal column tumors, high rates of local control were seen at 5 and 8 years posttreatment.27,28
Prior to this study, no biomarkers existed to guide risk stratification. We found that OS was significantly improved in patients with the A variant at rs230508 compared with those without, but this was not accounted for by local recurrence. Relatively little is understood regarding the molecular biology of chordomas compared with other bony malignancies. The T (brachyury) gene has been implicated in the pathogenesis of the disease, and the A variant at rs2305089, a common SNP found in 47% of individuals of northern and western European ancestry, is associated with sporadic chordoma risk. While brachyury is known to be overexpressed in almost all chordomas, it is also expressed in lung, colorectal, breast, and prostate cancers and is associated with a poor prognosis.5,29–31 Upregulation of brachyury results in upregulation of mesenchymal markers, downregulation of epithelial markers, and increased cell migration and invasion; ultimately, it is thought to promote the epithelial-mesenchymal transition. However, the genetic basis of brachyury overexpression appears complex and cannot be completely accounted for by somatic and germline alterations that have currently been identified. High-resolution array comparative genomic hybridization demonstrated the presence of germline duplications of a region of 6q27 containing only the T gene in 4 out of 7 multiplex families.6 Subsequently, somatic copy number gains, including minor allelic gain in 4.5% and amplification in 7% of 181 chordoma cases were identified, but studies have not revealed any mutations of the coding exons or promoter regions of the T gene.32
Pillay et al found that in copy number–neutral chordoma cases, the relative expression of T and its downstream targets was significantly higher in chordomas with the AA genotype compared with the GA genotype, and suggested that the rs2305089 SNP could alter the binding capacity of brachyury.7 However, these findings and the association of brachyury with cell migration and invasion appear incongruous with our results that patients with the A variant have a significantly improved prognosis compared with those without. It is possible that the SNP may simultaneously increase brachyury expression and increase chordoma vulnerability to innate antitumor mechanisms or certain treatment modalities. Alternatively, chordomas in patients without the SNP may rely on alternate mechanisms to upregulate brachyury or other pathways, which could result in a more aggressive phenotype. The ultimate impact of the rs2305089 SNP on brachyury is unknown and requires further investigation utilizing high quality, prospectively gathered samples; however, screening all patients for the SNP may still be instructive for risk stratification. A limitation to this study is that only DNA derived from archived FFPE samples was studied, limiting our ability to query for T-gene duplications, which could potentially impact overall survival.
Despite the use of large-scale population-based multicenter data, the current study remains limited by the rarity of chordomas and variability between institutions. The limited number of patients in our cohort without the A variant prevented us from exploring possible interactions among SNP status, adjuvant therapy, and other prognostic factors and their impact on OS. Further studies are needed to fully elucidate the significance of rs2305089 SNP status. Nevertheless, this study remains the largest contemporary series of spinal chordomas and is the first time the rs2305089 SNP has been implicated in the prognosis of individuals with chordoma.
Supplementary material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
Financial support was provided by AOSpine International.
Supplementary Material
Acknowledgments
AOSpine is a clinical division of the AO Foundation—an independent medically guided nonprofit organization. The AOSpine Knowledge Forums are pathology-focused working groups acting on behalf of AOSpine in their domain of scientific expertise. Each forum consists of a steering committee of up to 10 international spine experts who meet on a regular basis to discuss research, assess the best evidence for current practices, and formulate clinical trials to advance spine care worldwide. Study support is provided directly through AOSpine’s Research department and AO’s Clinical Investigation and Documentation unit. We would also like to thank Arpad Bozsodi for expert laboratory assistance.
Conflict of interest statement. No conflicts of interest to disclose.
References
- 1. Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20(3):417–426. [PubMed] [Google Scholar]
- 2. McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3. Potluri S, Jefferies SJ, Jena R, et al. Residual postoperative tumour volume predicts outcome after high-dose radiotherapy for chordoma and chondrosarcoma of the skull base and spine. Clin Oncol (R Coll Radiol). 2011;23(3):199–208. [DOI] [PubMed] [Google Scholar]
- 4. Stuer C, Schramm J, Schaller C. Skull base chordomas: management and results. Neurol Med Chir. 2006;46(3):118–124; discussion 124-115. [DOI] [PubMed] [Google Scholar]
- 5. Vujovic S, Henderson S, Presneau N, et al. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209(2):157–165. [DOI] [PubMed] [Google Scholar]
- 6. Yang XR, Ng D, Alcorta DA, et al. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41(11):1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pillay N, Plagnol V, Tarpey PS, et al. A common single-nucleotide variant in T is strongly associated with chordoma. Nat Genet. 2012;44(11):1185–1187. [DOI] [PubMed] [Google Scholar]
- 8. Kelley MJ, Shi J, Ballew B, et al. Characterization of T gene sequence variants and germline duplications in familial and sporadic chordoma. Hum Genet. 2014;133(10):1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stacchiotti S, Sommer J. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(2):e71–e83. [DOI] [PubMed] [Google Scholar]
- 10. Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31(4):493–503. [DOI] [PubMed] [Google Scholar]
- 11. Varga PP, Szoverfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014. [DOI] [PubMed] [Google Scholar]
- 12. Ruggieri P, Angelini A, Pala E, et al. Infections in surgery of primary tumors of the sacrum. Spine. 2012;37(5):420–428. [DOI] [PubMed] [Google Scholar]
- 13. Smoll NR, Gautschi OP, Radovanovic I, et al. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer. 2013;119(11):2029–2037. [DOI] [PubMed] [Google Scholar]
- 14. Samson IR, Springfield DS, Suit HD, et al. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75(10):1476–1484. [DOI] [PubMed] [Google Scholar]
- 15. Cheng EY, Ozerdemoglu RA, Transfeldt EE, et al. Lumbosacral chordoma. Prognostic factors and treatment. Spine. 1999;24(16):1639–1645. [DOI] [PubMed] [Google Scholar]
- 16. York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery. 1999;44(1):74–79; discussion 79–80. [DOI] [PubMed] [Google Scholar]
- 17. Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122–2134. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87(10):2211–2216. [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee D, Chaichana KL, Gokaslan ZL, et al. Survival of patients with malignant primary osseous spinal neoplasms: results from the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2003. J Neurosurg. Spine. 2011;14(2):143–150. [DOI] [PubMed] [Google Scholar]
- 20. Lee J, Bhatia NN, Hoang BH, et al. Analysis of prognostic factors for patients with chordoma with use of the California Cancer Registry. J Bone Joint Surg Am. 2012;94(4):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg oncol. 2010;17(1):211–219. [DOI] [PubMed] [Google Scholar]
- 22. Stener B, Gunterberg B. High amputation of the sacrum for extirpation of tumors. Principles and technique. Spine. 1978;3(4):351–366. [DOI] [PubMed] [Google Scholar]
- 23. Hsieh PC, Xu R, Sciubba DM, et al. Long-term clinical outcomes following en bloc resections for sacral chordomas and chondrosarcomas: a series of twenty consecutive patients. Spine. 2009;34(20):2233–2239. [DOI] [PubMed] [Google Scholar]
- 24. Kaiser TE, Pritchard DJ, Unni KK. Clinicopathologic study of sacrococcygeal chordoma. Cancer. 1984;53(11):2574–2578. [DOI] [PubMed] [Google Scholar]
- 25. Bjornsson J, Wold LE, Ebersold MJ, et al. Chordoma of the mobile spine. A clinicopathologic analysis of 40 patients. Cancer. 1993;71(3):735–740. [DOI] [PubMed] [Google Scholar]
- 26. Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet. Oncol. 2012;13(2):e69–76. [DOI] [PubMed] [Google Scholar]
- 27. Uhl M, Edler L, Jensen AD, et al. Randomized phase II trial of hypofractionated proton versus carbon ion radiation therapy in patients with sacrococcygeal chordoma-the ISAC trial protocol. Radiat Oncol. 2014;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol. 2014;110(2):115–122. [DOI] [PubMed] [Google Scholar]
- 29. Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13(8):2471–2478. [DOI] [PubMed] [Google Scholar]
- 30. Kilic N, Feldhaus S, Kilic E, et al. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 2011;47(7):1080–1085. [DOI] [PubMed] [Google Scholar]
- 31. Pinto F, Pertega-Gomes N, Pereira MS, et al. T-box transcription factor brachyury is associated with prostate cancer progression and aggressiveness. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(18):4949–4961. [DOI] [PubMed] [Google Scholar]
- 32. Presneau N, Shalaby A, Ye H, et al. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol. 2011;223(3):327–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.