Abstract
Liver injury after experimental acetaminophen treatment is mediated both by direct hepatocyte injury through a P450-generated toxic metabolite and indirectly by activated liver Kupffer cells and neutrophils. This study was designed to investigate the role of Notch signaling in the regulation of innate immune responses in acetaminophen (APAP)-induced liver injury. Using a mouse model of APAP-induced liver injury, wild-type (WT) and toll-like receptor 4 knockout (TLR4 KO) mice were injected intraperitoneally with APAP or PBS. Some animals were injected with γ-secretase inhibitor DAPT or DMSO vehicle. For the in vitro study, bone marrow-derived macrophages (BMMs) were transfected with Notch1 siRNA, TLR4 siRNA, and non-specific (NS) siRNA and stimulated with LPS. Indeed, paracetamol/acetaminophen-induced liver damage was worse after Notch blockade with DAPT in wild-type mice, which was accompanied by significantly increased ALT levels, diminished hairy and enhancer of split-1 (Hes1), phosphorylated Stat3 and Akt but enhanced high mobility group box 1 (HMGB1), TLR4, NF-κB, and NLRP3 activation after APAP challenge. Mice receiving DAPT increased macrophage and neutrophil accumulation and hepatocellular apoptosis. However, TLR4 KO mice received DAPT reduced APAP-induced liver damage, NF-κB, NLRP3, and cleaved caspase-1 activation. BMMs transfected with Notch1 siRNA reduced Hes1, phosphorylated Stat3 and Akt but augmented HMGB1, TLR4, NF-κB, and NLRP3. Furthermore, TLR4 siRNA knockdown resulted in decreased NF-κB, NLRP3, cleaved caspase-1, and IL-1β levels following LPS stimulation. These results demonstrate that Notch signaling regulates innate NLRP3 inflammasome activation through regulation of HMGB1/TLR4/NF-κB activation in APAP-induced liver injury. Our novel findings underscore the critical role of the Notch1-Hes1 signaling cascade in the regulation of innate immunity in APAP-triggered liver inflammation. This might imply a novel therapeutic potential for the drug-induced damage-associated lethal hepatitis.
Keywords: Notch, Hes1, DAPT, HMGB1, TLR4, liver inflammation
Introduction
An overdose of acetaminophen (N-acetyl-p-aminophenol, APAP) is the most common cause of severe liver damage, ultimately leading to acute liver failure (ALF) [1]. The chances of survival for advanced ALF are needed for liver transplantation [1, 2]. The mouse models of APAP-induced liver injury and clinical translational studies revealed that APAP-induced liver damage is not only influenced by the dose of APAP and the initial hepatocyte injury but also by the inflammatory response, which is recognized by activating hepatic macrophages (Kupffer cells) and neutrophils [3, 4].
The innate immune system constitutes the first line of defense against invading microbial pathogens and relies on a large family of pattern recognition receptors (PRRs). A range of pattern recognition receptors (PRRs) recognize specific pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). Among the PRRs, the Toll-like receptors (TLRs) are the first identified and the best characterized receptors that present at the surface of macrophages and neutrophils. Activation of macrophages and neutrophils leads to the release of pro-inflammatory mediators [5].
Notch signaling is highly conserved and critically involved in cell growth, differentiation, and survival [6]. The Notch signaling pathway regulates cell differentiation, proliferation, survival and development. In mammalian cells, four distinct Notch receptors (Notch 1–4) and five Notch ligands (Jagged1, Jagged2, Delta-like 1 (DLL1), DLL3, and DLL4) have been identified [7, 8]. The interaction between Notch receptors and their ligands leads to two proteolytic cleavage steps by a disintegrin and metalloprotease (ADAM) family proteases and the intracellular γ-secretase complex that releases the Notch intracellular domain (NICD). NICD then translocates to the nucleus, where it binds with RBP-J, a potent DNA-binding transcription factor that associates with a large number of chromatin regulators, corepressors, and coactivators [9]. This interaction results in activation of Notch target genes [10]. In the immune system, Notch signaling controls the homeostasis of several innate cell populations and regulates immune cell development and function [11]. Activation of Notch1 and its ligand Jagged-1 increases cell growth and differentiation during liver regeneration [12]. Disruption of Notch signaling increases intracellular ROS production and inflammatory response, leading to aggravated liver injury induced by ischemia and reperfusion [13].
The NLRP3 (nucleotide-binding domain leucine-rich repeat containing family, pyrin domain containing 3) inflammasome is a member of the NLR family of cytosolic pattern recognition receptors and can be activated by various pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) released from stressed or injured cells [14]. NLRP3 regulates the innate immune system through activation of caspase-1 to induce the maturation and secretion of IL-1 β [15]. Accumulated evidence demonstrates the important role of NLRP3 in the regulation of immune response associated with inflammatory diseases [16]. NLRP3 is critical for the activation of inflammatory response in Alzheimer’s disease [17]. Cholesterol crystals induce inflammation by activating NLRP3 in atherosclerotic lesions [18]. Moreover, recent reports in mouse models supported by human heritable and acquired diseases with dysregulated inflammasome activity strongly implies that NLRP3 initiates immune responses in metabolic disorders and neurodegenerative diseases [19–23]. We previously found that disruption of NLRP3 inhibited caspase-1 activity and IL-1β production, resulting in reduced liver inflammation in ischemia and reperfusion injury [24]. However, little is known about how Notch signaling may regulate NLRP3 activation during APAP-induced liver injury.
In the present study, we identified the novel regulatory role of Notch signaling in APAP-induced liver injury. We demonstrated that blocking Notch pathway triggers NLRP3 inflammasome activation through activation of HMGB1/TLR4/NF-κB signaling, which leads to augmented inflammatory responses and aggravated APAP-induced liver damage. Our data documents that Notch signaling is critical for the regulation of innate immunity in the mechanism of APAP-induced liver injury.
Materials and methods
Animals
Male C57BL/6 wild-type (WT) and TLR4 knockout (TLR4 KO, B6.B10ScN-Tlr4lps-del/JthJ) mice at - 6–8 weeks of age were used (The Jackson Laboratory, Bar Harbor, ME). All animals were housed in an animal facility under specific pathogen-free conditions. The animals were fed a laboratory diet with water and food and kept under constant environmental conditions with 12 hours light-dark cycles. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University, China, and the University of California at Los Angeles.
Mouse model and treatment
The Acetaminophen (APAP, Sigma-Aldrich) solution was freshly prepared for each experiment by dissolving acetaminophen in phosphate-buffered saline (PBS) at a concentration of 10 mg/ml and warmed to 40°C. Male mice were fasted for 16 hours and then treated intraperitoneally (i.p.) with 400 mg/kg of body weight APAP or PBS. In some experiments, mice were injected via tail vein with γ-secretase inhibitor DAPT (10 mg/kg, Sigma-Aldrich, MO) or DMSO vehicle at 30 min prior to APAP treatment. Animals were euthanized by ketamine/xylazine injection at the indicated time-point for collecting serum and liver tissues. Animal survival was observed every 4 hours for 72 hours until they became moribund.
Hepatocellular function assay
Serum alanine aminotransferase (sALT) levels, an indicator of hepatocellular injury induced by APAP treatment, were measured by IDEXX Laboratories (Westbrook, ME).
Histology and immunohistochemistry
The liver tissue from mice was harvested and rinsed with PBS, and then immersed into 10% of buffered formalin overnight. After processing for paraffin embedding, the liver sections (5μm) were stained with hematoxylin and eosin (H&E). Liver macrophages were detected using primary rat anti-mouse CD68 mAb (AbD Serotec, Raleigh, NC). The secondary, biotinylated goat anti-rat IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
Myeloperoxidase activity assay
The presence of myeloperoxidase (MPO) was used as an index of hepatic neutrophil accumulation [25]. The change in absorbance was measured spectrophotometrically at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide/min at 25°C per gram of tissue.
TUNEL assay
The Klenow-FragEL DNA Detection Kit (EMD Chemicals, Gibbstown, NJ) was used to detect the DNA fragmentation characteristic of apoptosis in formalin-fixed paraffin-embedded liver sections [26]. The results were scored semi-quantitatively by averaging the number of apoptotic cells/HPF (400×magnification). Ten fields were evaluated per tissue sample.
Quantitative RT-PCR analysis
The total RNA (2.5 μg) was reverse-transcribed into cDNA using the SuperScript™ III System (Invitrogen, CA). Quantitative real-time PCR was performed using a Platinum SYBR Green qPCR Kit (Invitrogen). The amplification conditions were 50°C (2min), 95°C (5min), followed by 40 cycles of 95°C (15sec) and 60°C (30sec). Primer sequences used for the amplification were shown in Supplementary Table 1.
Western blot analysis
Proteins (30μg/sample) from cell cultures or liver samples were subjected to 12% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). Monoclonal rabbit anti-mouse Hes1, HMGB1, TLR4, NLRP3, phos-Stat3, phos-Akt, NF-κB, cleaved caspase-1, cleaved caspase-3, and β-actin Abs (Cell Signaling Technology, Danvers, MA) were used. Relative quantities of protein were determined using a densitometer and expressed in absorbance units (AU).
BMM isolation and in vitro transfection
Murine bone marrow-derived macrophages (BMMs) were generated as described [26]. Bone marrow cells were removed from the femurs and tibias of WT mice and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS and 15% L929-conditioned medium. Cells (1×106/well) cultured for 7 days were transfected with 100 nM of Notch1 siRNA or TLR4 siRNA (Santa Cruz Biotechnology) using Lipojet™ In vitro Transfection Kit (SignaGen Laboratories, Rockville, MD). In some experiments, TLR4 siRNA-treated cells were incubated with 10 μM of DAPT (Sigma-Aldrich). The non-specific (NS) siRNA were used as controls. After 48 hours, the cells were supplemented with 100 ng/ml of LPS for an additional 6 hours.
Statistical analysis
Data are expressed as mean±SD and analyzed by Student’s t-tests. Multiple group comparisons were performed using one-way ANOVA with a post-hoc test. Per comparison two-sided p values less than 0.05 were considered statistically significant.
Results
Blockade of Notch signaling pathway aggravates APAP-induced liver damage
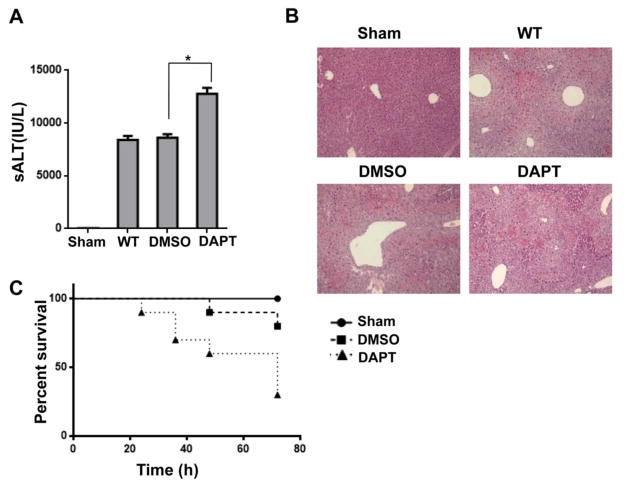
DAPT, a γ-secretase inhibitor, can block Notch signaling by preventing the final cleavage step of the precursor form of Notch to the active Notch intracellular domain (NICD) [27]. To investigate whether Notch signaling may affect inflammatory responses in mouse livers, we used a mouse model of APAP-induced liver injury. Mice treated with DAPT showed increased sALT levels compared to the DMSO-treated controls at 24 hours after APAP challenge (Fig. 1A, 12443±872 vs. 8621±322, p<0.01). In contrast, mice treated with vehicle DMSO controls showed less hepatic hemorrhage and necroinflammation after APAP challenge (Fig. 1B). Livers in mice revealed more hepatic hemorrhage and severe necrosis after receiving DAPT. To determine whether Notch signaling may be involved in animal survival, we monitored APAP-challenged mice for 72 hours after DAPT treatment. The mortality was dramatically increased in DAPT-treated mice compared to vehicle DMSO-treated controls (Fig. 1C). These results indicate that Notch signaling plays an important role in preventing APAP-induced liver injury.
Figure 1. Blockade of Notch signaling pathway aggravates APAP-induced liver damage.
Mice were subjected to injection of γ-secretase inhibitor DAPT (10 mg/kg, Sigma-Aldrich, MO) or DMSO vehicle via tail vein at 30 min prior to APAP (400 mg/kg) challenge. (A) Hepatocellular function, assessed by serum ALT levels (IU/L). Results expressed as mean±SD (n=4–6 samples/group), *p<0.01. (B) Representative histological staining (H&E) of APAP-conditioning liver tissue (n=4–6/group). Original magnification x100. (C) Animal survival curves after a single dose of APAP treatment over 72 hours (p< 0.05).
Blockade of Notch signaling pathway augments macrophage/neutrophil trafficking in APAP-induced liver injury
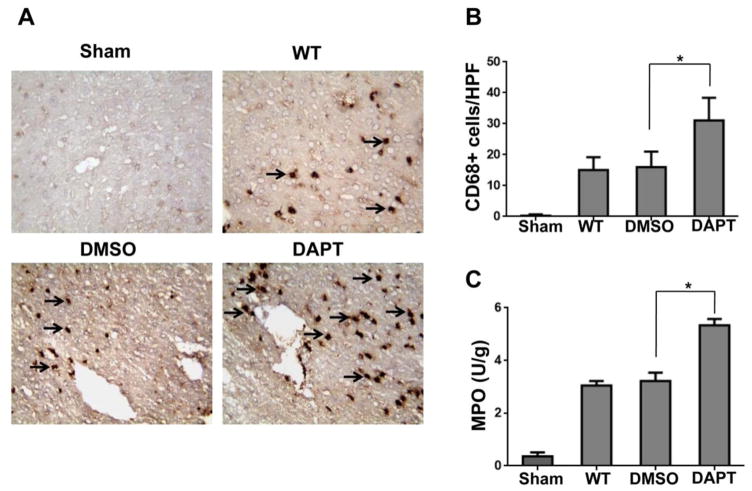
To determine whether Notch signaling might regulate inflammatory cell trafficking, we performed immunohistochemical staining of CD68+ macrophages in APAP-challenged livers. Indeed, DAPT treatment augmented CD68+ macrophage infiltration (Figure 2A and 2B, 31.1±7.3) compared to the DMSO controls (16.2±5.0, p<0.01) at 24 hours after APAP challenge. Moreover, the MPO assay, which reflects hepatic neutrophil activity, was increased in livers of mice subjected to DAPT condition (Figure 2C, 5.31±0.12) compared to the DMSO (3.27±0.21, p±0.05) or WT controls (3.05±0.13, p<0.05).
Figure 2. Blockade of Notch signaling pathway augments macrophage/neutrophil trafficking in APAP-induced liver injury.
(A) Liver macrophages were detected by immunohistochemical staining using mAb against mouse CD68+ in APAP-challenged livers after DAPT or DMSO treatment. (B) Quantification of CD68+ macrophages per high power field. Results scored semi-quantitatively by averaging the number of positively-stained cells (mean±SD)/field at 200× magnification. Representative of 4–6 mice/group. **p<0.01. (C) Liver neutrophil accumulation, analyzed by MPO activity (U/g). Mean±SD (n=4–6 samples/group). *p<0.05.
Blockade of Notch signaling pathway inhibits Hes1 and promotes HMGB1/TLR4/NF-κB and NLRP3 activation in APAP-induced liver injury
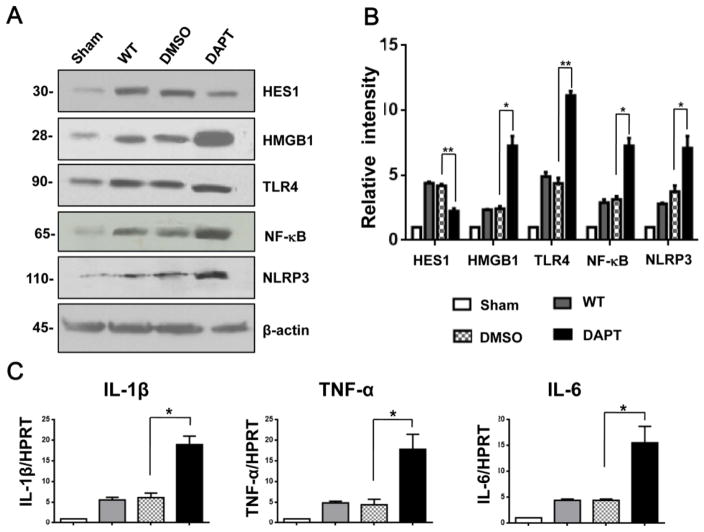
Next, we investigated whether disruption Notch signaling may affect innate immune responses in a mouse model of APAP-induced liver injury. We found that by 24 hours of APAP challenge, the protein expression of Hes1 was down-regulated in DAPT- but not in DMSO-treated livers (Fig. 3A and 3B). However, DAPT treatment up-regulated the protein expression of HMGB1, TLR4, NF-κB, and NLRP3 in APAP-challenged livers (Fig. 3A and 3B). Moreover, livers in mice receiving DAPT increased the mRNA levels of proinflammatory genes coding for IL-1β, TNF-α, and IL-6 compared to the DMSO controls (Fig. 3C).
Figure 3. Blockade of Notch signaling pathway inhibits Hes1 and induces HMGB1/TLR4 and NLRP3 activation in APAP-induced liver injury.
(A) Western-assisted expression of Hes1, HMGB1, TLR4, NF-κB, and NLRP3 in APAP-challenged livers with DAPT or DMSO precondition. β-actin served as an internal control. Data representative of three experiments. (B) Density ratios of Hes1, HMGB1, TLR4, NF-κB, and NLRP3. *p<0.05, **p<0.01. (C) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and IL-6 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05.
Blockade of Notch signaling pathway promotes APAP-induced hepatocellular apoptosis
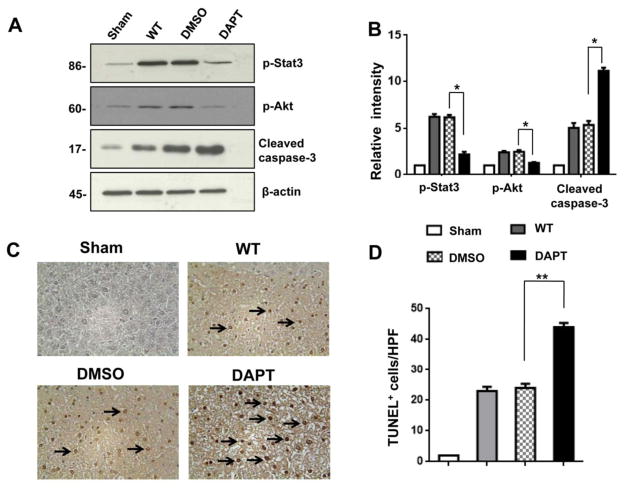
We used Western blots to analyze whether Notch signaling regulates cell apoptosis in APAP-challenged livers. Indeed, DAPT treatment inhibited phosphorylation of Stat3 and Akt compared to the DMSO controls (Fig. 4A and 4B). The increased expression of cleaved caspase-3 was observed in DAPT- but not in DMSO-treated livers (Fig. 4A and 4B). This result was further confirmed by TUNEL staining. We found that DAPT preconditioning augmented the frequency of TUNEL+ cells in APAP-challenged liver lobes (Fig. 4C and 4D, 44.5±1.4), as compared with DMSO (Fig. 4C and 4D, 24.2±0.9, p<0.001) or WT (Fig. 4C and 4D, 22.2±1.7, p<0.001) controls.
Figure 4. Blockade of Notch signaling pathway promotes APAP-induced hepatocellular apoptosis.
(A) Western blot analysis of phos-Stat3, phos-Akt, and cleaved caspase-3 in APAP-challenged livers after DAPT or DMSO treatment. β-actin served as an internal control. Data representative of three experiments. (B) Density ratios of Hes1, HMGB1, TLR4, and NLRP3. *p<0.05. (C) TUNEL-assisted detection of apoptosis in APAP-challenged liver tissue. Representative of 4–6 mice/group. (D) TUNEL+ cells were scored semi-quantitatively by averaging the number of apoptotic cells (mean±SD) per field at 200× magnification. A minimum of ten fields was evaluated per sample. *p<0.01. Representative of 4–6 mice/group.
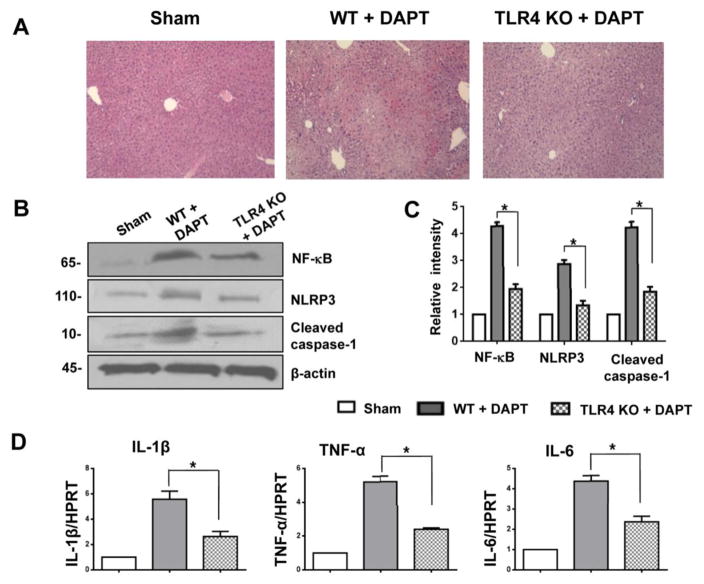
Disruption of TLR4 signaling ameliorates APAP-induced liver damage and inhibits NLRP3 activation
Because innate TLR4 signaling is critical for triggering inflammatory response and tissue injury [28], we then investigated whether TLR4 may affect Notch signaling in regulating NLRP3 activation in APAP-induced liver injury. At 24 hours of DAPT precondition, livers in WT mice showed severe necrosis (Fig. 5A). In contrast, livers in TLR4 KO mice after receiving DAPT revealed mild hemorrhage without necrosis (Fig. 5A). DAPT treatment increased the expression of NF-κB, NLRP3, and cleaved caspase-1 in APAP-challenged mice (Fig. 5B and 5C). However, disruption of TLR4 in DAPT-treated mice resulted in reduced NF-κB, NLRP3, and cleaved caspase-1 (Fig. 5B and 5C). The expression of pro-inflammatory IL-1β, TNF-α, and IL-6 consistently decreased in TLR4 KO but not in WT mice treated with DAPT (Fig. 5D). These results suggest that Notch signaling regulates NLRP3 activation via a TLR4-dependent pathway.
Figure 5. Disruption of TLR4 signaling ameliorates APAP-induced liver damage and inhibits NLRP3 activation.
TLR4 KO mice were injected via tail vein with γ-secretase inhibitor DAPT (10 mg/kg) or DMSO vehicle at 30 min prior to APAP challenge. (A) Representative histological staining (H&E) of ischemic liver tissue. Results representative of 4–6 mice/group; original magnification x100. (B) Western-assisted expression of NF-κB, HMGB1, NLRP3, and cleaved caspase-1 in APAP-challenged livers with DAPT or DMSO precondition. β-actin served as an internal control. Data representative of three experiments. (C) Density ratios of NF-κB, HMGB1, NLRP3, and cleaved caspase-1. *p<0.05. (D) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and IL-6 in mouse livers. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05.
Notch signaling regulates HMGB1/TLR4/NF-κB and NLRP3 activation in vitro
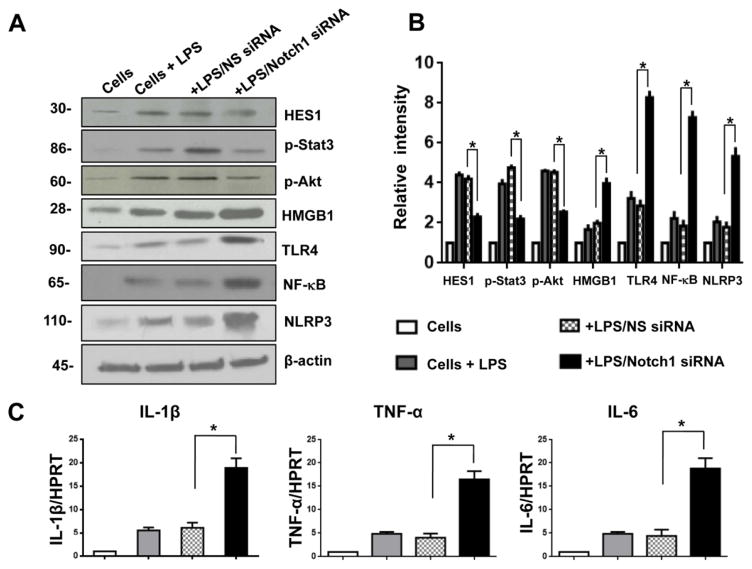
To elucidate the putative mechanisms by which Notch signaling might regulate NLRP3 activation during inflammatory response, we disrupted Notch1 in BMMs by using a small interfering RNA (Notch1 siRNA). Unlike in the control NS siRNA group, knockdown of Notch1 with Notch1 siRNA pretreatment depressed Hes1 expression and phosphorylated Stat3 and Akt in LPS-stimulated BMMs (Fig. 6A and 6B). However, increased HMGB1, TLR4, NF-κB, and NLRP3 expression was observed in Notch1 siRNA- but not in the NS siRNA-treated group (Fig. 6A and 6B). Moreover, Notch1 treatment augmented the mRNA levels of IL-1β, TNF-α, and IL-6 in BMMs after LPS stimulation compared to the NS siRNA-treated controls (Fig. 6C). These results indicate that the Notch receptor is a key regulator in the modulation of NLRP3 activation during inflammatory response.
Figure 6. Notch signaling regulates HMGB1/TLR4 and NLRP3 activation in vitro.
Bone marrow-derived macrophages (BMMs) were isolated from WT mice and transfected with Notch1 siRNA, and then stimulated with LPS for 6 h. The non-specific (NS) siRNA served as controls. (A) Western blot analysis of Hes1, phos-Stat3, phos-Akt, HMGB1, TLR4, NF-κB, and NLRP3 in LPS-stimulated macrophages. β-actin served as an internal control. Data representative of three experiments. (B) Density ratios of Hes1, phos-Stat3, phos-Akt, HMGB1, TLR4, NF-κB, and NLRP3. *p<0.05. (C) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and IL-6 in LPS-stimulated macrophages. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05.
TLR4 signaling is essential for Notch-mediated immune regulation of NLRP3 activation in vitro
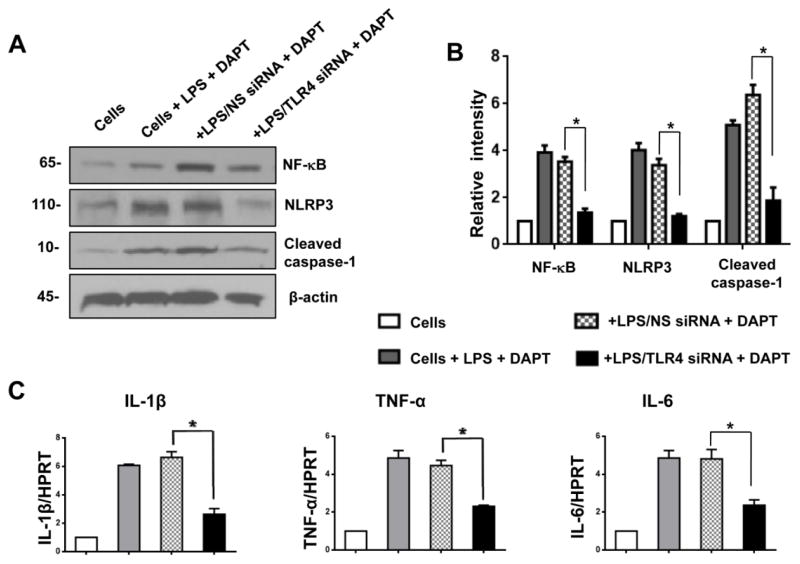
To further dissect the importance of TLR4 signaling in Notch-mediated immune regulation of NLRP3 activation during inflammatory response, we disrupted TLR4 with TLR4 siRNA after DAPT treatment in LPS-stimulated BMMs. DAPT precondition increased the expression of NF-κB, NLRP3 and cleaved caspase-1 in NS siRNA-transfected BMMs (Fig. 7A). However, knockdown of TLR4 by TLR4 siRNA transfection diminished NF-κB, NLRP3, and cleaved caspase-1 in DAPT-treated cells (Fig. 7A). Consistent with this data, knockdown of TLR4 resulted in reduced mRNA levels coding for IL-1β, TNF-α, and IL-6 in LPS-stimulated BMMs after DAPT treatment (Fig. 7C). These results imply that TLR4 signaling might be essential for the Notch-mediated immune regulation of NLRP3 activation during inflammatory response.
Figure 7. TLR4 signaling is essential for Notch-mediated immune regulation of NLRP3 activation in vitro.
Bone marrow-derived macrophages (BMMs) were isolated from WT mice, transfected with TLR4 siRNA, and then incubated with 10 μM of DAPT. After 48 hours, the cells were supplemented with 100 ng/ml of LPS for an additional 6 hours. The non-specific (NS) siRNA were used as controls. (A) Western blot analysis of NF-κB, NLRP3, and cleaved caspase-1 in LPS-stimulated macrophages. β-actin served as an internal control. Data representative of three experiments. (B) Density ratios of NF-κB, NLRP3, and cleaved caspase-1. *p<0.05. (C) Quantitative RT-PCR-assisted detection of IL-1β, TNF-α, and IL-6 in LPS-stimulated macrophages. Each column represents the mean±SD (n=3–4 samples/group). *p<0.05.
Discussion
This study demonstrates the important role of Notch signaling in the regulation of inflammatory responses induced by APAP-mediated liver injury. The principal findings of this study are as follows: (1) Blockade of Notch signaling aggravates APAP-induced liver damage, augments macrophage/neutrophil trafficking, and induces hepatocellular apoptosis, and (2) blockade of Notch signaling promotes HMGB1/TLR4/NF-κB and NLRP3 activation via a TLR4-dependent pathway. Thus, these findings document the key mechanism of Notch signaling in regulating innate immune responses in APAP-induced liver injury.
Since APAP-induced liver inflammation can be attributed to the innate immune response generated by Kupffer cells and neutrophils [29–31], the regulatory mechanisms of Notch signaling might be involved in the activation of multiple immune signaling pathways. DAPT is a γ-secretase inhibitor. γ-secretase mediates the cleavage of the Notch receptor and releases NICD. This allows the translocation of NICD into the nucleus, where it directly participates in a transcriptional complex with DNA-binding proteins to activate the transcription of target genes [9]. DAPT has been shown to inhibit Notch signaling in studies of autoimmune and lymphoproliferative diseases [32]. Inhibition of γ-secretase activity by DAPT increased LPS-induced inflammation through the activation of NF-κB signaling [33]. In agreement with these findings, we found that blocking Notch signaling exacerbated APAP-induced liver damage and augmented animal mortality following DAPT conditioning. Moreover, inhibition of Notch signaling increased macrophage accumulation and enhanced neutrophil activity after APAP challenge. This is consistent with our previous finding that Notch signaling regulated macrophage differentiation and neutrophil activation in other liver inflammation induced by ischemia and reperfusion [34].
Notch1 has been shown to function as an important regulator in the control of immune cell development and function [11]. Although APAP-induced liver injury upregulated Notch1 and Hes1 expression in our study, inhibition of Notch signaling reduced Hes1, which resulted in enhanced HMGB1 induction. Indeed, HMGB1, one of the key endogenous damage associated molecular pattern (DAMP) molecules, can activate inflammatory pathways. Macrophage-derived HMGB1 was shown to mediate acute inflammatory response and endotoxin lethality during acute lung injury in mice [35, 36]. HMGB1 can also be actively released by innate immune cells and contribute to innate immune response in APAP-induced liver injury [37, 38], suggesting its role as an endogenous danger signal or alarmin that may engage a diverse receptor repertoire, including TLR2, TLR4, and advanced glycation end products (RAGE), for the initiation of an array of inflammatory responses [39]. Consistent with these findings, our current data revealed that blocking Notch signaling by DAPT resulted in enhanced TLR4 activation, which is crucial for triggering innate immune response. Notably, DAPT treatment augmented NLRP3 inflammasome activation, which led to increased liver inflammation after APAP challenge. These results imply that there is a crosstalk between the HMGB1-TLR4 axis and NLRP3 activation in APAP-induced liver injury.
Direct evidence for a role of TLR4 in Notch signaling-mediated modulation of NLRP3 activation was obtained from TLR4 knockout (TLR4 KO) mice in APAP-challenged livers. We found that unlike DAPT-treated WT mice, which showed increased APAP-induced liver injury, TLR4 KO mice by DAPT showed markedly diminished liver damage with reduced NLRP3 and caspase-1 activation followed by APAP challenge. Indeed, TLR4 signaling is essential for HMGB1-mediated proinflammatory cytokine production [40]. HMGB1 mediates liver damage induced by ischemia and reperfusion injury through a TLR4-dependent pathway [41]. TLR4 deficiency inhibited HMGB1-mediated production of IL-23/IL-17A, which contributes to APAP-induced liver inflammation [42]. Moreover, TLRs and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are pattern recognition receptors (PRRs). NLRP3 is crucial for caspase-1-mediated IL-1β secretion via a TLR4/MyD88/NF-κB signaling pathway in response to LPS stimulation [43]. Macrophages deficient in NF-κB failed to enable NLRP3 activation upon LPS or ATP priming, suggesting the importance of NF-κB-dependent signals in the regulation of NLRP3 expression [44]. In agreement with these findings, we found that blocking Notch signaling by DAPT suppressed Hes1 expression, which may regulate TLR4 activation by targeting RBP-J via a feedback inhibitory loop [45]. Hes1 can also recruit IκBα and inhibit NF-κB transcription [46]. Thus, our results revealed an unexpected role of HES1 in controlling the dynamic crosstalk with NLRP3 in Notch signaling-mediated immune regulation.
We found that inhibition of Notch signaling by DAPT decreased Stat3 and Akt phosphorylation but increased cleaved caspase-3 in APAP-challenged livers. In fact, the emerging evidence suggests that Stat3 and Akt are involved in the intracellular apoptosis pathways [47, 48]. Activation of Notch signaling promoted Stat3 phosphorylation through a direct binding of the Stat3-specific promoter by Hes1 [49]. Inhibition of Stat3 signaling resulted in increased cell apoptosis [50]. Akt, an important signaling molecule in the cellular survival pathway, has been shown to promote cell survival by inhibiting apoptotic processes [51]. Activation of Notch signaling increased Akt phosphorylation and cell survival through modulation of regulator of G protein signaling 19 (RGS19) [52]. Moreover, Hes1 essentially maintains cell survival by preventing apoptosis [53]. Thus, Notch signaling promotes hepatocellular survival by regulating Hes1/Stat3 and Akt transcription. Our TUNEL assay provided further evidence for the increased frequency of apoptotic cells in DAPT-conditioned livers. Although the role of apoptosis in APAP-induced liver injury is still controversial, intracellular signaling and regulatory mechanisms leading to liver cell death is important for the crosstalk between apoptosis and necrosis. Correspondingly, alterations in mitochondrial permeabilization might be the key to understanding these differences [54, 55].
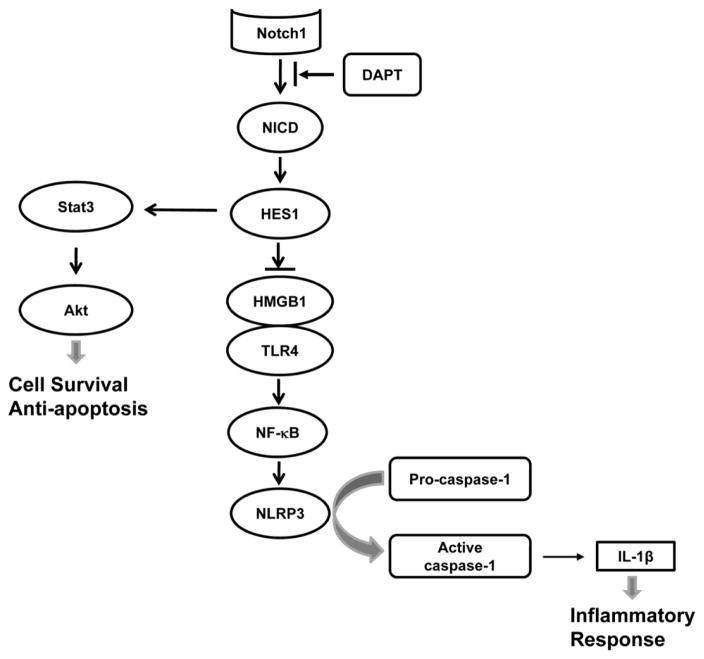
Figure 8 depicts putative molecular mechanisms by which Notch signaling may regulate NLRP3 activation in APAP-induced liver injury. Blocking Notch signaling by γ-secretase inhibitor DAPT suppresses the cleavage of Notch receptor to NICD, which translocates into the nucleus to activate Notch target gene Hes1. Inhibition of Hes1 promotes the HMGB1-TLR4 axis, which in turn triggers NLRP3 activation, leading to augmented caspase-1-mediated IL-1β maturation and secretion in APAP-induced liver inflammation. In addition, inhibition of Hes1 diminishes Stat3 and Akt phosphorylation, resulting in increased cell apoptosis/necrosis and reduced cell survival in APAP-challenged livers.
Figure 8. Schematic illustration of signaling pathways by which Notch signaling may regulate NLRP3 inflammasome activation in APAP-induced liver injury.
Blocking Notch signaling by γ-secretase inhibitor DAPT suppresses the cleavage of Notch1 to NICD, which translocates into the nucleus to activate Notch target gene Hes1. Inhibition of Hes1 promotes HMGB1-TLR4 axis, which in turn triggers NLRP3 activation, leading to augmented caspase-1-mediated IL-1β secretion in APAP-induced liver inflammation. In addition, inhibition of Hes1 diminishes Stat3 and Akt phosphorylation, resulting in increased cell apoptosis/necrosis and reduced cell survival in APAP-challenged livers.
In conclusion, we demonstrated that Notch signaling regulates NLRP3 activation via inhibiting the HMGB1-TLR4 axis in APAP-induced liver injury. Disruption of Notch signaling reduces Hes1 expression, which in turn increases HMGB1 release and triggers TLR4/NF-κB signaling, resulting in activating NLRP3 to induce caspase-1-mediated IL-1β secretion. By identifying a novel Hes1-dependent crosstalk between the integrated Notch signaling network and the NLRP3-mediated innate immune response, this study provides the rationale for refined therapeutic approaches to managing liver inflammation in APAP-induced liver injury.
Supplementary Material
Primer sequences for the amplification
Acknowledgments
This work was supported in part by the NIH grant (R21AI112722 to B.K.), the China Mega-Project for Infectious Diseases (2016ZX10002005-002-005 to J.L.), the National Natural Science Foundation of China (No. 81573213 to X.D.), and the National Science Foundation of Jiangsu Province (No. BK20161059, BE2012770 to L.J. BK20151089 to X.D.).
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Chun LJ, Tong MJ, Busuttil RW, Hiatt JR. Acetaminophen hepatotoxicity and acute liver failure. J Clin Gastroenterol. 2009;43:342–9. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JE, McKenzie TJ, Lillegard JB, Yu Y, Juskewitch JE, Nedredal GI, et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J Surg Res. 2013;180:147–55. doi: 10.1016/j.jss.2012.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012;56:735–46. doi: 10.1002/hep.25657. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–65. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 8.Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14:320–8. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–33. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 11.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–37. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 12.Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–65. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu HC, Qin HY, He F, Wang L, Fu W, Liu D, et al. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. 2011;54:979–88. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 15.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 16.Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140:784–90. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue S, Zhu J, Zhang M, Li C, Zhou X, Zhou M, et al. The myeloid heat shock transcription factor 1/beta-catenin axis regulates NLR family, pyrin domain-containing 3 inflammasome activation in mouse liver ischemia/reperfusion injury. Hepatology. 2016;64:1683–98. doi: 10.1002/hep.28739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke B, Shen XD, Gao F, Ji H, Qiao B, Zhai Y, et al. Adoptive transfer of ex vivo HO-1 modified bone marrow-derived macrophages prevents liver ischemia and reperfusion injury. Mol Ther. 2010;18:1019–25. doi: 10.1038/mt.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, et al. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol. 2012;56:359–66. doi: 10.1016/j.jhep.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–66. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 29.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–50. [PubMed] [Google Scholar]
- 30.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–95. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke H, Liu J. Neutrophil depletion protects against murine acetaminophen hepatotoxicity: another perspective. Hepatology. 2007;45:1588–9. doi: 10.1002/hep.21549. author reply 9. [DOI] [PubMed] [Google Scholar]
- 32.Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, et al. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111:705–14. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasoohi S, Hemmati AA, Moradi F, Ahmadiani A. The gamma-secretase blocker DAPT impairs recovery from lipopolysaccharide-induced inflammation in rat brain. Neuroscience. 2012;210:99–109. doi: 10.1016/j.neuroscience.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Shen XD, Yue S, Zhu J, Gao F, Zhai Y, et al. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Mol Med. 2014;20:448–55. doi: 10.2119/molmed.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 36.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Hou W, Zhang Q, Kang R, Fan XG, Tang D. Emerging role of high-mobility group box 1 (HMGB1) in liver diseases. Mol Med. 2013;19:357–66. doi: 10.2119/molmed.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–4. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–84. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 43.Zhang A, Wang P, Ma X, Yin X, Li J, Wang H, et al. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol Immunol. 2015;66:253–62. doi: 10.1016/j.molimm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29:691–703. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc Natl Acad Sci USA. 2004;101:16537–42. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choudhari SR, Khan MA, Harris G, Picker D, Jacob GS, Block T, et al. Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther. 2007;6:112–21. doi: 10.1158/1535-7163.MCT-06-0561. [DOI] [PubMed] [Google Scholar]
- 48.Shih WL, Liao MH, Lin PY, Chang CI, Cheng HL, Yu FL, et al. PI 3-kinase/Akt and STAT3 are required for the prevention of TGF-beta-induced Hep3B cell apoptosis by autocrine motility factor/phosphoglucose isomerase. Cancer Lett. 2010;290:223–37. doi: 10.1016/j.canlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6:547–54. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 50.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–62. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–44. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- 52.Sangphech N, Osborne BA, Palaga T. Notch signaling regulates the phosphorylation of Akt and survival of lipopolysaccharide-activated macrophages via regulator of G protein signaling 19 (RGS19) Immunobiology. 2014;219:653–60. doi: 10.1016/j.imbio.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, et al. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J Cell Biol. 2006;173:333–9. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 55.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences for the amplification