Abstract
Background.
Platelet-derived growth factor (PDGF) signaling is important in gliomagenesis and PDGF receptor-β is expressed on most endothelial cells in glioblastoma specimens.
Methods.
We report the results of feasibility, phase I, and phase II studies of tandutinib (MLN518), an orally bioavailable inhibitor of type III receptor tyrosine kinases including PDGF receptor-β, Fms-like tyrosine kinase 3, and c-Kit in patients with recurrent glioblastoma.
Results.
In an initial feasibility study, 6 patients underwent resection for recurrent glioblastoma after receiving tandutinib 500mg twice daily for 7 days. The mean ratio of tandutinib concentration in brain tumor-to-plasma was 13.1±8.9 in 4 of the 6 patients. In the phase I study, 19 patients were treated at 500, 600, and 700mg twice daily dose levels. The maximum tolerated dose was found to be 600mg twice daily, and 30 patients were treated with this dose in the phase II study. The trial was closed after interim analysis, as the prespecified goal of patients alive and progression-free survival at 6 months was not achieved. Biomarker studies suggested that tandutinib treatment could lead to vascular disruption rather than normalization, which was associated with rapid progression.
Conclusions.
Tandutinib readily distributed into the brain following oral administration and achieved concentrations within the tumor that exceed the corresponding concentration in plasma. The phase II study was closed at interim analysis due to lack of efficacy, although this study was not enriched for glioblastomas with alterations of the PDGF pathway.
Keywords: glioblastoma, overall survival, platelet-derived growth factor receptor, progression-free survival, tandutinib.
Despite recent advances in radiation and neurosurgical techniques and the approval of new medical therapies, glioblastoma, the most common primary malignant brain tumor in adults, causes significant neurological morbidity and is associated with survival of <2 years.1,2 At the time of disease recurrence, options are limited and progression-free survival (PFS) is typically <6 months.3 Platelet-derived growth factor (PDGF) signaling is implicated in gliomagenesis and is the predominant driver of the proneural molecular subtype of glioblastoma.4 The biological effects of PDGF signaling range from autocrine-stimulated cancer cell growth to paracrine effects on adjacent stroma and vasculature.5,6 PDGF receptor-beta (PDGFR-β) is expressed on >90% of endothelial cells in glioblastoma specimens.7 Consequently, inhibition of PDGFR-β potentially disrupts glioblastoma proliferation and angiogenesis. Tandutinib is an orally bioavailable, quinazoline-based inhibitor of type III receptor tyrosine kinases, including PDGFR-β, Fms-like tyrosine kinase 3, and c-Kit with an elimination half-life of 6.4 days.8 The drug has similar activity against these 3 receptor tyrosine kinases with in vitro, cell based half-maximal inhibitory concentrations of ~200nM (100ng/mL).8,9
Importance of the study
In patients with recurrent glioblastoma, we evaluated the distribution of tandutinib to brain tumors by obtaining surgical specimens, the impact of the drug on physiological tumor and vascular parameters with MRI and blood biomarkers, and clinical outcomes such as disease progression and overall survival. Our findings show that despite achieving pharmacologically relevant intratumoral concentrations, tandutinib had minimal impact on tumor vasculature or growth. This study highlights the feasibility of a paradigm of combination phase 0, I, and II studies as a model for future neuro-oncology clinical trials to better understand why drugs do or do not work.
Tandutinib is not subject to extensive metabolism in humans, suggesting that its pharmacokinetics and clinical effects may not be altered when coadministered with other drugs that modulate hepatic drug metabolizing enzymes.8 The compound is a substrate of P-glycoprotein and breast cancer resistance protein, which effectively limit the oral absorption and CNS penetration of numerous anticancer drugs.10 Nevertheless, tandutinib exhibits excellent oral availability and achieves concentrations in brain tissue that are comparable to plasma concentrations in mice and rats.10 Tandutinib inhibits the growth of C6 glioma xenografts in a dose-dependent fashion. The dose-limiting toxicity (DLT) of tandutinib in human studies is reversible, generalized muscle weakness, probably due to disruption of the neuromuscular junction.11 A feasibility trial followed by a phase I/II multicenter clinical trial of tandutinib for patients with recurrent glioblastoma was conducted in the National Cancer Institute–sponsored Adult Brain Tumor Consortium (ABTC).
Materials and Methods
Patients
Patients with recurrent glioblastoma were the target population for this study (NCT00379080). Inclusion criteria included age ≥18 years, pathological diagnosis of glioblastoma, prior treatment with radiation, Karnofsky performance status (KPS) ≥60, Mini-Mental Status Examination (MMSE) score ≥15, and measurable disease on MRI. Exclusion criteria included any prior anti-PDGF therapy, cranial radiation within 3 months prior to study entry, the use of enzyme-inducing anti-epileptic drugs, or prior treatment with >2 chemotherapy regimens. All patients were required to sign an informed consent form approved by the institutional review board of the enrolling institution. Patients were required to maintain a drug diary and if compliance was <80% of the planned dose, the patient was removed from study.
Treatment Protocol
The primary objective of the feasibility study was to determine if the concentration of tandutinib in brain tumor tissue was 33% or greater than the corresponding concentration in plasma in at least 3 of 6 patients. The target brain tumor-to-plasma concentration ratio (B/P) of 0.33 was based upon the concentration of tandutinib required to inhibit PDGFR phosphorylation by 50% in vitro (~100ng/mL) relative to the average steady-state minimum concentration of the drug in plasma for cancer patients treated orally with the 525mg maximum tolerated dose (MTD) for continuous twice daily administration (~300ng/mL).8,9
Study subjects undergoing resection for recurrent glioblastoma received 500mg tandutinib orally twice daily for 7 days. The surgical procedure was performed at least 6 hours after taking the last dose of drug. A single intact section of tumor tissue (0.5–1.0cm3) was rinsed with ice-cold phosphate buffered saline, blotted on filter paper, and stored at −80°C. Peripheral blood samples (6mL) were obtained immediately before and after the surgical resection in tubes containing spray-coated sodium heparin and centrifuged (1300 g, 10min, 4°C). The plasma was removed and stored at −80°C. Homogenates were prepared from the brain tumor samples as previously described.12,13 The concentration of tandutinib in the plasma samples and tumor homogenates was determined by reversed-phase high performance liquid chromatography with tandem mass spectrometric detection (LC-MS/MS). The analytical method was adapted from a previously reported assay for the drug and validated according to current recommendations.10,14 Tandutinib was determined with an interday accuracy of 108.4% and a precision of 7.1% at the 25.0ng/mL lower limit of quantitation. The concentration of tandutinib in tumor tissue, expressed as ng/g tissue weight, was calculated by multiplying the assayed drug concentration in the homogenate by the dilution factor for the volume of water added to prepare the homogenate assuming a density of 1.0g/mL for tumor tissue. The average concentration of tandutinib in plasma during the tissue resection was calculated from the determinations made in the samples obtained before and after the surgical procedure. Blood to plasma ratio was calculated by dividing the tandutinib concentration in brain tumor tissue (ng/g) by its average concentration in plasma during the surgical procedure (ng/mL).
The primary aim of the phase I study was to define the MTD in the recurrent glioblastoma population. The primary aims of the phase II study were to determine the proportion of patients with objective radiographic responses and to determine the proportion of patients alive without disease progression at 6 months (PFS6). Radiographic response was determined by the treating physician using Macdonald criteria, since this study predated RANO criteria publication.15,16 We also did not anticipate seeing a significant impact on T2/fluid attenuation inversion recovery (FLAIR) progression, so we continued to use Macdonald criteria for the analysis of the study once RANO was published. The phase II study was a planned 2-stage design with interim analysis for efficacy after enrollment of 31 patients in stage 1.
Correlative Imaging
Twenty patients in the phase II portion of the study were to undergo advanced MRI scans at select ABTC sites. These scans were performed on day −3, day −1 (double baseline scans), cycle 1 day 10, and cycle 2 day 1. The acquisition protocol was standardized across sites and included the following sequences: standard pre- and postcontrast images, FLAIR, dynamic contrast enhanced (DCE) images, dynamic susceptibility contrast (DSC) images, and diffusion weighted images (see Supplementary material). All data were analyzed at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital as previously described.7 DCE images were used to calculate ktrans (the volume transfer coefficient) and Ve (the volume of extravascular extracellular space). DSC images were used to calculate cerebral blood volume (CBV) in small vessels (based on a spin echo sequence) or all vessels (based on a gradient echo sequence).
Correlative Circulating Blood Biomarkers
Peripheral blood was obtained from patients enrolled in the phase II portion of the study (n=20), and evaluated as previously described.7 Blood samples were collected in EDTA-containing tubes at baseline; during the first cycle of tandutinib treatment on days 2, 8, and 10; and on day 1 of cycle 2. Plasma samples were obtained by centrifugation and aliquoted and stored at −80ºC. Multiplex array (Meso-Scale Discovery) was used to measure plasma vascular endothelial growth factor (VEGF), soluble VEGF receptor-1 (sVEGFR1), placental growth factor (PlGF), and basic fibroblast growth factor (bFGF). Single analyte enzyme-linked immunosorbent assay kits (R&D Systems) were used to measure stromal-derived factor (SDF)1α, carbonic anhydrase (CA)IX, angiopoietin 2 (Ang-2), and sVEGFR2. All samples were run in duplicate.
Pharmacokinetic Studies
Blood samples were obtained from all patients enrolled in each component of the clinical trial shortly before dosing and at 0.5, 1, 2, 4, 6, 8, and 24h relative to the first dose of cycle 1. Patients received a single dose of tandutinib on day 1 with twice daily dosing beginning on day 2 after collecting the 24h blood sample. Steady-state trough concentrations of tandutinib were monitored in predose samples obtained during the regularly scheduled visits for evaluations on days 8, 15, and 21 of cycle 1 and day 1 of cycle 2. The procedures used to collect, process, store, and assay the pharmacokinetic samples are the same as described in the above. Pharmacokinetic parameters were estimated by standard noncompartmental methods using WinNonlin Professional software (Pharsight) and reported as the geometric mean ± SD of the values for individual patients at each dose level.
Statistical Methods
In the phase I study, 3 patients per cohort were treated at the starting dose of 500mg b.i.d. with escalation in subsequent dose cohorts to 600mg b.i.d., 700mg b.i.d., then subsequent 200mg b.i.d. increments in a stepwise fashion, with potential expansion of each dose cohort up to 6 patients until the MTD was defined. The MTD was defined as (i) the dose producing DLT in 2/6 patients, but the 2 could not both be grade 4 toxicities (if both were grade 4, then the dose level below would be defined as the MTD) or (ii) the dose level below which DLTs were observed in ≥2 out of 3 patients or in ≥3 out of 6 patients.
In the phase II study, the primary endpoint was radiographic response as determined by Macdonald criteria.15 A Simon 2-stage design was used to test a null hypothesis of 10% (null hypothesis, H0) versus 25% (alternative hypothesis, HA) response with a 90% power, alpha = 0.05. In the first stage, 31 patients would be enrolled and the trial would be terminated if 3 or fewer patients demonstrated objective responses (partial response or complete response). However, to allow for stable disease which may also be clinically beneficial, if 9 of 30 (30%) patients were alive and without disease progression at 6 months (PFS6), the trial would proceed to the second stage with accrual of 24 additional subjects even if 3 patients had not demonstrated an objective response. Tandutinib would be deemed a promising agent worthy of a comparative trial if ≥10 radiographic responses were observed in the entire cohort. Central review of pathology and neuroimaging was mandated for all patients with a documented complete or partial response. Survival probability and median time of survival were calculated using the Kaplan‒Meier method. Biomarker outcomes were presented as descriptive summaries. Changes from baseline were analyzed using paired comparison. Possible association between survival and biomarker change from baseline was estimated using a Cox regression model. All P values reported are 2-sided. No adjustment was made for multiple testing. All analyses were performed with the use of SAS software v9.2.
Results
A total of 56 patients were enrolled on all phases of the study. Overall patient and disease characteristics at baseline are summarized in Table 1. In the feasibility study, 6 patients were treated at a dose of 500mg twice daily based on prior studies in non–brain tumor subjects demonstrating the safety and tolerability of this dose.9 Brain tumor tissue and plasma samples were obtained from a total of 6 patients, although samples from 2 of the patients were thawed upon receipt by the analytical laboratory and the results from the analysis of these samples were considered to be unacceptable. Results for each of the other 4 patients are presented in Table 2. The tumor sections from these patients were excised 6.4±3.8h (average ± SD) after taking the last dose of tandutinib. The intratumoral concentration of tandutinib was greater than the corresponding average concentration in plasma during the surgical procedure in all 4 patients. The mean (± SD) concentration of the drug in plasma during the surgical procedure was 604±247ng/mL, and the mean concentration of drug in tumor tissue was 6860±2834ng/g, yielding a mean B/P of 13.1±8.9. The criteria for proceeding to the phase I part of the clinical trial, by demonstrating that the B/P of tandutinib was ≥0.33 in at least 3 of 6 patients, was achieved.
Table 1.
Baseline characteristics of study subjects
| Phase 0–I | Phase II | Total | |
|---|---|---|---|
| N | 25 | 31 | 56 |
| Age, year | |||
| Median | 58 | 54 | 56 |
| Range | 42–77 | 24–69 | 24–77 |
| Gender, no. (%) | |||
| Male | 19 (76) | 24 (77) | 43 (77) |
| Female | 6 (24) | 7 (23) | 13 (23) |
| KPS status | |||
| Median | 90 | 90 | 90 |
| Range | 60–100 | 60–100 | 60–100 |
| MMSE | |||
| Median | 29 | 29 | 29 |
| Range | 15–30 | 20–30 | 15–30 |
| Steroids, no. (%) | |||
| Yes | 15 (60) | 8 (26) | 23 (41) |
| No | 10 (40) | 23 (74) | 33 (59) |
| Pre Avastin, no. (%) | |||
| Yes | 8 (26) | 8 (26) | |
| No | 23 (74) | 23 (74) | |
Phase 0 indicates the feasibility cohort (N=6).
Table 2.
Tandutinib concentrations in brain tumor tissue and plasma samples
| Patient No. | Intratumoral Conc. (ng/g) | Average Conc. in Plasma during Surgery (ng/mL) | B/P Ratio |
|---|---|---|---|
| 1 | 10008 | 911 | 11.0 |
| 2 | 9593 | 366 | 26.2 |
| 3 | 4729 | 533 | 8.9 |
| 4 | 4878 | 748 | 6.5 |
| Mean | 6860 | 604 | 13.1 |
| SD | 2834 | 247 | 8.9 |
In the phase I study, 3 dose levels (500mg b.i.d., 600mg b.i.d., and 700mg b.i.d.) were assessed in 19 patients. Four patients were replaced due to early withdrawal unrelated to toxicity (patients refused further treatment). DLTs were observed in 1/6 patients at 500mg b.i.d. (grade 3 phosphorus, grade 3 fatigue, grade 3 somnolence in 1 patient); 1/6 patients at 600mg b.i.d. (grade 3 phosphorus); 2/3 patients at 700mg b.i.d. (grade 3 fatigue, grade 3 weakness)—so 600mg b.i.d. was declared the MTD and the phase II study was opened using this dose.
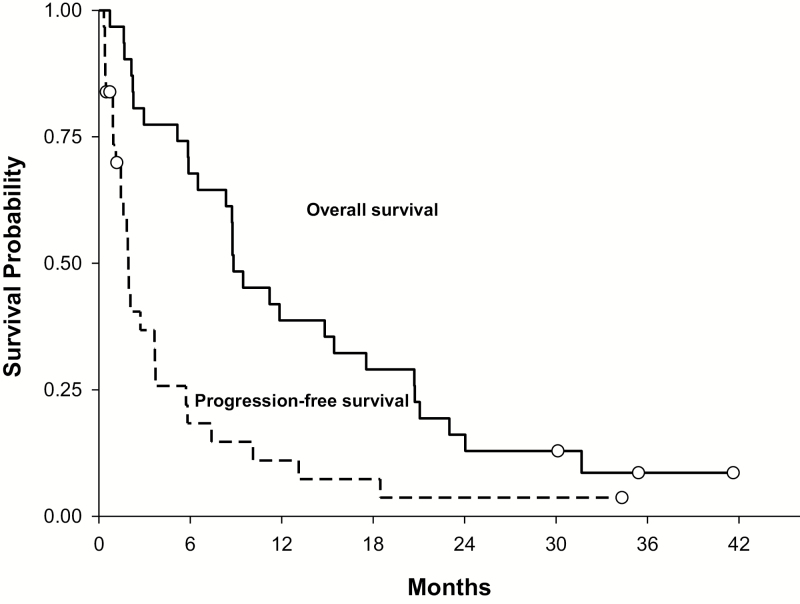
In the 2-stage phase II study, 31 patients were treated in the first stage; PFS and overall survival (OS) are summarized in Fig. 1. The median PFS for all these patients was 1.9 months (95% CI: 1.5–3.7 mo), the PFS6 was 16% (95% CI: 6%–34%), and the median OS was 8.8 months (95% CI: 5.9–15.4 mo). At the time of analysis after the first stage of the Simon 2-stage design, there was one complete response (3%) and 5 patients reached PFS6 (16%). Thus, the study did not meet the prespecified efficacy thresholds for response rate of 13% or PFS6 rate of 30% and the study was terminated (central review was not performed on the 1 patient achieving a complete response because the study was halted). Eight of 31 patients (26%) had received prior treatment with anti-VEGF therapy (bevacizumab or aflibercept). None of these 8 patients reached PFS6. Comparison of enrolled patients who had received no prior anti-VEGF therapy (N = 23) versus those who did receive prior anti-VEGF therapy (N = 8) revealed median PFS of 2.1 months (95% CI: 1.4–5.7 mo) versus 1 month (95% CI: 0.3–1.8 mo), P = .0075, and median OS of 9.5 months (95% CI: 6.5–20.7) versus 5.5 months (95% CI: 0.7–11.9 mo), P = .019.
Fig. 1.
Overall survival and progression-free survival in phase II cohort (N =31).
Mean pharmacokinetic parameters for tandutinib in the groups of patients evaluated at each dose level are summarized in Supplementary Table 1. Mean values of the parameters for patients treated with the 600mg MTD were comparable to data that were previously reported for a phase I clinical trial of single-agent tandutinib in patients with hematological malignancies.9
The correlative imaging was performed in 19 patients (Table 3). As expected, larger tumor volume at baseline was significantly associated with worse OS and PFS. In addition, increasing tumor volume at cycle 1 day 10 and cycle 2 day 1 were associated with worse PFS, and the increase at cycle 2 day1 was also significantly associated with worse OS. The MRI vascular parameters demonstrated that CBV in small vessels increased significantly from baseline to cycle 2 day 1 (Table 3), and higher baseline CBV in small vessels was also associated with a worse OS and PFS (Table 4). An increase in ktrans from baseline to cycle 2 day 1 was associated with worse PFS.
Table 3.
Change in imaging biomarkers during therapy
| Imaging Parameters | Baseline | Cycle 1, Day 10 | Cycle 2, Day 1 |
|---|---|---|---|
| T1CE Volume | 17.47 (6.55, 46.94) | 18 (6.07, 56.04) | 17.51 (5.65, 42.56) |
| N = 19 | N = 19 | N = 16 | |
| P value | NA | 0.40 | 0.07 |
| FLAIR Volume | 95.5 (39.87, 138.62) | 103.35 (38.74, 145.35) | 82.27 (30.9, 117.53) |
| N = 18 | N = 19 | N = 16 | |
| P value | NA | 0.39 | 0.3 |
| CBV_SE | 1.55 (1.24, 2.49) | 1.69 (1.37, 2.55) | 1.77 (1.38, 2.72) |
| N = 19 | N = 18 | N = 15 | |
| P value | NA | 0.15 | 0.04 |
| CBV_GE | 0.99 (0.82, 1.47) | 1.06 (0.93, 1.28) | 1.01 (0.82, 1.27) |
| N = 19 | N = 17 | N = 15 | |
| P value | NA | 0.96 | 0.56 |
| Mean ADC within FLAIR | 0.89 (0.80, 0.95) | 0.92 (0.82, 0.97) | 0.91 (0.85, 0.93) |
| N = 18 | N = 19 | N = 16 | |
| P value | NA | 0.32 | 0.85 |
| Mean FA within FLAIR | 0.22 (0.20, 0.27) | 0.23 (0.19, 0.27) | 0.22 (0.2, 0.27) |
| N = 18 | N = 19 | N = 16 | |
| P value | NA | 0.26 | 0.19 |
| Ktrans | 0.01 (0.004, 0.02) | 0.01 (0.004, 0.04) | 0.01 (0.004, 0.03) |
| N = 17 | N = 15 | N = 14 | |
| P value | NA | 0.08 | 0.45 |
| Ve | 0.59 (0.48, 0.86) | 0.68 (0.59, 0.83) | 0.75 (0.47, 0.85) |
| N = 18 | N = 16 | N = 14 | |
| P value | NA | 0.72 | 0.64 |
Abbreviations: T1CE weighted contrast enhanced; CBV_ SE, cerebral blood flow within all vessels within contrast enhancement; CBV_GE, cerebral blood flow within all vessels within contrast enhancement; ADC, apparent diffusion coefficient; FA, fractional anisotropy; Ve, volume of extravascular extracellular space
Data are shown as medians and interquartile ranges and are compared with baseline levels. Wilcoxon signed rank test was used for imaging parameter comparison at cycle 1 day 10 or cycle 2 day 1 from baseline. The P values are two-sided. Significant changes are bolded.
Table 4.
Association between change in imaging parameter from baseline to progression-free survival or overall survival
| Imaging Parameter | Baseline | Change at C1D10 from Baseline | Change at C2D1 from Baseline | |||
|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | OS | PFS | |
| CE_T1_Volume | 1.02 (1.01,1.04) | 1.03 (1.01,1.05) | 1.3 (0.99,1.7) | 1.31 (1.04,1.65) | 1.23 (1.03,1.48) | 1.2 (1.03,1.39) |
| N = 19 | N = 19 | N = 19 | N = 19 | N = 16 | N = 16 | |
| P value | 0.004 | 0.01 | 0.06 | 0.02 | 0.02 | 0.02 |
| FLAIR_Volume | 1.01 (1,1.02) | 1 (1,1.01) | 1.01 (0.93,1.09) | 0.96 (0.87,1.06) | 1.06 (1,1.12) | 1.03 (0.99,1.07) |
| N = 18 | N = 18 | N = 18 | N = 18 | N = 15 | N = 15 | |
| P value | 0.01 | 0.22 | 0.88 | 0.38 | 0.06 | 0.17 |
| CBV_SE | 2.12 (1.07,4.2) | 2.63 (1.18,5.84) | 0.45 (0.12,1.7) | 0.9 (0.37,2.2) | 1.9 (0.33,11.11) | 1.87 (0.26,13.61) |
| N = 19 | N = 19 | N = 18 | N = 18 | N = 15 | N = 15 | |
| P value | 0.03 | 0.02 | 0.24 | 0.82 | 0.48 | 0.54 |
| CBV_GE | 0.91 (0.39,2.12) | 1.38 (0.65,2.96) | 1.51 (0.47,4.86) | 1 (0.38,2.65) | 2.05 (0.05,93.57) | 1.03 (0.02,62.94) |
| N = 19 | N = 19 | N = 17 | N = 17 | N = 15 | N = 15 | |
| P value | 0.82 | 0.41 | 0.49 | 1 | 0.71 | 0.99 |
| Mean ADC within FLAIR | 0.04 (0, 1.2) | 0.004 (0, 0.41) | 11.37 (0.22, 585) | 26.37 (0.22, 3193) | 617 (0.08,4.58e6) | 960 (0.05,1.76e7) |
| N = 18 | N = 18 | N = 18 | N = 18 | N = 15 | N = 15 | |
| P value | 0.06 | 0.02 | 0.23 | 0.18 | 0.16 | 0.17 |
| Mean FA within FLAIR | 54.77 (0,1.94e6) | 1.38e6 (1.01,1.88e12) | <.0001 (0,6085) | <.0001 (0,<.0001) | .0003 (0,8896) | <.0001 (0,32.3) |
| N = 18 | N = 18 | N = 18 | N = 18 | N = 15 | N = 15 | |
| P value | 0.45 | 0.05 | 0.2 | 0.005 | 0.35 | 0.08 |
| Median Ktrans | 0.004 (0,3.86e8) | 35.97 (0,1.87e12) | 8018 (0.09,7.33e8) | 143 (0,5.49e7) | 72.32 (0.87,5986) | 233 (1.68,3.23e4) |
| N = 17 | N = 17 | N = 14 | N = 14 | N = 13 | N = 13 | |
| P value | 0.67 | 0.78 | 0.12 | 0.45 | 0.06 | 0.03 |
| Median Ve | 0.52 (0.07,3.93) | 3.25 (0.31,33.53) | 0.07 (0,6.87) | .0002 (0,0.16) | 11.82 (0.25,562) | 10.11 (0.24,427) |
| N = 18 | N = 18 | N = 15 | N = 15 | N = 13 | N = 13 | |
| P value | 0.52 | 0.32 | 0.26 | 0.01 | 0.21 | 0.23 |
T1CE –T1 weighted contrast enhanced; CBV_ SE – cerebral blood flow within all vessels within contrast enhancement; CBV_GE – cerebral blood flow within all vessels within contrast enhancement; ADC – apparent diffusion coefficient; FA – fractional anisotropy; Ve – volume of extravascular extracellular space
Data from are shown as hazard ratios and 95% confidence interval. No adjustment for multiple testing was performed. The p values are two-sided. Significant changes are bolded.
Circulating biomarker analyses showed that tandutinib treatment significantly decreased plasma PlGF (at days 8 and 10 and cycle 2 day 1), SDF1α (at day 8), VEGF (at day 8 and cycle 2 day 1), and sVEGFR2 (at cycle 2 day 1), and increased plasma CAIX (at days 8 and 10 and cycle 2 day 1), and Ang-2 (at day 8) (Table 5). Greater decreases in plasma PlGF (hazard ratio [HR] = 11.8; P = .04) and CAIX (HR = 1.74; P = .02) at day 10 and in plasma sVEGFR1 (HR = 61.5; P = .05) at cycle 2 day 1 were associated with longer PFS (Supplementary Table 2). A greater decrease in plasma sVEGFR1 (HR = 524; P = 0.01) and increase in plasma sVEGFR2 (HR = 0.001; P = 0.05) at cycle 2 day 1 were associated with longer OS.
Table 5.
Blood biomarker data are shown as medians and interquartile ranges (in square brackets) and are compared with baseline levels. Significant changes are bolded
| Biomarker | Baseline | C1D2 | C1D8 | C1D10 | C2D1 |
|---|---|---|---|---|---|
| SDF_1a |
1727 [1463, 1967]
(N = 20) |
1668 [1518, 1828] (N = 19) |
1452 [1340, 1657]
(N = 17) |
1661 [1348, 1823] (N = 11) |
1479 [1398, 1691] (N = 13) |
| P value | NA | 0.47 | 0.008 | 0.07 | 0.06 |
| bFGF | 35 [25, 74] (N = 20) |
35 [23, 52] (N = 19) |
36 [20, 55] (N = 17) |
34 [14, 67] (N = 11) |
35 [23, 56] (N = 14) |
| P value | NA | 0.89 | 0.61 | 0.52 | 0.81 |
| PlGF | 26 [21, 38] (N = 20) |
26 [22, 41] (N = 19) |
19 [15, 20]
(N = 17) |
17 [12, 33]
(N = 11) |
17 [15, 25]
(N = 14) |
| P value | NA | 0.49 | 0.001 | 0.003 | 0.001 |
| sFLT_1 | 114 [84, 139] (N = 20) |
101 [73, 146] (N = 19) |
111 [86, 131] (N = 17) |
117 [97, 171] (N = 11) |
101 [80, 133] (N = 14) |
| P value | NA | 0.12 | 0.40 | 0.97 | 0.33 |
| VEGF | 156 [86, 272] (N = 20) |
119 [68, 286] (N = 19) |
119 [83, 141]
(N = 17) |
141 [66, 229] (N = 11) |
86 [70, 130]
(N = 14) |
| P value | NA | 0.26 | 0.04 | 0.08 | 0.02 |
| CAIX | 45 [26, 108] (N = 19) |
45 [30, 74] (N = 19) |
111 [49, 196]
(N = 17) |
144 [81, 206]
(N = 12) |
155 [75, 218]
(N = 13) |
| P value | NA | 1.00 | 0.005 | 0.005 | 0.001 |
| VEGFR2 | 9471 [7868, 10867] (N = 20) |
9476 [7894, 10480] (N = 19) |
8088 [6857, 10152] (N = 17) |
8008 [7276, 10852] (N = 12) |
7680 [7306, 9657]
(N = 13) |
| P value | NA | 0.80 | 0.07 | 0.30 | 0.03 |
| Ang_2 | 2328 [1806, 3219] (N = 20) |
2508 [1734, 3012] (N = 19) |
2810 [1975, 4145]
(N = 17) |
3241 [2640, 5554] (N = 12) |
2534 [2402, 3679] (N = 13) |
| P value | NA | 0.31 | 0.02 | 0.052 | 0.59 |
Discussion
The present study was designed to determine whether or not pharmacologically relevant concentrations of tandutinib were achieved in recurrent glioblastoma following the treatment of patients with the drug as a requirement for undertaking a formal phase I trial in this patient population. The concentration of drug in brain tumor samples obtained from all 4 evaluable patients exceeded its corresponding average concentration in plasma by factors ranging from 6.5- to 26-fold. This is an exceptional finding, especially for a compound that is a known substrate of the P-glycoprotein and breast cancer resistance protein drug efflux transporters that are expressed in the blood‒brain barrier and limit the distribution of many xenobiotics into the central nervous system.17 An important consideration is that the tissue samples were excised from a contrast enhancing region of the tumors in which it is well established that the functional integrity of the blood‒brain barrier is compromised.18 Consistent with these findings, it has been demonstrated that tandutinib achieved concentrations in normal brain tissue in P-glycoprotein knockout mice that were 2 to 3 times greater than wild-type animals and 13 times greater in P-glycoprotein/breast cancer resistance protein double knockout animals.10
Although the target of tandutinib is expressed on both tumor cells and tumor endothelium and the drug achieves adequate blood-tumor penetration, there was no efficacy of this PDFGR tyrosine kinase inhibitor in the recurrent glioblastoma patient population. This is largely consistent with the outcomes observed in other studies of inhibitors of the PDGF signal transduction pathway, including imatinib and dasatinib.19–25 Although some responses have been observed with dual VEGF/PDGFR inhibitors (cediranib, vatalanib), these radiographic responses are probably mediated through the anti-VEGF effect of these agents.26,27 It is noteworthy that only one complete response was observed in this study of tandutinib, in contrast to prior trials of the dual VEGF/PDGFR inhibitors, cediranib and vatalanib, in which radiographic response proportions of 12%–26% were observed. Given the high expression levels of the target of tandutinib, PDGFR-β, on glioblastoma endothelium this agent appears to have minimal activity as an anti-angiogenic therapeutic—a fact confirmed by the lack of significant change in imaging biomarkers of vessel modulation such as CBV or ktrans that would suggest improved vessel structure and function. In fact, CBV worsened with treatment. Circulating biomarkers showed decreases in plasma PlGF, VEGF, and SDF1α, and transient increase in Ang-2, which is in contrast to the changes seen after treatment with dual inhibitors of VEGFR and PDGFR, again supporting the absence of a beneficial impact on the tumor or its vasculature.26,27
The lack of efficacy of PDGF inhibitors in glioblastoma noted to date could be due to several factors. Thus far, none of the clinical trials have been selective for the proneural subtype of glioblastoma, in which PDGF signaling may predominate. One limitation of our study was not preselecting patients for PDGFR expression to enrich for patients more likely to respond. As molecular profiling of cancers is now feasible, it should be possible to study this class of agents in this subtype of glioblastoma, potentially in combination with standard chemoradiation. However, even in the proneural glioblastoma subtype, other signal transduction pathways (c-methionine (MET), epidermal growth factor receptor [EGFR]) may be coactivated within the same tumor, likely rendering a strategy focused solely on the PDGF pathway ineffective. Studies have demonstrated that 13% of glioblastomas with EGFR, PDGFR-α, or MET amplification have multiple receptor tyrosine kinase amplifications.28,29 In one example there was spatial heterogeneity of EGFR amplified and PDGFR-α–amplified cells within the brain.28 Such genetic mosaicism may ultimately require combinations of agents targeting different signal transduction pathways.
The relative specificity of PDGFR inhibitors for the α and β receptors should be considered in assessment of trials, since PDGFR-α and PDGFR-β expression vary in glioblastoma. PDGFR-α is highly expressed (>90%) on both tumor cells and tumor endothelium, while PDGFR-β is primarily expressed on tumor endothelium (100%) and to a lesser extent on tumor cells (37%).7,30 Tandutinib and dasatinib are both potent inhibitors of the PDGFR-β receptor, while imatinib is a potent inhibitor of both PDGFR-β and PDGFR-α. However, none of these agents has achieved any significant antitumor or anti-angiogenic activity against glioblastoma. In fact, despite being a PDGFR-β receptor inhibitor more likely to impact endothelium, “vascular abnormalization” was observed in this study after tandutinib treatment—suggested by increases in ktrans (a parameter associated with vascular surface area and permeability) and in the hypoxia marker (plasma CAIX)—and was associated with shorter PFS. The vascular abnormalization likely reflects persistent tumor growth and was not likely a rebound effect from prior anti-VEGF treatment, as only 5/19 patients with perfusion imaging had received prior anti-VEGF therapy. The lack of PDGFR-α inhibition on glioma cells by tandutinib may also explain its minimal efficacy.
While tumor delivery of the drug is always a consideration in the interpretation of glioma trials, this factor is not an explanation for the lack of efficacy of tandutinib observed in this study, as the concentrations of the drug achieved in brain tumor samples obtained from patients treated with the drug were more than sufficient for inhibiting the tyroisne kinase activity of PDGFR in vitro. However, we did not measure the physiological impact on PDGFR or downstream signaling cascades to determine if these targets were sufficiently impacted. Moreover, another study demonstrated similar results regarding adequate brain penetration with imatinib in glioblastoma patients.13 Finally, the inclusion of patients who had received prior anti-VEGF therapy in this trial may have biased this study toward a negative outcome. It is well documented that recurrent glioblastoma patients who have received treatment with bevacizumab or other anti-VEGF agents are resistant to subsequent therapies.31 However, the lack of a significant number of radiographic responses in any component of this study (feasibility, phase I, phase II) and the poor PFS6 in the patient population without prior exposure to anti-VEGF agents suggest that the poor observed outcomes were a feature of the drug and not the patient population.
In summary, while tandutinib achieved adequate concentrations to achieve PDGFR-β inhibition in glioblastoma at tolerable doses, there was no efficacy of this targeted agent in the recurrent glioblastoma population treated in this study. Moreover, the mechanism of action of tandutinib significantly differs from that of agents targeting VEGFRs, and may lead to “vascular abnormalization,” which is associated with rapid tumor progression in this patient population. While this study is negative and argues against further development of tandutinib in this patient population, we contend that the study design, starting with a feasibility cohort focused on tumor drug delivery followed by phase I and phase II studies incorporating correlative studies, is a rational and informative method by which to assess novel agents in the glioblastoma population.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/)
Funding
This work was supported by the National Cancer Institute, Adult Brain Tumor Consortium (5U01CA137443-05), Millennium.
Supplementary Material
Acknowledgments
Partial results of this trial were previously presented at the 2009 American Society of Clinical Oncology and the 2011 Society for Neuro-Oncology meetings. We thank Dominique Jennings for help with image analysis.
Conflicts of interest statement. Tracy T. Batchelor: Research Funding: National Cancer Institute, Millennium, Pfizer, AstraZeneca; Consulting: Roche/Genentech, Merck, Oxigene, Upsher
Patrick Wen: Consultant: Cavion, Cortice Biosciences, Genentech/Roche, Monteris, Novocure, Regeneron, Vascular Biogenic, VBI vaccines. Speaker: Merck; Research support: Acerta, Agios, Angiochem, Astra Zeneca, Genentech/Roche, GlaxoSmithKline, Karyopharm, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics
Jeffrey G. Supko: Consultant: Millennium Pharmaceuticals, Inc.
All other authors: none.
References
- 1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 5. Nazarenko I, Hede SM, He X, et al. PDGF and PDGF receptors in glioma. Ups J Med Sci. 2012;117(2):99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westermark B. Platelet-derived growth factor in glioblastoma-driver or biomarker? Ups J Med Sci. 2014;119(4):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pandey A, Volkots DL, Seroogy JM, et al. Identification of orally active, potent, and selective 4-piperazinylquinazolines as antagonists of the platelet-derived growth factor receptor tyrosine kinase family. J Med Chem. 2002;45(17):3772–3793. [DOI] [PubMed] [Google Scholar]
- 9. DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108(12):3674–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang JJ, Milton MN, Yu S, et al. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab Lett. 2010;4(4):201–212. [DOI] [PubMed] [Google Scholar]
- 11. Lehky TJ, Iwamoto FM, Kreisl TN, et al. Neuromuscular junction toxicity with tandutinib induces a myasthenic-like syndrome. Neurology. 2011;76(3):236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akingbemi BT, Braden TD, Kemppainen BW, et al. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology. 2007;148(9):4475–4488. [DOI] [PubMed] [Google Scholar]
- 13. Holdhoff M, Supko JG, Gallia GL, et al. Intratumoral concentrations of imatinib after oral administration in patients with glioblastoma multiforme. J Neurooncol. 2010;97(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Department of Health and Human Services FaDAGfI, Bioanalytical Method Validation http://www.fda.gov/cder/guidance/index.htm 2001. [DOI] [PubMed]
- 15. Macdonald DR Cascino TL Schold SC Jr.et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65(24):11419–11428. [DOI] [PubMed] [Google Scholar]
- 18. Jain R. Measurements of tumor vascular leakiness using DCE in brain tumors: clinical applications. NMR Biomed. 2013;26(8):1042–1049. [DOI] [PubMed] [Google Scholar]
- 19. Morris PG, Abrey LE. Novel targeted agents for platelet-derived growth factor receptor and c-KIT in malignant gliomas. Target Oncol. 2010; 5(3):193–200. [DOI] [PubMed] [Google Scholar]
- 20. Razis E, Selviaridis P, Labropoulos S, et al. Phase II study of neoadjuvant imatinib in glioblastoma: evaluation of clinical and molecular effects of the treatment. Clin Cancer Res. 2009;15(19):6258–6266. [DOI] [PubMed] [Google Scholar]
- 21. Reardon DA, Dresemann G, Taillibert S, et al. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101(12):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12(16):4899–4907. [DOI] [PubMed] [Google Scholar]
- 23. Lassman AB, Pugh SL, Gilbert MR, et al. Phase 2 trial of dasatinib in target-selected patients with recurrent glioblastoma (RTOG 0627). Neuro Oncol. 2015;17(7):992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galanis E, Anderson SK, Anastasiadis P, et al. NCCTG N0872 (Alliance): A randomized placebo-controlled phase II trial of bevacizumab plus dasatinib in patients with recurrent glioblastoma (GBM). J Clin Oncol. 2015;33 (suppl; abstr 2004). [Google Scholar]
- 25. Laack NN, Galanis E, Anderson SK, et al. Randomized, placebo-controlled, phase II study of dasatinib with standard chemo-radiotherapy for newly diagnosed glioblastoma (GBM), NCCTG N0877 (Alliance). J Clin Oncol 2015;33 (suppl; abstr 2013). [Google Scholar]
- 26. Batchelor TT, Duda DG, di Tomaso E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerstner ER, Eichler AF, Plotkin S, et al. Phase I study of vatalanib (PTK787) in combination with standard radiation and temozolomide in patients with newly diagnosed glioblastoma. J Neurooncol. 2010:DOI: 10.1007/s11060-11010-10390-11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. [DOI] [PubMed] [Google Scholar]
- 29. Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52(11):3213–3219. [PubMed] [Google Scholar]
- 31. Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.