Abstract
Background:
Matrix-assisted autologous chondrocyte transplantation (MACT) is a surgical treatment option for articular cartilage lesions of the knee joint.
Purpose:
To investigate mid- to long-term clinical outcomes of MACT in the patellofemoral (PF) and tibiofemoral (TF) joints.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
A systematic review was performed by searching PubMed, Embase, and the Cochrane Library to find studies evaluating minimum 5-year clinical outcomes of patients undergoing MACT in the knee joint. Search terms used were knee, matrix, and autologous chondrocyte. Patients were evaluated based on treatment failure rates, magnetic resonance imaging, and subjective outcome scores. Study methodology was assessed using the Modified Coleman Methodology Score (MCMS).
Results:
Ten studies (two level 1, one level 2, one level 3, and six level 4 evidence) were identified that met inclusion and exclusion criteria, for a total of 442 TF patients and 136 PF patients. Treatment failure occurred in 9.7% of all patients, including 4.7% of PF patients and 12.4% of TF patients (P = .037). Weighted averages of subjective outcome scores, including Knee injury and Osteoarthritis Outcome Score, Short Form–36 Health Survey, and Tegner scores, improved from baseline to latest follow-up in both TF and PF patients. The mean MCMS was found to be 57.4, with a standard deviation of 18.5.
Conclusion:
Patients undergoing MACT in the knee show favorable mid- to long-term clinical outcomes. A significantly higher treatment failure rate was found in patients undergoing MACT in the TF joint compared with the PF joint.
Keywords: matrix-assisted autologous chondrocyte transplantation, matrix-assisted chondrocyte implantation, knee, articular cartilage
Articular cartilage has limited to no ability for spontaneous repair after injury.6 If left untreated, full-thickness articular cartilage lesions can lead to symptoms such as pain, swelling, and joint dysfunction.7 Articular cartilage injuries have been found in up to 63% of patients undergoing arthroscopic knee procedures, with a prevalence of 32% in patients aged 20 to 29 years and 46% in those aged 30 to 39 years.8,10 Several surgical procedures, such as marrow-stimulation techniques (MST) and autologous chondrocyte implantation (ACI), have been developed to treat articular cartilage lesions. MST is a reparative treatment that stimulates subchondral bone, resulting in the formation of fibrocartilage tissue at the site of the lesion.26 ACI, a restorative treatment option, produces a repair tissue more similar to hyaline cartilage compared with MST, which may be better able to restore the natural function of the knee.16
ACI is a 2-step procedure in which chondrocytes are first arthroscopically harvested and cultured in vitro. The second procedure involves injecting the chondral defect with the cultured cells and then covering them with a periosteal patch (first-generation ACI) or collagen membrane (second-generation ACI).4,27 Issues negatively affecting clinical outcomes, such as periosteal patch hypertrophy associated with first-generation ACI22 and extensive suturing and cell leakage associated with second-generation ACI,2 have led to the development of third-generation ACI, otherwise known as matrix-assisted autologous chondrocyte transplantation (MACT).18 In MACT, cultured chondrocytes are seeded into a matrix scaffold and then fixed to the chondral defect with fibrin glue.27 MACT was first introduced into clinical practice in Europe in 1998. Third-generation ACI was just recently approved by the Food and Drug Administration in the United States, with several additional clinical trials currently underway.21
Several systematic reviews have evaluated outcomes after treatment with MACT.3,8,24 However, each of these reviews has included studies with short-term outcomes. Furthermore, these reviews did not calculate an overall failure rate of MACT at follow-up. A recent systematic review comparing minimum 5-year outcomes of ACI versus microfracture surgery (MFx)20 showed no differences in clinical outcomes, although this review included mostly first-generation ACI studies. The purpose of this systematic review was therefore to evaluate the current literature in order to assess mid- to long-term outcomes of MACT and to compare outcomes in patients with patellofemoral (PF) versus tibiofemoral (TF) chondral lesions. We hypothesized that patients would have favorable mid- to long-term outcomes after MACT and that patients with TF chondral lesions would have better outcomes than those with PF lesions.
Methods
A systematic review of multiple databases was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two independent reviewers searched PubMed, Embase, and the Cochrane Library up to October 4, 2016. The electronic search strategy used was knee AND matrix AND “autologous chondrocyte.” A total of 332 studies were reviewed by title and/or abstract to determine study eligibility based on inclusion/exclusion criteria. Inclusion criteria included studies that reported clinical outcomes of MACT at a minimum 5-year follow-up and studies that reported clinical outcomes of MACT specific to either the PF or TF joint. Studies with a level of evidence from 1 to 4 were included. Studies were not excluded if patients had existing arthritis, although those that included skeletally immature patients were excluded. Additionally, studies were excluded if they did not specify outcomes based on lesion location or if they reported outcomes of MACT when performed with specific concurrent procedures. Disparities in eligible studies were resolved by discussion between the 2 reviewers.
Reporting Outcomes
Patients were divided into 2 groups: those undergoing MACT in the PF joint (PF group) and those undergoing MACT in the TF joint (TF group). For the purposes of this systematic review, outcome measures were included which allowed for comparison between patients who had MACT in PF versus TF joints. Outcomes assessed included treatment failure rate, magnetic resonance imaging (MRI) composite scores derived from the magnetic resonance observation of cartilage repair tissue (MOCART) score, and patient-reported outcomes, including the Knee injury and Osteoarthritis Outcome Score (KOOS), Tegner score, and Short Form–36 Health Survey (SF-36) physical and mental component scores. Treatment failure definitions varied among studies and are explained in the Results section. The MOCART score assesses 8 parameters of graft repair, with each parameter being scored from 1 to 4 (1 = poor; 2 = fair; 3 = good; 4 = excellent).11
Study Methodology Assessment
The Modified Coleman Methodology Score (MCMS) was used to evaluate study methodology quality.9 The MCMS has a scaled potential score ranging from 0 to 100. Scores ranging from 85 to 100 are excellent, 70 to 84 are good, 55 to 69 are fair, and less than 55 are poor.
Statistical Analysis
A weighted average was calculated for numerical demographics (age, defect size) and outcome scores based on the included studies. A chi-square test was used to determine significant differences in treatment failure rates between the PF and TF groups.
Results
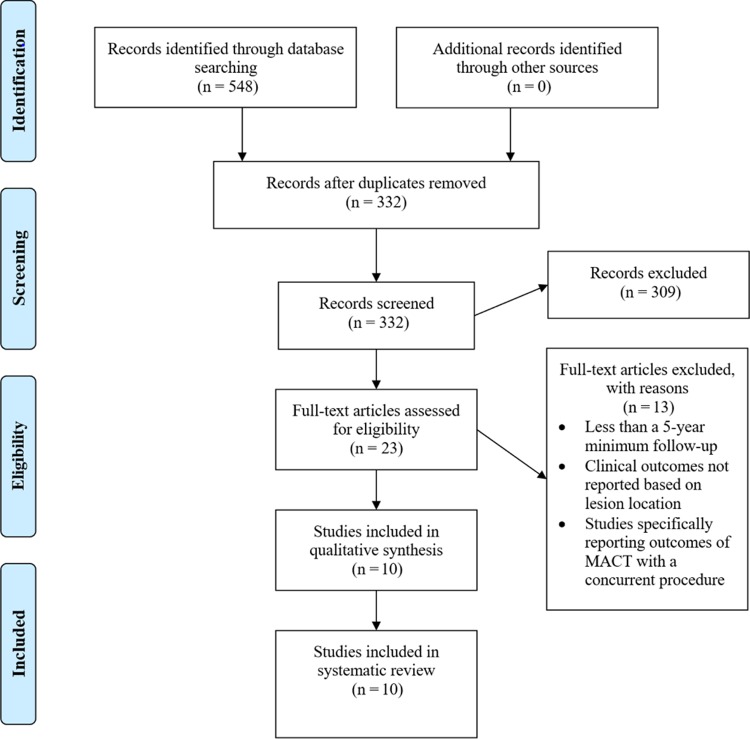
Ten studies5,11–15,19,23,28,29 met inclusion and exclusion criteria (Figure 1) (two with level 1 evidence,12,28 one level 2,14 one level 3,13 and six level 45,11,15,19,23,29). Of these, 3 studies14,19,23 reported outcomes specific to the PF joint, 4 studies11–13,28 reported outcomes specific to the TF joint, and 3 studies5,15,29 reported outcomes specific to both PF and TF joints. Nine studies11–15,19,23,28,29 excluded patients with diffuse or inflammatory/metabolic arthritis. One study5 included patients with early osteoarthritis and kissing lesions.
Figure 1.
Search strategy using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. MACT, matrix-assisted autologous chondrocyte transplantation.
All 10 studies used the standard MACT procedure as described above. However, there were variations in the method of fixation used. There were variations in matrix scaffold type and manufacturer used, as shown in Table 1. One study29 did not use the same matrix in all patients.
TABLE 1.
Matrix-Assisted Autologous Chondrocyte Transplantation Techniques
| Study | Matrix Scaffold | |
|---|---|---|
| Brix et al, 20145 Filardo et al, 201414 Filardo et al, 201415 Kon et al, 201619 |
Hyalograft C Hyalograft C Hyalograft C Hyalograft C |
|
| Ebert et al, 201711 Ebert et al, 201212 Ebert et al, 201313 Meyerkort et al, 201423 |
Type I/III collagen membrane Type I/III collagen membrane Type I/III collagen membrane Type I/III collagen membrane |
|
| Wondrasch et al, 201528 | Type I collagen membrane or Hyalograft C | |
| Zak et al, 201229 | Type I/III collagen membrane or type I collagen membrane or Hyalograft C |
aManufacturers: Hyalograft C (Fidia Advanced Biopolymers); type I/III collagen membrane (Genzyme); type I collagen membrane (Arthro Kinetics Biotechnology GmbH).
Table 2 depicts the characteristics of the sample population from the 10 included studies. Overall, 587 patients with 442 TF defects and 136 PF defects were compared.
TABLE 2.
Population Characteristicsa
| Study | n | Age, y | Defect Size, cm2 | Minimum Follow-up, y | Lesion Location,b n |
|---|---|---|---|---|---|
| Brix et al, 20145 | 53 | 32.0 ± 12.0 | 4.4 ± 1.9 | 5 | LFC: 8 MFC: 44 PF: 2 TP: 1 |
| Ebert et al, 201711 | 31 | 35.3 | 2.52 | 5 | LFC: 7 MFC: 18 TP: 6 |
| Ebert et al, 201212 | 63 | 38.2 | 3.3 | 5 | TF: 63 |
| Ebert et al, 201313 | 104 | 37.9 ± 11.6 | 3.2 ± 2.3 | 5 | LFC:27 MFC: 73 TP: 4 |
| Filardo et al, 201414 | 49 | 31.5 ± 99 | 3.0 ± 1.4 | 5 | PF: 49 |
| Filardo et al, 201415 | 131 | 29.2 ± 11.1 | 2.3 ± 1.0 | 7 | MFC: 82 LFC: 36 PF:14 |
| Kon et al, 201619 | 32 | 31.3 ± 10.1 | 4.5 ± 2.1 | 10 | PF: 32 |
| Meyerkort et al, 201423 | 23 | 42.3 ± 11.6 | 3.5 ± 1.4 | 5 | PF: 24 |
| Wondrasch et al, 201528 | 31 | 33.0 | 4.9 | 5 | LFC: 10 MFC: 22 |
| Zak et al, 201229 | 70 | 34.9 ± 8.6 | 5.3 ± 2.9 | 5 | TF: 40 PF: 15 Multiplec: 15 |
| Total | 587 | 34.0 | 3.5 | 5 | LFC: 88 MFC: 239 TF: 104 TP: 11 PF: 136 Multiplec: 15 |
aAge and defect size are reported as a mean ± standard deviation (when available). If available, TF lesion locations were specified by TP, MFC, or LFC. TF refers to tibiofemoral lesions that were not further specified. LFC, lateral femoral condyle; MFC, medial femoral condyle; PF, patellofemoral; TF, tibiofemoral; TP; tibial plateau.
bSeveral patients had more than 1 lesion.
cPatients with multiple lesions of unspecified locations.
Seven studies5,12,14,15,19,23,29 performed concomitant procedures (Table 3). Additionally, 2 studies11,23 reported postoperative complications, including 1 patient with a deep venous thrombosis23 and 10 patients with graft hypertrophy.11,23
TABLE 3.
Number of Concomitant Procedures Performeda
| Study | ACLR | HTO | TTT | PCLR | Lateral Release | Meniscectomy | Trochleoplasty | Meniscal Sutures | Collagen Meniscal Implants | Osteotomy | Realignment Procedure | Patellar Tendon Scarification | LBR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brix et al, 20145 | 4 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ebert et al, 201711 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ebert et al, 201212 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ebert et al, 201313 | 6 | 2 | 2 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Filardo et al, 201414 | 2 | 0 | 0 | 0 | 13 | 7 | 1 | 0 | 0 | 1 | 6 | 1 | 0 |
| Filardo et al, 201415 | 29 | 0 | 0 | 2 | 2 | 34 | 0 | 3 | 4 | 5 | 1 | 1 | 15 |
| Kon et al, 201619 | 1 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 10 | 0 | 0 |
| Meyerkort et al, 201423 | 0 | 0 | 7 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wondrasch et al, 201528 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zak et al, 201229 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
aACLR, anterior cruciate ligament reconstruction; HTO, high tibial osteotomy; LBR, loose body removal; PCLR, posterior cruciate ligament reconstruction; TTT, tibial tubercle transfer.
Table 4 shows the MCMS scores from the 10 included studies. Two studies13,28 achieved good scores, while 8 studies5,11,12,14,15,19,23,29 achieved fair scores. The mean score was 57.4, with a standard deviation of 18.5.
TABLE 4.
Modified Coleman Methodology Score (MCMS)
Seven studies5,11,12,14,15,19,23 reported treatment failures. Two studies5,23 did not provide a specific definition of treatment failure, 2 studies11,12 did not provide a clear definition of treatment failure beyond graft failure, and 3 studies14,15,19 defined treatment failure as the need for reoperation due to symptoms caused by the primary defect. Overall, 9.7% of patients failed treatment, including 12.4% in the TF group and 4.7% in the PF group (P = .037) (Table 5). One study23 did not state that any patients failed treatment, although 3 patients in this study exhibited graft hypertrophy as detected by MRI, and 1 patient underwent a subsequent arthroscopic debridement.
TABLE 5.
Treatment Failuresa
| Study | TF | PF | Total |
|---|---|---|---|
| Brix et al, 20145 | 11/51 (21.6) | 1/2 (50.0) | 12/53 (22.6) |
| Ebert et al, 201711 | 2/31 (3.2) | — | 2/31 (3.2) |
| Ebert et al, 201212 | 5/63 (7.9) | — | 5/63 (7.9) |
| Filardo et al 201414 | — | 0/49 (0.0) | 0/49 (0.0) |
| Filardo et al, 201415 | NR | NR | 14/131 (10.7) |
| Kon et al, 201619 | — | 4/32 (12.5) | 4/32 (12.5) |
| Meyerkort et al, 201423 | — | 0/23 (0.0) | 0/23 (0.0) |
| Total | 18/145 (12.4) | 5/106 (4.7) | 37/382 (9.7) |
aFailures are reported as number of failures/total number of patients (%). NR, not reported; PF, patellofemoral; TF, tibiofemoral.
MRI composite scores are presented in Table 6. Overall, the PF group had a higher MRI composite score at latest follow-up, although only 1 study included these scores for PF patients.
TABLE 6.
Magnetic Resonance Imaging Composite Scorea
| Study | TF (n = 197) | PF (n = 23) |
|---|---|---|
| Ebert et al, 201711 | 3.14 (n = 30) | — |
| Ebert et al, 201212 | 2.96 (n = 63) | — |
| Ebert et al, 201313 | 3.00 (n = 104) | — |
| Meyerkort et al, 201423 | — | 3.38 (n = 23) |
| Weighted average | 3.01 | 3.38 |
aPF, patellofemoral; TF, tibiofemoral.
Subjective outcome scores, including Tegner, SF-36 physical component summary (PCS) and mental component summary (MCS) are presented in Table 7. Mean Tegner scores at latest follow-up were higher for the PF group, although no baseline Tegner score was reported for TF patients. In both groups, SF-36 PCS and MCS improved at follow-up.
TABLE 7.
Subjective Outcome Scoresa
| TF | PF | |||
|---|---|---|---|---|
| Study | Preoperative Score | Postoperative Score | Preoperative Score | Postoperative Score |
| Tegner | ||||
| Ebert et al, 201711 | 2.7 ± 0.3 (n = 31) | 5.5 ± 0.5 (n = 30) | — | — |
| Filardo et al, 201414 | — | — | 1.9 ± 1.2 (n = 49) | 4.7 ± 2.0 (n = 49) |
| Kon et al, 201619 | — | — | 2.5 ± 1.4 (n = 32) | 4.4 ± 1.5 (n = 32) |
| Wondrasch et al, 201528 | NR | 4.0 (n = 31) | — | — |
| Zak et al, 201229 | NR | 4.0 ± 1.6 (n = 40) | NR | 4.3 ± 1.6 (n = 15) |
| Weighted average | 2.7 (n = 31) | 4.5 (n = 101) | 2.1 (n = 81) | 4.5 (n = 96) |
| Weighted improvement | 2.8 (n = 30) | 2.4 (n = 81) | ||
| SF-36 PCS | ||||
| Ebert et al, 201711 | 39.1 ± 1.9 (n = 31) | 51.0 ± 1.4 (n = 30) | — | — |
| Ebert et al, 201212 | 39.3 (n = 63) | 48.3 (n = 63) | — | — |
| Meyerkort et al, 201423 | — | — | 36.4 (n = 23) | 45.1 (n = 23) |
| Weighted average | 39.2 (n = 94) | 48.8 (n = 93) | 36.4 (n = 23) | 45.1 (n = 23) |
| Weighted improvement | 9.9 (n = 93) | 8.7 (n = 23) | ||
| SF-36 MCS | ||||
| Ebert et al, 201711 | 50.9 ± 1.5 (n = 31) | 54.6 ± 1.4 (n = 30) | — | — |
| Ebert et al, 201212 | 51.7 (n = 63) | 54.7 (n = 63) | — | — |
| Meyerkort et al, 201423 | — | — | 51.2 (n = 23) | 57.3 (n = 23) |
| Weighted average | 51.4 (n = 94) | 54.3 (n = 93) | 51.2 (n = 23) | 57.3 (n = 23) |
| Weighted improvement | 3.2 (n = 93) | 6.1 (n = 23) | ||
aScores are listed as a mean ± standard deviation (when available). MCS, mental component summary; NR, not reported; PCS, physical component summary; PF, patellofemoral; SF-36, Short Form–36 Health Survey; TF, tibiofemoral.
KOOS subscale outcomes are presented in Table 8. The PF group had a lower average baseline for all KOOS subscales. Patients in both groups showed improvement in all 5 KOOS subscales at latest follow-up.
TABLE 8.
KOOS Subscale Outcomesa
| TF | PF | |||
|---|---|---|---|---|
| Study | Preoperative Score | Postoperative Score | Preoperative Score | Postoperative Score |
| KOOS-SR | ||||
| Ebert et al, 201711 | 32.4 ± 4.4 (n = 31) | 71.5 ± 4.7 (n = 30) | — | — |
| Ebert et al, 201212 | 26.1 (n = 63) | 67.1 (n = 63) | — | — |
| Ebert et al, 201313 | 23.6 (n = 104) | 63.1 (n = 104) | — | — |
| Meyerkort et al, 201423 | — | — | 23.0 (n = 23) | 50.2 (n = 23) |
| Wondrasch et al, 201528 | 25.2 (n = 31) | 73.7 (n = 31) | — | — |
| Zak et al, 201229 | NR | 67.4 ± 30.0 (n = 40) | NR | 61.3 ± 23.0 (n = 15) |
| Weighted average | 25.7 (n = 229) | 66.9 (n = 268) | 23.0 (n = 23) | 54.6 (n = 38) |
| Weighted improvement | 41.1 (n = 228) | 27.2 (n = 23) | ||
| KOOS-QOL | ||||
| Ebert et al, 201711 | 29.1 ± 3.1 (n = 31) | 67.5 ± 4.6 (n = 30) | — | — |
| Ebert et al, 201212 | 33.4 (n = 63) | 62.6 (n = 63) | — | — |
| Ebert et al, 201313 | 29.4 (n = 104) | 58.5 (n = 104) | — | — |
| Meyerkort et al, 201423 | — | — | 19.5 (n = 23) | 50.8 (n = 23) |
| Wondrasch et al, 201528 | 29.3 (n = 31) | 64.9 (n = 31) | — | — |
| Weighted average | 30.4 (n = 229) | 61.7 (n = 228) | 19.5 (n = 23) | 50.8 (n = 23) |
| Weighted improvement | 31.2 (n = 228) | 31.3 (n = 23) | ||
| KOOS-Pain | ||||
| Ebert et al, 201711 | 59.6 ± 3.9 (n = 31) | 91.2 ± 1.8 (n = 30) | — | — |
| Ebert et al, 201212 | 68.9 (n = 63) | 85.8 (n = 63) | — | — |
| Meyerkort et al, 201423 | — | — | 60.0 (n = 23) | 80.6 (n = 23) |
| Wondrasch et al, 201528 | 60.0 (n = 31) | 83.6 (n = 31) | — | — |
| Weighted average | 64.4 (n = 125) | 86.6 (n = 124) | 60.0 (n = 23) | 80.6 (n = 23) |
| Weighted improvement | 22.1 (n = 124) | 20.6 (n = 23) | ||
| KOOS-Symptoms | ||||
| Ebert et al, 201711 | 62.3 ± 3.4 (n = 31) | 85.6 ± 2.1 (n = 30) | — | — |
| Ebert et al, 201212 | 71.6 (n = 63) | 85.0 (n = 63) | — | — |
| Meyerkort et al, 201423 | — | — | 62.4 (n = 23) | 84.0 (n = 23) |
| Wondrasch et al, 201528 | 53.2 (n = 31) | 64.9 (n = 31) | — | — |
| Weighted average | 64.7 (n = 125) | 80.1 (n = 124) | 62.4 (n = 23) | 84.0 (n = 23) |
| Weighted improvement | 15.4 (n = 124) | 21.6 (n = 23) | ||
| KOOS-ADL | ||||
| Ebert et al, 201711 | 75.8 ± 3.6 (n = 31) | 94.1 ± 1.6 (n = 30) | — | — |
| Ebert et al, 201212 | 80.1 (n = 63) | 92.8 (n = 63) | — | — |
| Meyerkort et al, 201423 | — | — | 69.3 (n = 23) | 88.3 (n = 23) |
| Wondrasch et al, 201528 | 63.1 (n = 31) | 87.9 (n = 31) | — | — |
| Weighted average | 74.8 (n = 125) | 91.9 (n = 124) | 69.3 (n = 23) | 88.3 (n = 23) |
| Weighted improvement | 17.1 (n = 124) | 19.0 (n = 23) | ||
aScores are reported as a mean ± standard deviation (when available). ADL, activities of daily living; KOOS, Knee injury and Osteoarthritis Outcome Score; NR, not reported; PF, patellofemoral; QOL, quality of life; SR, sports and recreation; TF, tibiofemoral.
Discussion
This systematic review is the first to specifically evaluate mid- to long-term clinical outcomes after MACT in the knee. Based on this review, patients undergoing MACT have a 9.7% treatment failure rate at a minimum 5-year follow-up. There was a significantly higher failure rate at latest follow-up in patients undergoing MACT in the TF joint compared with the PF joint (P = .037). Of the studies that discussed specific reasons for treatment failure, the most commonly reported reasons included progressing osteoarthritis,5 dislocation or delamination of graft,5,12 and lack of a clinically significant improvement in symptoms.19 It should be noted that there was significant heterogeneity among treatment failure definitions, and these differences may have significantly biased the true failure rate.
Three published systematic reviews have evaluated mid- to long-term outcomes of other common surgical treatments used for chondral defects of the knee joint, including MFx,17 osteochondral allograft transplantation,1 and osteochondral autograft transplantation (OAT).25 Assenmacher et al1 found that, at an average follow-up of 12.3 years, patients who had OAT had an overall failure rate of 25%, with worse clinical outcomes demonstrated in patients with PF lesions. Pareek et al25 found that, at an average follow-up of 10.2 years, patients who had OAT had an overall failure rate of 28%. In a systematic review evaluating mid- to long-term patient outcomes after MFx, Goyal et al17 found 5-year failure rates as high as 23% and 10-year failure rates as high as 38%. The overall failure rate found in the current systematic review on MACT was lower than that reported in any of these studies, although this may partially be due to a shorter follow-up period in our study.
Of the 6 studies in our review that evaluated using KOOS scores,11–13,23,28,29 average baseline values for all KOOS subscales were found to be lower in the PF group. Additionally, 2 studies12,23 found that baseline SF-36 PCS and MCS scores were lower for the PF group. These findings suggest that PF lesions may be more debilitating than TF lesions, although further studies are necessary to directly compare baseline subjective characteristics between these groups. All studies in this review showed improvements in patient-reported outcomes at follow-up in both TF and PF groups after MACT. Of the 8 patient-reported outcome scores displayed in Tables 5 and 6, the TF group had a greater mean improvement in 5 of the scores (Tegner, SF-36 PCS, KOOS–Sports and Recreation, KOOS–Pain, and KOOS–Activities of Daily Living), while the PF group had a greater mean improvement in 3 of the scores (SF-36 MCS, KOOS–Quality of Life, and KOOS–Symptoms).
There are several limitations to this systematic review. First, level 1 to 4 evidence studies were included. Although 587 patients were included in this review, not all patients were evaluated using the same outcome measures, and therefore sample sizes were limited for particular outcomes. Of the defects compared, there was a significant disparity in defect numbers between those in the TF group (442) and those in the PF group (136). Additionally, 2 studies12,13 included some overlapping patients. The authors of 3 included studies14,15,19 work at the same research center, and while no mention of overlapping patients was made in these studies, it is possible there may be some overlap. Another limitation includes the variation in different scaffold types being used in the studies included in this review; some studies used type I/III collagen membranes while others used just type I collagen membranes. Finally, some studies were excluded for not reporting outcomes specifically with regard to cartilage lesion location.
In conclusion, this systematic review supports the view that patients undergoing MACT in the knee have favorable mid- to long-term clinical outcomes. Significantly higher failure rates were demonstrated in patients undergoing MACT in the TF joint compared with the PF joint. Further studies are necessary to compare long-term outcomes between MACT and other surgical treatment options for chondral lesions in the knee.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
References
- 1. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32:2160–2168. [DOI] [PubMed] [Google Scholar]
- 2. Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. [DOI] [PubMed] [Google Scholar]
- 3. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–1271. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed] [Google Scholar]
- 5. Brix MO, Stelzeneder D, Chiari C, et al. Treatment of full-thickness chondral defects with Hyalograft C in the knee: long-term results. Am J Sports Med. 2014;42:1426–1432. [DOI] [PubMed] [Google Scholar]
- 6. Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. [DOI] [PubMed] [Google Scholar]
- 7. Chubinskaya S, Haudenschild D, Gasser S, Stannard J, Krettek C, Borrelli J., Jr Articular cartilage injury and potential remedies. J Orthop Trauma. 2015;29(suppl 12):S37–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ciccotti MC, Kraeutler MJ, Austin LS, et al. The prevalence of articular cartilage changes in the knee joint in patients undergoing arthroscopy for meniscal pathology. Arthroscopy. 2012;28:1437–1444. [DOI] [PubMed] [Google Scholar]
- 9. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2–11. [DOI] [PubMed] [Google Scholar]
- 10. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456–460. [DOI] [PubMed] [Google Scholar]
- 11. Ebert JR, Fallon M, Wood DJ, Janes GC. A prospective clinical and radiological evaluation at 5 years after arthroscopic matrix-induced autologous chondrocyte implantation. Am J Sports Med. 2017;45:59–69. [DOI] [PubMed] [Google Scholar]
- 12. Ebert JR, Fallon M, Zheng MH, Wood DJ, Ackland TR. A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Am J Sports Med. 2012;40:1527–1537. [DOI] [PubMed] [Google Scholar]
- 13. Ebert JR, Smith A, Edwards PK, Hambly K, Wood DJ, Ackland TR. Factors predictive of outcome 5 years after matrix-induced autologous chondrocyte implantation in the tibiofemoral joint. Am J Sports Med. 2013;41:1245–1254. [DOI] [PubMed] [Google Scholar]
- 14. Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42:626–634. [DOI] [PubMed] [Google Scholar]
- 15. Filardo G, Kon E, Andriolo L, Di Matteo B, Balboni F, Marcacci M. Clinical profiling in cartilage regeneration: prognostic factors for midterm results of matrix-assisted autologous chondrocyte transplantation. Am J Sports Med. 2014;42:898–905. [DOI] [PubMed] [Google Scholar]
- 16. Gillogly SD, Myers TH, Reinold MM. Treatment of large full-thickness chondral defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 2006;36:751–764. [DOI] [PubMed] [Google Scholar]
- 17. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579–1588. [DOI] [PubMed] [Google Scholar]
- 18. Kon E, Filardo G, Di Matteo B, Perdisa F, Marcacci M. Matrix assisted autologous chondrocyte transplantation for cartilage treatment: a systematic review. Bone Joint Res. 2013;2:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kon E, Filardo G, Gobbi A, et al. Long-term results after hyaluronan-based MACT for the treatment of cartilage lesions of the patellofemoral joint. Am J Sports Med. 2016;44:602–608. [DOI] [PubMed] [Google Scholar]
- 20. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes [published online April 1, 2017]. Am J Sports Med. doi:10.1177/0363546517701912. [DOI] [PubMed] [Google Scholar]
- 21. Lyman S, Nakamura N, Cole BJ, Erggelet C, Gomoll AH, Farr J., 2nd Cartilage-repair innovation at a standstill: methodological and regulatory pathways to breaking free. J Bone Joint Surg Am. 2016;98:e63. [DOI] [PubMed] [Google Scholar]
- 22. Marlovits S, Zeller P, Singer P, Resinger C, Vécsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57:24–31. [DOI] [PubMed] [Google Scholar]
- 23. Meyerkort D, Ebert JR, Ackland TR, et al. Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:2522–2530. [DOI] [PubMed] [Google Scholar]
- 24. Oussedik S, Tsitskaris K, Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy. 2015;31:732–744. [DOI] [PubMed] [Google Scholar]
- 25. Pareek A, Reardon PJ, Maak TG, Levy BA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral autograft transfer: a systematic review at mean follow-up of 10.2 years. Arthroscopy. 2016;32:1174–1184. [DOI] [PubMed] [Google Scholar]
- 26. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. [DOI] [PubMed] [Google Scholar]
- 27. Steinwachs M. New technique for cell-seeded collagen-matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25:208–211. [DOI] [PubMed] [Google Scholar]
- 28. Wondrasch B, Risberg MA, Zak L, Marlovits S, Aldrian S. Effects of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle: a prospective, randomized controlled study presenting MRI-based and clinical outcomes after 5 years. Am J Sports Med. 2015;43:146–153. [DOI] [PubMed] [Google Scholar]
- 29. Zak L, Aldrian S, Wondrasch B, Albrecht C, Marlovits S. Ability to return to sports 5 years after matrix-associated autologous chondrocyte transplantation in an average population of active patients. Am J Sports Med. 2012;40:2815–2821. [DOI] [PubMed] [Google Scholar]