See article in this issue by Hardcastle et al., pp. 493–502.
One of the most important questions in cancer therapeutics at this time is whether it will be possible to extend the remarkable initial results seen in immune checkpoint blockade studies1 to more patients, and across more tumor types, including brain tumors like glioblastoma. Approaches in which immune checkpoint blocking antibodies are combined with other immunotherapeutic modalities are therefore under intense investigation.2 These approaches are designed to stimulate the immune system and overcome tumor immunosuppressive mechanisms that may prevent sustained anti-tumor immune responses. Here, I will briefly discuss the mechanisms, the potential, and the challenges involved in the combination of local oncolytic virus (OV)-mediated immunostimulation and immune checkpoint blockade for glioblastoma treatment. This type of approach has shown efficacy in two recent preclinical glioblastoma studies,3,4 and clinical trials are beginning.
Immunosuppression is a multi-faceted process by which tumor cells evade recognition and elimination by the host immune system.5 For example, T cell activation is under tight physiological control via an intricate network of co-stimulatory and inhibitory signals that can shut down cytotoxic T cells by causing “T cell exhaustion”. Cancer cells hijack these mechanisms to avoid elimination by anti-tumor T cells, and immune checkpoint blocking therapeutic antibodies, which prevent key receptor-ligand interactions (e.g., anti-CTLA4, anti-PD-1, and anti-PD-L1), have led to remarkable effects in responsive tumors including advanced melanoma and non-small cell lung cancer. However, typically less than 30% of patients exhibit a durable response to single-agent immune checkpoint blockade. Thus, it is tempting to speculate that combination therapies may increase the applicability of this approach. Indeed, combining two separate immune checkpoint approaches increases response rates in melanoma, but not without an increase in side effects.1 A key advantage of local delivery of an OV is that the toxic effects of systemic therapeutic combinations may be avoided.
Glioblastoma has a relatively low mutation rate (therefore fewer neoantigens for T cells to target) and it is not yet known whether immune checkpoint blockade will be effective. In two patients it has been shown that anti-PD1 immune checkpoint blockade can lead to responses in DNA mismatch repair-deficient glioblastoma.6 These cases, which have an extremely high mutation rate, are quite rare but this observation supports the principle that immune checkpoint blockade can work in the context of the brain.
Oncolytic viruses are attracting interest as local immunostimulatory agents that could offer a therapeutic advantage in combination with immune checkpoint inhibition. Many OVs already have an established safety profile in human patients, offering potential opportunities for tailored combination therapies according to tumor type and immune status. OVs have been developed from a wide range of viruses and can be either wild type or engineered to improve tumor targeting, immunostimulation, and other properties.7 In general, OVs are thought to work via the combination of tumor cell-specific lysis and immunostimulation. The biggest breakthrough so far in the field came in 2015 with FDA approval of the OV “T-VEC” following a successful phase IIl trial in advanced melanoma (16% durable response rate). T-VEC is an immunostimulatory oncolytic Herpes virus and is now marketed as Imlygic. This agent has already been tested in combination with ipilimumab (anti-CTLA4) in human advanced melanoma patients with a durable response rate of 44%; the authors concluded that T-VEC with ipilimumab had a tolerable safety profile, and the combination appeared to have greater efficacy than either T-VEC or ipilimumab monotherapy.8
OVs have been tested extensively in glioblastoma, which has established their safety and shown occasional remarkable responses. However, trials thus far generally have not been successful despite supporting preclinical data. The reasons for this are likely many, but certainly rapid and selective immune-mediated elimination of the OV is a factor along with the failure to overcome the strongly immunosuppressive glioblastoma microenvironment. Data are eagerly awaited from various next-generation OVs with increased potency that are now in development.
OV/immune checkpoint combination preclinical data from mouse models have recently started to appear in the literature. These studies have employed a range of checkpoint blockade strategies, cancer models, and OVs (e.g., 3, 4, 9, 10), and have shown that OV/checkpoint combinations lead to better outcomes (often including complete remission) compared with single-agent treatments, and that the response is dependent on CD8+ T cells. These preclinical studies also showed that OVs can overcome systemic tumor resistance to immune checkpoint blockade immunotherapy,9 have provided mechanistic insights, and have suggested optimal dosing regimens.
In glioblastoma, two recent studies have shown the effectiveness of OV/immune checkpoint combination therapy in the GL261 syngeneic mouse glioma model. Cockle et al.3 used intravenously administered vesicular stomatitis virus and showed enhanced animal survival in combination with anti-CTLA4 and anti-PD1 treatment. The triple combination of virus, anti-CTLA4, and anti-PD1 gave the best survival advantage in this model. In this issue of Neuro-Oncology, Hardcastle et al.4 report the combination of an oncolytic EGFR-targeted measles virus with anti-PD1 immune checkpoint blockade. The authors showed that in vitro infection of GL261 cells with the measles OV led to a pro-inflammatory response and increased microglia activation; in some cell lines the OV also upregulated PD-L1 levels. When combined in vivo, the OV/anti-PD1 combined treatment showed a synergistic enhancement, with increased CD8+ T cell influx into treated brains. In fact, neither single agent had a great effect on survival in this study (no long-term survivors), but the combination gave 60% long-term survivors; a remarkable effect. However, one general caveat for the glioblastoma immunotherapy field is the heavy reliance in studies like these on a single model of glioblastoma – GL261. This cell line was established via chemical mutagenesis and is known to be immunogenic and therefore responsive to immunotherapies. Although GL261 is undoubtedly a useful tool, multiple syngeneic mouse lines should be used, if possible, in these kinds of studies.
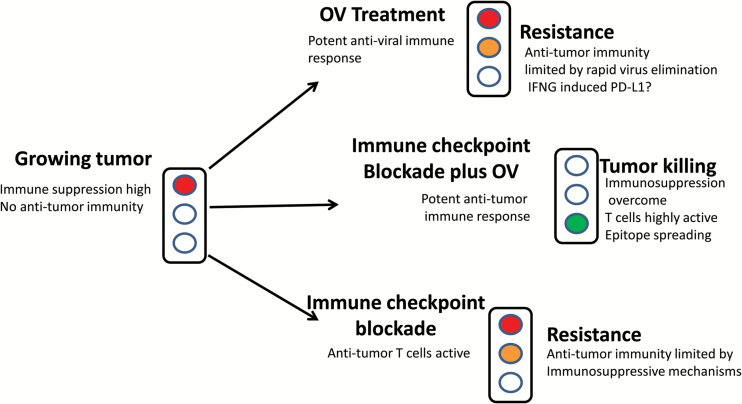
Mechanistically, there is much to be learned, and things are complicated further when the number of possible virus/checkpoint blockade/tumor combinations is considered. In our favor, there are a number of readily conceivable ways that OVs could synergize with immune checkpoint blockade: In general terms, OV-mediated immunostimulation could be seen as allowing the opening of a door (the door being opened by the anti-viral immune response) to allow intratumoral infiltration of immune cells, “shifting the balance of power” in the immune system against the tumor. Also, the release of tumor antigens during oncolysis may act as an in situ vaccine to generate a stronger T cell response. So far, pre-clinical studies indicate that OVs may upregulate the T cell immune checkpoint ligand PD-L1 and also cause epitope spreading, allowing T cells to recognize a wider range of tumor antigens.10Figure 1 illustrates how these therapies may work together to improve anti-tumor T cell responses.
Fig. 1.
Potential mechanisms involved in the effectiveness of OV/immune checkpoint blockade combination therapy. The figure indicates that the therapeutic combination will lead to an improved outcome by leading to an elevated anti-tumor CD8+ T cell response. OV, oncolytic virus.
As we learn more about the interactions between these two therapies we will be able to design improved tumor and patient-tailored combinations. Our preclinical models will assist in further understanding of mechanisms, safety, dosing, optimizing combinations, and providing potential biomarkers as well as inspiring further virus engineering.
In glioblastoma we still await many answers from ongoing immunotherapy trials before making any assumptions regarding our patients. However, on this winter evening as I stare out across the Boston skyline from my office window and try to imagine what the future of glioblastoma treatment will look like, I see a glimmer of something interesting in the distance…. is it coming towards us?
References
- 1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–584. [DOI] [PubMed] [Google Scholar]
- 3. Cockle JV, Rajani K, Zaidi S, et al. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro Oncol. 2016;18(4):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hardcastle J, Mills L, Malo CS, et al. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro Oncol. 2016;19(4):XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palucka AK, Coussens LM. The Basis of Oncoimmunology. Cell. 2016;164(6):1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 7.Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review [published online ahead of print July 21]. JAMA Oncol. 2016. doi:10.1001/jamaoncol.2016.2064. [DOI] [PubMed] [Google Scholar]
- 8. Puzanov I, Milhem MM, Minor D, et al. Talimogene Laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woller N, Gürlevik E, Fleischmann-Mundt B, et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol Ther. 2015;23(10):1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]