Summary
The development of pharmaceutical agents such as sucralfate, histamine 2 (H2) receptor blockers and proton pump inhibitors (PPIs) reducing gastric acidity has been a mile stone for treatment of dyspeptic disorders. However, due to current prescription habits resulting in overuse of these potent drugs as well as over-the-counter (OTC) availability associated with self-medication, substantial health concern is related to the mechanisms of drug action as well as known side effects influencing gastrointestinal physiology.
More than a decade ago the first study appeared reporting an association between anti-ulcer drug intake and food allergy development. Ever since this first report several experimental as well as human studies verified this correlation, demonstrating that acid suppressive drugs not only influence the sensitization capacity of orally ingested proteins, but also represent a risk factor for food allergy patients. Additionally, gastric acid suppression was reported to increase the risk for development of drug hypersensitivity reactions. These consequences of anti-ulcer drug intake might on the one hand be associated with direct influence of these drugs on immune responses. On the other hand reduction of gastric acidity leads to impaired gastrointestinal protein degradation. Nevertheless, also disruption of the gastrointestinal barrier function, changes in microbiome or lack of tolerogenic peptic digests might contribute to the connection between anti-ulcer drug intake and allergic reaction. Therefore, these drugs should only be prescribed based on a precise gastroenterological diagnosis taking into consideration allergological mechanisms to ensure patients’ safety.
Keywords: gastric acid suppression medication, proton pump inhibitors, H2 receptor blockers, food allergy, drug allergy, sensitization
Introduction
Until the 1970s treatment of diseases associated with inadequate gastric acid secretion was limited to the use of antacids neutralizing excessive gastric acid and partial surgical elimination of the stomach. As this treatment was associated with insufficient mucosal healing and as surgical interventions expectably had severe side effects, effective pharmacological control of gastric acid secretion was a desirable goal of pharmaceutical research. In 1972 the first H2 receptor blocker buimamide was developed. This major pharmaceutical break-through was followed by development of the widely prescribed H2 receptor antagonist cimetidine in 1975.[1] The major advantage of both compounds was the direct interference with the acid stimulating capacity of histamine via its receptor on parietal cells.
Only a few years later, first studies on the ulcer-healing and pepsin-binding properties of the basic aluminum sucrose sulfate complex termed sucralfate were published and this drug class was approved for the treatment of acute ulcers as well as continuous maintenance therapy for disease recurrence prevention.[2, 3]
In parallel pharmaceutical companies continued their efforts to develop novel therapeutics to reduce gastric acid output. In 1988 the PPI omeprazole was approved in Europe, which was followed by marketing of the same compound two years later in the US.[4] This and the other drugs with proton pump inhibitory action being subsequently released were quickly accepted as clinically superior to previous antisecretory medication.[5]
Besides their substantial benefit for management of patients with dyspeptic disorders gastric acid suppressive drugs can be considered a goldmine for the pharmaceutical industry. Even at the end of the patent protection period the PPI esomeprazole is still among the top selling drugs worldwide and the first PPI on the market omeprazole and esomeprazole were recently ranked among the all-time best selling drugs.[6, 7]
Besides an indicated long term use of this medication for relapse prevention in case of gastro-esophageal reflux (GERD), reflux esophagitis and non-erosive reflux disease (NERD) [8] there is substantial health and also economical concern about overuse and the inadequate prescription habits of acid suppression drugs. Several studies revealed that around 70% of patients admitted to nursing facilities or being hospitalized were on acid suppressive medication with more than 50% of them without an appropriate diagnosis justifying prescription.[9, 10] Another major health concern is the current inappropriate self-medication habit. Approximately 80% of patients with dyspeptic disorders of minor severity do not seek medical advice and simply use OTC available acid suppressive drugs.[11]
Mechanisms of action and side effects of anti-ulcer drugs
From a chemical point of view antacids are weak bases, such as hydroxides or carbonates, which are used to neutralize hydrochloric acid in the stomach.[12] They are formulated as salts of an alkaline ion in combination with one polyvalent cation such as calcium, aluminum and magnesium. Even though they are rarely prescribed by physicians, antacids are frequently used without prescription due to OTC availability. Major concerns regarding safety of these drugs are the observed pH dependent or chelation dependent drug interactions.[13]
Being a derivative of aluminum hydroxide containing antacids, sucralfate is a salt of sucrose sulfate and aluminum hydroxide. The drug was demonstrated to be activated in the presence of acidity. Upon release of aluminum, the molecule obtains a negative charge interacting with positively charged chemical groups in its environment such as e.g. H+ ions, drugs or mucins. It is known for its pepsin binding capacity, thereby reducing pepsin concentration in gastric juices.[14, 15] Moreover, sucralfate has a mucosal protective effect by forming a physical barrier in the form of complex gels and interacts with high affinity with normal as well as inflamed mucosa.[16, 17, 18] In post-marketing evaluations sucralfate was described as an efficient and safe drug with limited side effects,[19, 20] with obstipation reported as the most common undesired effect occurring in up to 15% of patients.[16, 21]
As histamine has an acid secretion activation function via its H2 receptor on parietal cells, interference with this receptor by antagonists, was the first available causative treatment option for dyspeptic disorders. Later it was found that the acid secretion antagonizing effect of H2 receptor blockers is not only due to inhibition of histamine stimulation, but also due to interference with carbachol and gastrin stimulation which both act via H2 receptors.[22] Regarding side effects of these drugs the initial fear of many physicians was predisposition to bacterial overgrowth and consequently elevated intragastric levels of N-nitrosoamine, a known carcinogen. However, long-term clinical experience proofed these drugs not to harbor cancer inducing properties.[23] Regarding bacterial overgrowth and infections, these drugs seem to pose a minor risk compared to PPIs as reviewed below, probably due to the observed circadian changes in acid control by H2 receptor blockers.[22] Nevertheless, it is well accepted that H2 receptor blockers strongly interfere with cytochrome P450 (CPY450) enzymes, which requires special caution regarding interaction with other pharmaceuticals.[24]
PPIs were designed to acquire their potential acid suppressive function via specific and irreversible inhibition of the H+K+ATPase located in the secretory membrane of the parietal cells.[25, 26, 27] Influencing function and bioavailability different PPI subclasses revealed variations in speed and degree of gastric acid reduction but also in metabolism by hepatic CYP450 enzymes.[28] One side effect of PPIs and other acid-reducing drugs is the lowered bioavailability of drugs depending on acidic intragastric pH for absorption. PPIs may further alter the first pass metabolism and hepatic drug elimination.[29] PPIs are known to not only change intestinal bacterial composition [30] but also to be associated with intragastric bacterial overgrowth [31] which might lead to lung colonization and subsequently pneumonia by aspiration.[32] In general hospital settings but also in intensive care units (ICUs) gavage of PPI to critically ill patients for stress ulcer prophylaxis was associated with an increased risk for Clostridium difficile associated diarrhea.[33, 34] In contrast, PPIs might have an anti-microbial effect on some pathogens by blocking the proton pump in the membrane of certain bacteria and fungi.[35, 36] Furthermore, rare cases of anaphylactic reactions upon intake of omeprazole were reported in literature, with potential for crossreactivity between different PPI subclasses as indicated by intradermal skin testing.[37, 38]
The impact of acid reduction drugs on digestive capacity and function of the gastrointestinal tract
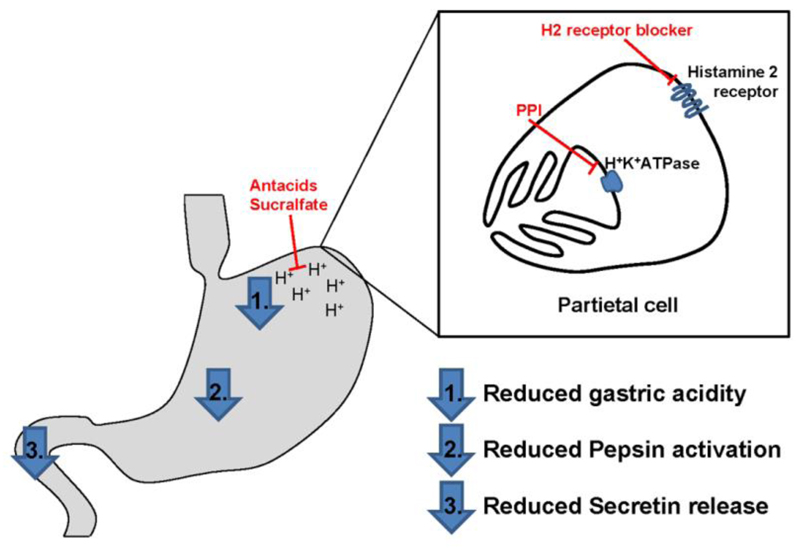
As indicated above acid-reduction medication either binds protons in the gastric lumen or substantially reduces the gastric acid output, which is the therapeutic goal for treatment of acid related disorders. Acidity itself is not only required for protection against infectious agents, but also initiates protein degradation by influencing food protein-food matrix and protein-protein interactions as well as structural properties of proteins. Additionally, acidity is a prerequisite for activation of the major gastric enzyme pepsin. Pepsin, produced by gastric chief cells, is secreted into the gastric lumen as the inactive proenzyme pepsinogen.[39] Only at low intragastric pH levels the prosegment is released making the binding cleft available for protein interaction.[40] As pepsin is the first protein degrading enzyme during gastrointestinal transit, interference with its function has major impact on protein digestion.[41] Acidity of the chyme has a second main influence on gastrointestinal protein digestion. Upon arrival in the duodenum the acidic chyme represents the main stimulus for secretin release by S-cells mainly found in the duodenal mucosa (Fig. 1).[42] Secretin is essential to stimulate pancreatic secretion of the proteases trypsin, chymotrypsin and carboxypeptidases.[43] These proteases and peptidases are responsible for further peptide digestion into single amino acids or small peptides which are subsequently taken up by enterocytes for nutrition of the human body. Besides its influence on the gastric acidity, the PPI esomeprazole was even demonstrated to induce a significant transmucosal leak by compromising the barrier function of the upper gastrointestinal tract already after 5 days of medication.[44] However, in patients with eosinophilic esophagitis (EoE) mucosal integrity of the esophagus was restored after high-dose treatment with PPIs in tissue samples from patients with PPI-responsive eosinophilia (PPI-REE).[45] Thus, additional studies are needed to evaluate whether anti-ulcer drug intake might be associated not only with compromised digestive capacity of the gastrointestinal tract, but also with a leaky barrier function of the gastrointestinal tract with major implications on health and disease.
Figure 1.
Mechanisms of action of acid suppression medication. While antacids and sucralfate directly bind protons in the gastric lumen, other drugs interfere with the gastric acid production of parietal cells. H2 receptor antagonists block the activating function of histamine via its H2 receptor. PPIs irreversibly inhibit the proton pump in the secretory cell membrane of parietal cells. The resulting elevated gastric pH leads to reduced pepsin activation in the stomach as well as duodenal secretin release, which substantially interferes with the gastrointestinal protein digestion.
The influence of acid-suppression medication on the immune response
Already during the first years after approval for clinical practice studies appeared that suggested an influence of sucralfate on the immune response. Sucralfate was reported to activate the mucosal cyclooxygenase directly affecting the arachidonic acid metabolism and leading to elevated synthesis and release of leukotriene C4.[46, 47] Moreover, an enhanced production of prostaglandins from the gastric mucosa and activation of macrophages were described.[48] Additionally, sucralfate, but also simple antacids are aluminum containing drugs, which might per se exert a substantial influence on the immune response.[49] Despite common knowledge the first focused study evaluating the impact of the aluminum containing drug sucralfate on the immune response appeared in 2007. The authors revealed a Th2 shift after systemic application in an experimental model and speculated on a possible impact of aluminum on the observed immune response deviation. [50]
The biogenic amine histamine exerts an effect in a large variety of different cells including important immune cells via its 4 receptors.[51] Out of these 4 the H2 receptor is not only expressed on gastric parietal cells, but additionally also on brain tissue, cardiac tissue, smooth muscle cells, T- and B-cells and dendritic cells.[52] Especially for dendritic cells histamine interaction via its H2 receptor was proposed to alter response to microbial ligands and promote T regulatory cells.[51, 53] Furthermore, it was proposed that H2 receptor activation might shift the immune response towards Th2 dominance. Thus, it is not surprising that H2 receptor antagonists have an eminent effect on the immune response with an overall immune-activating potential as reviewed by Biwas and colleagues.[54] In context with these above mentioned findings, it is of special interest that usage of H2 receptor antagonists in inflammatory bowel disease patients were reported to increase the risk for hospitalization.[55]
Also for PPI a reasonable number of studies report a direct influence on the immune response unrelated to the inhibition of gastric acid. PPIs were shown to have anti-oxidative effects in vitro and in vivo.[56, 57] As PPIs interfere with the proton pump, effects on other, non-gastric cells expressing the H+K+ATPase on extracellular membranes and intracellular organelles like neutrophils and endothelial cells can be expected.[58] It is known that PPIs decrease expression of intercellular adhesion molecules leading to reduced neutrophil accumulation as well as to inhibited oxidative burst of human neutrophils.[59] Additionally, PPIs were demonstrated to inhibit lysosomal enzymes with broad implication in infectious as well as tumor immune defense.[60] In contrast other studies even proposed an association between the intake of PPIs and decreased tumorigenesis, which in some of the studies was beyond the mucosa healing effect of the drugs.[61, 62] Furthermore, Lansoprazole treatment was described to down-regulate Th1 and Th2 signaling pathways in the human gastric mucosa [63] and to be associated with decreased levels of proinflammatory cytokines such as IL-6, IL-8 and TNF-α in cultured tracheal epithelial cells.[64] Interestingly, PPIs were also reported to inhibit IL-4 and IL-13 signaling via STAT6 [65] and in PPI-REE a marked reduction of eotaxin-3, IL-13, IL-5 mRNA expression was observed after PPI treatment.[66]
The association of anti-ulcer drug intake and allergic reactions
Based on the above reviewed data it is tempting to speculate that acid-reduction medication might also have an important influence on the allergic immune response. However, the first definitive indication of a correlation between anti-ulcer drug intake and food allergy development was the case history of a patient diagnosed with caviar allergy at our institution.[67] He reported to have been on acid-reduction medication during the first symptom-free consumption of Beluga caviar. Two years later he developed severe clinical symptoms including anaphylaxis at the second ingestion of caviar. Based on this knowledge we performed experimental studies confirming the correlation of gastric acid-reduction by anti-ulcer drug intake and the development of food allergy irrespective of the type of medication used (i.e. sucralfate, H2-receptor blockers or PPIs).[68, 69] The influence on IgE induction and skewing the immune response towards Th2 was confirmed for another subclass of H2-receptor blockers.[70] Furthermore, also less potent acid reduction medication, such as antacids and base powder, were found to be associated with food allergy development.[71] These results were confirmed in human studies evaluating the influence of long-term anti-ulcer drug intake on food sensitization and allergy induction. A cohort of 152 adult patients treated for dyspeptic disorder with either H2-recptor blockers or PPIs was subjected to an allergological screening before and after a 3 month course of acid-reduction treatment. In these patients we observed a boost of preexisting food-specific IgE antibodies in 10% or de novo IgE formation towards regular constituents of the daily diet in 15% of all patients. Even though food specific IgE levels decreased in some patients 5 months after discontinuation of anti-ulcer medication intake, sensitization was still confirmed by positive skin tests.[72] Additionally, food allergy was diagnosed in a patients’ subgroup developing hazelnut specific IgE antibodies during anti-ulcer treatment by double-blind, placebo-controlled food challenges.[68] This correlation is not only of importance for adult patients but also for other age groups. Already during pregnancy intake of acid-suppression medication represents a risk for maternal food allergy development, but also shifts the immune response of the off-springs towards Th2 in an experimental model.[73] These results were underlined by a population-based birth register study revealing a link between maternal acid suppression therapy during pregnancy and the development of childhood asthma.[74] In line with these data intake of acid-suppression medication during childhood was suggested to be associated with an increasing prevalence of food allergy.[75, 76] However, also during immunosenescence in elderly patients gastric acid reduction is a risk for food allergy as indicated by experimental as well as human studies.[77, 78] Moreover, clinical studies revealed impairment of gastric digestion by gastric pH elevation to represent a causative factor for allergic reactions at lower amounts of ingested allergens in already food allergic patients.[79, 80]
Not only for food allergy acid-suppression medication seems to be risk factor. We additionally reported a correlation between gastric acid suppression and development of IgE mediated hypersensitivity towards diclofenac.[81] In line with these findings, a retrospective chart review study reported PPI intake to increase the risk for the development of drug hypersensitivity reactions (DHR).[82] From this study no conclusion is possible on the influence of route of drug administration and drug metabolism on DHR induction during PPI treatment.
Of interest, in a subgroup of patients with EoE, a chronic immune-mediated disorder of the esophagus associated with eosinophilic infiltrates as well as eotaxin and Th2 cytokine overexpression, a therapeutic role to PPIs is increasingly accepted.[83] Even though mechanisms are not fully understood and therapeutic applications are controversially discussed,[84] an inhibitory action of PPI on eotaxin 3 expression by blocking STAT6 promotor binding in esophageal cells from patients with EoE was reported.[85] For a better mechanistic understanding, it is of special interest to investigate pathophysiological similarities and differences between PPI-REE and EoE as well as other Th2 driven diseases.
Discussion of causal relation between gastric acid reduction and allergy development
The connection between gastric acid reduction and allergy development can be seen in association with several mechanisms of actions or side-effects of these potent drugs.
First of all, acid-suppression medication substantially interferes with the digestive function of the gastrointestinal tract. A recent study in morbid obese patients undergoing bariatic surgery underlined the important gate-keeping function of gastric digestion in allergy development. The patients’ sensitization profiles substantially changed after elimination of adequate digestion by gastric bypass surgery.[86] Moreover, at least for food allergy induction resistance of food proteins to gastric digestive enzymes seems to be decisive for allergenicity and has for a long time been regarded as predictive for their sensitizing capacity.[41] Digestion experiments with simulated gastric fluid were even considered to distinguish between potentially allergenic and non-allergenic food compounds and digestion labile food proteins were termed non-sensitizing elicitors.[87, 88] However, during the past decades it has become evident that major food allergens inducing allergic sensitization via the oral route are readily degraded by gastric enzymes under physiological conditions.[89] Especially for these food proteins interference with the gastrointestinal digestion as it is the case under acid-reduction medication, might have major consequences increasing the risk for food allergy development.[41]
Moreover, gastric digestion itself is considered to contribute to tolerance induction via the oral route. The concept of tolerogenic properties of peptic fragments of food proteins goes back to the late 1970ies. At that time first studies reported peptic fragments of the model allergen bovine serum albumin (BSA) to be capable of suppressing an antibody response in immunization experiments due to T-cell interaction.[90, 91] Pepsin and trypsin digest of certain food allergens might still have retained immunogenic properties of fragments especially with regards to T-cell activation.[92, 93] Furthermore, digestion-resistant food proteins might even have an intact IgE binding capacity after pepsin incubation.[94] Based on this knowledge experimental trails evaluated pepsinized food protein digests for the development of novel therapeutic approaches in food allergy and demonstrated beneficial effects on cashew nut and cow’s milk allergy.[95, 96] Thus, hindrance of gastric digestion under anti-ulcer medication might not only increase the sensitization capacity of food proteins by leaving them undigested but additionally might reduce the availability of tolerogenic digestion fragments.
Another risk factor for allergy induction under acid reduction medication might be its interference with epithelial barrier function. The integrity of the gastrointestinal epithelium gains increasing attention as dysfunction of the intestinal epithelial barrier is currently discussed as a contributing factor in development and progression of various diseases including food allergy.[97, 98] Interestingly, high levels of total and food-specific IgE were measured in the gastrointestinal mucosa of peptic ulcer patients which was discussed as a result of enhanced mucosal permeability in H. pylori infection.[99, 100] Also for other diseases of the upper gastrointestinal tract such as Barrett’s esophagus an increased transepithelial leak was described.[101] In 2008 a first report appeared linking PPI intake with increased transmucosal leak in the upper gastrointestinal tract.[44] However, in patients with PPI-REE integrity of the esophageal mucosa was restored after PPI treatment. Due to the different outcome in these studies and due to the lack of information on the influence of acid suppressive drugs on barrier function of other parts of the gastrointestinal tract, further studies are needed to evaluate a possible contribution to allergy development. Last but not least acid suppression drug might influence the gastrointestinal microbiome. Gastric acid reduction is known to change the intestinal microbial composition.[102] On the one hand PPIs substantially increase the number of bacteria in the oral cavity and the upper gastrointestinal tract due to lack of gastric acidity.[103, 104] One the other hand PPIs might have an anti-microbial effect on certain microbes by inhibiting the H+K+ATPase found in bacterial and fungal cell membranes.[35, 36] Microbiome changes during anti-ulcer drug treatment and the known influence of the intestinal bacterial composition on food allergy [105, 106] could offer a further mechanistic explanation for the observed association between pharmaceutical gastric acid-suppression and allergy development. Without any doubt additional research is needed to in-depth evaluate this possible interaction.
Conclusions and clinical out-look
The functional homeostasis of the gastrointestinal tract is essential for overall immunological health and allergy prevention. Even though treatment with anti-ulcer drugs has an essential health benefit for patients with acid related disorders and is increasingly recognized for treatment of a subset of patients with EoE, it has to be taken into consideration that acid suppression medication substantially interferes with the physiological properties of the gastrointestinal tract. Several mechanisms of action or side effects of these potent drugs could beneficially or detrimentally influence ongoing immune responses. So far experimental as well as clinical studies outlined an association between gastric acid suppression and allergy induction. The causative factors for this correlation probably go beyond the influence of acid reduction medication on gastrointestinal protein digestion and might explain the observed variations between patients regarding allergy induction. Therefore, prescription of this medication should be based on a clear gastroenterological diagnosis with avoidance of long-term intake, if possible. Additionally, it seems to be crucial to consider the immunological impact of these drugs to ensure full therapeutic benefit and patients’ safety. Additional mechanistic studies as well as extensive interdisciplinary exchange are clearly needed and will be essential in the future.
Acknowledgements
This work was supported by grants KLI284-B00 and WKP39 of the Austrian Science fund FWF.
References
- 1.Henn RM, Isenberg JI, Maxwell V, Sturdevant RAL. Inhibition of Gastric Acid Secretion by Cimetidine in Patients with Duodenal Ulcer. N Engl J Med. 1975;293:371–5. doi: 10.1056/NEJM197508212930802. [DOI] [PubMed] [Google Scholar]
- 2.Blum AL, Bethge H, Bode JC, Domschke W, Feurle G, Hackenberg K, et al. Sucralfate in the treatment and prevention of gastric ulcer: multicentre double blind placebo controlled study. Gut. 1990;31:825–30. doi: 10.1136/gut.31.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borella LE, Seethaler K, Lippmann W. Sucralfate: antipeptic, antiulcer activities and antagonism of gastric emptying. Arzneimittelforschung. 1979;29:793–8. [PubMed] [Google Scholar]
- 4.Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov. 2003;2:132–9. doi: 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]
- 5.Andersson T. Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet. 1996;31:9–28. doi: 10.2165/00003088-199631010-00002. [DOI] [PubMed] [Google Scholar]
- 6. [last accessed 15.04.2015]; http://www.medscape.com/viewarticle/820011.
- 7. [last accessed 15.04.2015]; http://www.forbes.com/sites/simonking/2013/01/28/the-best-selling-drugs-of-all-time-humira-joins-the-elite/
- 8.Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol. 2010;16:2323–30. doi: 10.3748/wjg.v16.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glew CM, Rentler RJ. Use of proton pump inhibitors and other acid suppressive medications in newly admitted nursing facility patients. J Am Med Dir Assoc. 2007;8:607–9. doi: 10.1016/j.jamda.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Gupta Ruchi, Garg Praveen, Kottoor Ravi, Munoz Juan Carlos, Jamal M Mazen, L Louis R, et al. Overuse of Acid Suppression Therapy in Hospitalized Patients. South Med J. 2010;103:207–11. doi: 10.1097/SMJ.0b013e3181ce0e7a. [DOI] [PubMed] [Google Scholar]
- 11.Corder AP, Jones RH, Sadler GH, Daniels P, Johnson CD. Heartburn, oesophagitis and Barrett's oesophagus in self-medicating patients in general practice. Br J Clin Pract. 1996;50:245–8. [PubMed] [Google Scholar]
- 12.Feurle GE. Effect of rising intragastric pH induced by several antacids on serum gastrin concentrations in duodenal ulcer patients and in a control group. Gastroenterology. 1975;68:1–7. [PubMed] [Google Scholar]
- 13.Ogawa R, Echizen H. Clinically significant drug interactions with antacids: an update. Drugs. 2011;71:1839–64. doi: 10.2165/11593990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Samloff IM, O'Dell C. Inhibition of peptic activity by sucralfate. Am J Med. 1985;79:15–8. doi: 10.1016/0002-9343(85)90566-2. [DOI] [PubMed] [Google Scholar]
- 15.Venables CW. Mucus, pepsin, and peptic ulcer. Gut. 1986;27:233–8. doi: 10.1136/gut.27.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy DM. Sucralfate. N Engl J Med. 1991;325:1017–25. doi: 10.1056/NEJM199110033251407. [DOI] [PubMed] [Google Scholar]
- 17.Nagashima R. Mechanisms of action of sucralfate. J Clin Gastroenterol. 1981;3:117–27. [PubMed] [Google Scholar]
- 18.Slomiany BL, Piotrowski J, Okazaki K, Grzelinska E, Slomiany A. Nature of the enhancement of the protective qualities of gastric mucus by sucralfate. Digestion. 1989;44:222–31. doi: 10.1159/000199915. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RS. Sucralfate: a review of drug tolerance and safety. J Clin Gastroenterol. 1981;3:181–4. [PubMed] [Google Scholar]
- 20.Ishimori A. Safety experience with sucralfate in Japan. J Clin Gastroenterol. 1981;3:169–73. [PubMed] [Google Scholar]
- 21.Marks IN. The efficacy, safety and dosage of sucralfate in ulcer therapy. Scand J Gastroenterol Suppl. 1987;140:33–8. [PubMed] [Google Scholar]
- 22.Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 2003;98:109–27. doi: 10.1016/s0163-7258(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 23.Rossing MA, Scholes D, Cushing-Haugen KL, Voigt LF. Cimetidine use and risk of prostate and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:319–23. [PubMed] [Google Scholar]
- 24.Rendic S. Drug interactions of H2-receptor antagonists involving cytochrome P450 (CYPs) enzymes: from the laboratory to the clinic. Croat Med J. 1999;40:357–67. [PubMed] [Google Scholar]
- 25.Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjostrand SE, et al. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature. 1981;290:159–61. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg P, Nordberg P, Alminger T, Brandstrom A, Wallmark B. The mechanism of action of the gastric acid secretion inhibitor omeprazole. J Med Chem. 1986;29:1327–9. doi: 10.1021/jm00158a001. [DOI] [PubMed] [Google Scholar]
- 27.Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+,K+-ATPase activity. J Biol Chem. 1985;260:13681–4. [PubMed] [Google Scholar]
- 28.Robinson M. Proton pump inhibitors: update on their role in acid-related gastrointestinal diseases. Int J Clin Pract. 2005;59:709–15. doi: 10.1111/j.1368-5031.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 29.Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29:769–84. doi: 10.2165/00002018-200629090-00002. [DOI] [PubMed] [Google Scholar]
- 30.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 31.Del Piano M, Pagliarulo M, Tari R, Carmagnola S, Balzarini M, Lorenzini P, et al. Correlation between chronic treatment with proton pump inhibitors and bacterial overgrowth in the stomach: any possible beneficial role for selected lactobacilli? J Clin Gastroenterol. 2014;48(Suppl 1):S40–6. doi: 10.1097/MCG.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 32.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–60. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 33.Buendgens L, Bruensing J, Matthes M, Duckers H, Luedde T, Trautwein C, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care. 2014;29:696 e11–5. doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243–5. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 35.Monk BC, Mason AB, Abramochkin G, Haber JE, Seto-Young D, Perlin DS. The yeast plasma membrane proton pumping ATPase is a viable antifungal target. I. Effects of the cysteine-modifying reagent omeprazole. Biochim Biophys Acta. 1995;1239:81–90. doi: 10.1016/0005-2736(95)00133-n. [DOI] [PubMed] [Google Scholar]
- 36.Nakao M, Malfertheiner P. Growth inhibitory and bactericidal activities of lansoprazole compared with those of omeprazole and pantoprazole against Helicobacter pylori. Helicobacter. 1998;3:21–7. doi: 10.1046/j.1523-5378.1998.08024.x. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez P, Soriano V, Lopez P, Niveiro E. Anaphylaxis to proton pump inhibitors. Allergol Immunopathol (Madr) 2002;30:342–3. doi: 10.1016/s0301-0546(02)79150-7. [DOI] [PubMed] [Google Scholar]
- 38.Kollmeier AP, Eddleston J, Zuraw BL, Christiansen SC. Recurrent anaphylaxis linked to pantoprazole. J Allergy Clin Immunol. 2004;114:975–7. doi: 10.1016/j.jaci.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 39.Etherington DJ, Taylor WH. The pepsins from human gastric mucosal extracts. Biochem J. 1970;118:587–94. doi: 10.1042/bj1180587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815–36. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Untersmayr E, Jensen-Jarolim E. The role of protein digestibility and antacids on food allergy outcomes. J Allergy Clin Immunol. 2008;121:1301–8. doi: 10.1016/j.jaci.2008.04.025. quiz 9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chey WY, Chang TM. Neural control of the release and action of secretin. J Physiol Pharmacol. 2003;54(Suppl 4):105–12. [PubMed] [Google Scholar]
- 43.Pali-Schöll I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy. 2011;66:469–77. doi: 10.1111/j.1398-9995.2010.02511.x. [DOI] [PubMed] [Google Scholar]
- 44.Mullin JM, Valenzano MC, Whitby M, Lurie D, Schmidt JD, Jain V, et al. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28:1317–25. doi: 10.1111/j.1365-2036.2008.03824.x. [DOI] [PubMed] [Google Scholar]
- 45.van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, et al. Proton pump inhibitors partially restore mucosal integrity in patients with proton pump inhibitor-responsive esophageal eosinophilia but not eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12:1815–23 e2. doi: 10.1016/j.cgh.2014.02.037. [DOI] [PubMed] [Google Scholar]
- 46.Ligumsky M, Karmeli F, Rachmilewitz D. Sucralfate protection against gastrointestinal damage: possible role of prostanoids. Isr J Med Sci. 1986;22:801–6. [PubMed] [Google Scholar]
- 47.Wallace JL, Morris GP, Beck PL, Williamson TE, Gingras GR. Effects of sucralfate on gastric prostaglandin and leukotriene synthesis: relationship to protective actions. Can J Physiol Pharmacol. 1988;66:666–70. doi: 10.1139/y88-105. [DOI] [PubMed] [Google Scholar]
- 48.Morris GP, Keenan CM, MacNaughton WK, Wallace JL, Williamson TE. Protection of rat gastric mucosa by sucralfate. Effects of luminal stasis and of inhibition of prostaglandins synthesis. Am J Med. 1989;86:10–6. doi: 10.1016/0002-9343(89)90150-2. [DOI] [PubMed] [Google Scholar]
- 49.Jensen-Jarolim E. Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ J. 2015;8:7. doi: 10.1186/s40413-015-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunner R, Wallmann J, Szalai K, Karagiannis P, Kopp T, Scheiner O, et al. The impact of aluminium in acid-suppressing drugs on the immune response of BALB/c mice. Clin Exp Allergy. 2007;37:1566–73. doi: 10.1111/j.1365-2222.2007.02813.x. [DOI] [PubMed] [Google Scholar]
- 51.O'Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128:1153–62. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 52.Smolinska S, Jutel M, Crameri R, O'Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–81. doi: 10.1111/all.12330. [DOI] [PubMed] [Google Scholar]
- 53.Frei R, Ferstl R, Konieczna P, Ziegler M, Simon T, Rugeles TM, et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013;132:194–204. doi: 10.1016/j.jaci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Biswas S, Benedict SH, Lynch SG, LeVine SM. Potential immunological consequences of pharmacological suppression of gastric acid production in patients with multiple sclerosis. BMC Med. 2012;10:57. doi: 10.1186/1741-7015-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juillerat P, Schneeweiss S, Cook EF, Ananthakrishnan AN, Mogun H, Korzenik JR. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:239–47. doi: 10.1111/j.1365-2036.2012.05173.x. [DOI] [PubMed] [Google Scholar]
- 56.Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993–1001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- 57.Lapenna D, de Gioia S, Ciofani G, Festi D, Cuccurullo F. Antioxidant properties of omeprazole. FEBS Lett. 1996;382:189–92. doi: 10.1016/0014-5793(96)00155-x. [DOI] [PubMed] [Google Scholar]
- 58.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–7. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki M, Nakamura M, Mori M, Miura S, Tsuchiya M, Ishii H. Lansoprazole inhibits oxygen-derived free radical production from neutrophils activated by Helicobacter pylori. J Clin Gastroenterol. 1995;20(Suppl 2):S93–6. doi: 10.1097/00004836-199506002-00025. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, Baker SS, Trinidad J, Burlingame AL, Baker RD, Forte JG, et al. Inhibition of lysosomal enzyme activities by proton pump inhibitors. J Gastroenterol. 2013;48:1343–52. doi: 10.1007/s00535-013-0774-5. [DOI] [PubMed] [Google Scholar]
- 61.Han YM, Hahm KB, Park JM, Hong SP, Kim EH. Paradoxically augmented anti-tumorigenic action of proton pump inhibitor and GastrininAPCMin/+ intestinal polyposis model. Neoplasia. 2014;16:73–83. doi: 10.1593/neo.131510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Milito A, Marino ML, Fais S. A rationale for the use of proton pump inhibitors as antineoplastic agents. Curr Pharm Des. 2012;18:1395–406. doi: 10.2174/138161212799504911. [DOI] [PubMed] [Google Scholar]
- 63.Larussa T, Suraci E, Leone I, Nazionale I, Abenavoli L, Galasso O, et al. Short-term therapy with celecoxib and lansoprazole modulates Th1/ Th2 immune response in human gastric mucosa. Helicobacter. 2010;15:449–59. doi: 10.1111/j.1523-5378.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki T, Yamaya M, Yasuda H, Inoue D, Yamada M, Kubo H, et al. The proton pump inhibitor lansoprazole inhibits rhinovirus infection in cultured human tracheal epithelial cells. Eur J Pharmacol. 2005;509:201–10. doi: 10.1016/j.ejphar.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 65.Cortes JR, Rivas MD, Molina-Infante J, Gonzalez-Nunez MA, Perez GM, Masa JF, et al. Omeprazole inhibits IL-4 and IL-13 signaling signal transducer and activator of transcription 6 activation and reduces lung inflammation in murine asthma. J Allergy Clin Immunol. 2009;124:607–10, 10 e1. doi: 10.1016/j.jaci.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Molina-Infante J, Rivas MD, Hernandez-Alonso M, Vinagre-Rodriguez G, Mateos-Rodriguez JM, Duenas-Sadornil C, et al. Proton pump inhibitor-responsive oesophageal eosinophilia correlates with downregulation of eotaxin-3 and Th2 cytokines overexpression. Aliment Pharmacol Ther. 2014;40:955–65. doi: 10.1111/apt.12914. [DOI] [PubMed] [Google Scholar]
- 67.Untersmayr E, Focke M, Kinaciyan T, Poulsen LK, Boltz-Nitulescu G, Scheiner O, et al. Anaphylaxis to Russian Beluga caviar. J Allergy Clin Immunol. 2002;109:1034–5. doi: 10.1067/mai.2002.124893. [DOI] [PubMed] [Google Scholar]
- 68.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 69.Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 70.Arae K, Oboki K, Ohno T, Hirata M, Nakae S, Taguchi H, et al. Cimetidine enhances antigen-specific IgE and Th2 cytokine production. Allergol Int. 2011;60:339–44. doi: 10.2332/allergolint.10-OA-0255. [DOI] [PubMed] [Google Scholar]
- 71.Pali-Schöll I, Herzog R, Wallmann J, Szalai K, Brunner R, Lukschal A, et al. Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin Exp Allergy. 2010;40:1091–8. doi: 10.1111/j.1365-2222.2010.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 73.Schöll I, Ackermann U, Ozdemir C, Blumer N, Dicke T, Sel S, et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a Th2-bias in their offspring. FASEB J. 2007;21:1264–70. doi: 10.1096/fj.06-7223com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dehlink E, Yen E, Leichtner AM, Hait EJ, Fiebiger E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy. 2009;39:246–53. doi: 10.1111/j.1365-2222.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 75.DeMuth K, Stecenko A, Sullivan K, Fitzpatrick A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013;34:227–32. doi: 10.2500/aap.2013.34.3657. [DOI] [PubMed] [Google Scholar]
- 76.Trikha A, Baillargeon JG, Kuo YF, Tan A, Pierson K, Sharma G, et al. Development of food allergies in patients with gastroesophageal reflux disease treated with gastric acid suppressive medications. Pediatr Allergy Immunol. 2013;24:582–8. doi: 10.1111/pai.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Untersmayr E, Diesner SC, Brämswig KH, Knittelfelder R, Bakos N, Gundacker C, et al. Characterization of intrinsic and extrinsic risk factors for celery allergy in immunosenescence. Mech Ageing Dev. 2008;129:120–8. doi: 10.1016/j.mad.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 78.Bakos N, Scholl I, Szalai K, Kundi M, Untersmayr E, Jensen-Jarolim E. Risk assessment in elderly for sensitization to food and respiratory allergens. Immunol Lett. 2006;107:15–21. doi: 10.1016/j.imlet.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Untersmayr E, Poulsen LK, Platzer MH, Pedersen MH, Boltz-Nitulescu G, Skov PS, et al. The effects of gastric digestion on codfish allergenicity. J Allergy Clin Immunol. 2005;115:377–82. doi: 10.1016/j.jaci.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 80.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711–7. doi: 10.1016/j.jaci.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riemer AB, Gruber S, Pali-Schöll I, Kinaciyan T, Untersmayr E, Jensen-Jarolim E. Suppression of gastric acid increases the risk of developing immunoglobulin E-mediated drug hypersensitivity: human diclofenac sensitization and a murine sensitization model. Clin Exp Allergy. 2010;40:486–93. doi: 10.1111/j.1365-2222.2009.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramirez E, Cabanas R, Laserna LS, Fiandor A, Tong H, Prior N, et al. Proton pump inhibitors are associated with hypersensitivity reactions to drugs in hospitalized patients: a nested case-control in a retrospective cohort study. Clin Exp Allergy. 2013;43:344–52. doi: 10.1111/cea.12034. [DOI] [PubMed] [Google Scholar]
- 83.Molina-Infante J, Hernandez-Alonso M, Vinagre-Rodriguez G, Martin-Noguerol E. Proton pump inhibitors therapy for esophageal eosinophilia: simply following consensus guidelines. J Gastroenterol. 2011;46:712–3. doi: 10.1007/s00535-011-0388-8. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- 84.Asher Wolf W, Dellon ES. Eosinophilic esophagitis and proton pump inhibitors: controversies and implications for clinical practice. Gastroenterol Hepatol. 2014;10:427–32. [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shakeri-Leidenmühler S, Lukschal A, Schultz C, Bohdjalian A, Langer F, Birsan T, et al. Surgical elimination of the gastric digestion by Roux-en-Y gastric bypass impacts on food sensitization – a pilot study. Obes Surg. 2015 Apr 25; doi: 10.1007/s11695-015-1689-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 88.Aalberse RC. Food allergens. Environ Toxicol Pharmacol. 1997;4:55–60. doi: 10.1016/s1382-6689(97)10042-4. [DOI] [PubMed] [Google Scholar]
- 89.Fu TJ. Digestion stability as a criterion for protein allergenicity assessment. Ann N Y Acad Sci. 2002;964:99–110. doi: 10.1111/j.1749-6632.2002.tb04135.x. [DOI] [PubMed] [Google Scholar]
- 90.Muckerheide A, Pesce AJ, Michael JG. Immunosuppressive properties of a peptic fragment of BSA. J Immunol. 1977;119:1340–5. [PubMed] [Google Scholar]
- 91.Zhang Z, Apple RJ, Pesce A, Michael JG. Peptic fragments of bovine serum albumin bind antigen-specific T suppressor cells from orally tolerized mice. Cell Immunol. 1987;104:426–33. doi: 10.1016/0008-8749(87)90044-x. [DOI] [PubMed] [Google Scholar]
- 92.Eiwegger T, Rigby N, Mondoulet L, Bernard H, Krauth MT, Boehm A, et al. Gastro-duodenal digestion products of the major peanut allergen Ara h 1 retain an allergenic potential. Clin Exp Allergy. 2006;36:1281–8. doi: 10.1111/j.1365-2222.2006.02565.x. [DOI] [PubMed] [Google Scholar]
- 93.Hong SJ, Michael JG, Fehringer A, Leung DY. Pepsin-digested peanut contains T-cell epitopes but no IgE epitopes. J Allergy Clin Immunol. 1999;104:473–8. doi: 10.1016/s0091-6749(99)70396-9. [DOI] [PubMed] [Google Scholar]
- 94.Akkerdaas JH, Wensing M, Asero R, Fernandez Rivas M, Knulst AC, Bolhaar S, et al. IgE binding to pepsin-digested food extracts. Int Arch Allergy Immunol. 2005;138:203–8. doi: 10.1159/000088720. [DOI] [PubMed] [Google Scholar]
- 95.Kulis M, Macqueen I, Li Y, Guo R, Zhong XP, Burks AW. Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in mice with cashew allergy. J Allergy Clin Immunol. 2012;130:716–23. doi: 10.1016/j.jaci.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meulenbroek LA, van Esch BC, Hofman GA, den Hartog Jager CF, Nauta AJ, Willemsen LE, et al. Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow's milk allergy. Pediatr Allergy Immunol. 2013;24:656–64. doi: 10.1111/pai.12120. [DOI] [PubMed] [Google Scholar]
- 97.Price D, Ackland L, Suphioglu C. Nuts 'n' guts: transport of food allergens across the intestinal epithelium. Asia Pac Allergy. 2013;3:257–65. doi: 10.5415/apallergy.2013.3.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 99.De Lazzari F, Mancin O, Plebani M, Venturi C, Battaglia G, Vianello F, et al. High IgE serum levels and "peptic" ulcers: clinical and functional approach. Ital J Gastroenterol. 1994;26:7–11. [PubMed] [Google Scholar]
- 100.Matysiak-Budnik T, Coffin B, Lavergne-Slove A, Sabate JM, Megraud F, Heyman M. Helicobacter pylori increases the epithelial permeability to a food antigen in human gastric biopsies. Am J Gastroenterol. 2004;99:225–32. doi: 10.1111/j.1572-0241.2004.04080.x. [DOI] [PubMed] [Google Scholar]
- 101.Mullin JM, Valenzano MC, Trembeth S, Allegretti PD, Verrecchio JJ, Schmidt JD, et al. Transepithelial leak in Barrett's esophagus. Dig Dis Sci. 2006;51:2326–36. doi: 10.1007/s10620-006-9478-5. [DOI] [PubMed] [Google Scholar]
- 102.Bajaj JS, Cox IJ, Betrapally NS, Heuman DM, Schubert ML, Ratneswaran M, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014;307:G951–7. doi: 10.1152/ajpgi.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verdu E, Viani F, Armstrong D, Fraser R, Siegrist HH, Pignatelli B, et al. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut. 1994;35:455–60. doi: 10.1136/gut.35.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vesper BJ, Jawdi A, Altman KW, Haines GK, 3rd, Tao L, Radosevich JA. The effect of proton pump inhibitors on the human microbiota. Curr Drug Metab. 2009;10:84–9. doi: 10.2174/138920009787048392. [DOI] [PubMed] [Google Scholar]
- 105.Noval Rivas M, Burton OT, Wise P, Zhang YQ, Hobson SA, Garcia Lloret M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131:201–12. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111:13145–50. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]