Abstract
Background
Coordination between breathing and swallowing helps prevent aspiration of foreign material into the respiratory tract. We examined the effects of anesthesia, and hypercapnia on swallowing-breathing coordination.
Methods
In a randomized controlled cross-over study, general anesthesia with propofol or sevoflurane was titrated using an up-down method to identify the threshold for suppression of the motor response to electrical stimulation of the forearm. Additional measurements included bispectral index, genioglossus electromyogram, ventilation (pneumotachometer), and hypopharyngeal pressure. During wakefulness and at each level of anesthetic, carbon dioxide was added to increase its end tidal pressure by 4 and 8 mmHg. A swallow was defined as increased genioglossus activity with deglutition apnea and an increase in hypopharyngeal pressure. Spontaneous swallows were categorized as physiological (during expiration or followed by expiration), or pathological (during inspiration or followed by an inspiration).
Results
A total of 224 swallows were analyzed. Anesthesia increased the proportion of pathological swallows (25.9% versus 4.9%), and decreased the number of swallows per hour (1.7 ± 3.3 versus 28.0 ± 22.3) compared to wakefulness. During anesthesia, hypercapnia decreased hypopharyngeal pressure during inspiration (-14.1±3.7 versus -8.7±2 mmHg), and increased minute ventilation the proportion of pathological swallows (19.1% versus 12.3%), and the number of swallows per hour (5.5 ±17.0. versus 1.3 ± 5.5).
Conclusions
Anesthesia impaired the coordination between swallowing and respiration. Mild hypercapnia increased the frequency of swallowing during anesthesia and the likelihood of pathological swallowing. During anesthesia, the risk for aspiration may be further increased when ventilatory drive is stimulated.
Introduction
The coordination between breathing and swallowing is important to prevent the aspiration of foreign material into the respiratory tract.1 In healthy, conscious adults, swallowing occurs during or immediately before the expiratory phase of respiration.2, 3 However, in patients with neurodegenerative diseases4,5 and chronic obstructive pulmonary disease6, impaired coordination between breathing and swallowing has been observed, characterized by inspiration after swallowing. This pathological swallowing pattern is associated with an increased risk for aspiration.7
Anesthesia is associated with a higher risk of aspiration compared to wakefulness,8 and one purpose of this study was to evaluate if general anesthesia, like neurodegenerative or respiratory diseases, can impair the coordination between breathing and swallowing. Previous studies have shown that a hypercapnia-induced increase in ventilatory drive can inhibit airway protective reflexes, similar to the effect of anesthetics.9 In addition, hypercapnia disrupts the physiological coordination between swallowing and breathing.10 Since variable levels of hypercapnia occur during anesthesia, an additional question of interest was whether the addition of hypercapnia during anesthesia further impairs the coordination of breathing and swallowing compared with the effects of anesthesia alone. We hypothesized that:
Propofol or sevoflurane anesthesia increase the proportion of pathological swallows compared to the awake state.
The administration of carbon dioxide during anesthesia further increases the proportion of pathological swallows.
Materials and methods
Subjects
Following approval by the Partners Human Research Committee (Boston, MA), 11 American Society of Anesthesiologists I healthy volunteers were studied in this randomized, crossover, nested protocol. All subjects provided written informed consent prior to participation. Subjects were recruited through a recurring broadcast e-mail advertisement to employees at Massachusetts General Hospital. Eligible subjects were 18-45 with a Body Mass Index of 18.5-28 kg/m2 and with no history of dysphagia. Prior to enrollment, a preliminary history and physical was performed. All experiments were conducted at Massachusetts General Hospital in a research facility that was hospital-certified as an anesthetizing location. The study area was equipped with a standard anesthesia workstation with automated recordkeeping and resuscitation equipment.
Propofol was administered to target concentrations using a Fresenius target controlled infusion Pump (Injectomat TIVA Agilia, Fresenius Kabi, Brezins, France). Two board-certified anesthesiologists were present for the duration of each experiment, and one of them was assigned to monitor and care for the subject as his only responsibility. All subjects received standard anesthesia monitoring (electrocardiogram, pulse oximetry, capnography, and oscillometric blood pressure measurements).
Equipment and Techniques
For measurements of genioglossus activity, breathing, and upper airway closing pressure, subjects were prepared as described previously.11 Briefly, one nostril was decongested with oxymetazoline and anesthetized with 4% lidocaine spray prior to insertion of a Millar pressure catheter (Millar Instruments, Houston, TX) nasally into the hypopharyngeal area. Correct placement was confirmed visually (oropharyngeal inspection), and by confirmation of a spike in hypopharyngeal pressure upon asking the subject to swallow. The catheter was taped to the nose and then secured to a nasal continuous positive airway pressure mask (Philips Respironics, Murrysville, PA) which was connected to a high flow anesthesia circuit. For measurement of electromyogram, two 30mm needles were used to insert 27 gauge stainless steel wire electrodes into the genioglossus muscle transcutanously, as described previously.12 These electrodes were referenced to a ground electrode on the sternum.
The genioglossus electromyogram signal was filtered with a band-pass filter (200 – 1000 Hz, transition width 40 Hz) and displayed as a moving time average (time constant 100 ms). Both the raw signal and moving time average were recorded for the duration of the experiment. Correct placement of the genioglossus electromyogram electrodes was confirmed by an increase in activity during inspiration and a burst in electromyogram activity upon asking the subject to press the tongue against the teeth.
Ventilatory flow was measured with a pneumotachograph (Hans Rudolph, Kansas City, MO) and tidal volume was obtained by electrical integration of the inspiratory flow signal. End-tidal PCO2 (PETCO2) was measured through a port in the nasal mask. A 100% carbon dioxide tank attached to the inspiratory limb of the breathing circuit permitted steady-state PETCO2 to be increased by 4mmHg or 8mmHg above baseline. All data were recorded, processed and filtered using LabChart software (AD Instruments, Colorado Springs, CO). Anesthetic sedative effects were measured with the bispectral index (BIS) (Covidien, Boston, MA) using a smoothing window of 15 seconds and recorded at one minute intervals.
Experimental Protocol
Subjects were fasted for at least 8 hours prior to the start of the experiment. The study was a randomized-crossover study, with nested design. Each subject received propofol and sevoflurane sequentially, randomized for order. The nested design specified three anesthetic conditions (wakefulness, propofol, or sevoflurane); three PETCO2 conditions (baseline, +4mmHg, and +8mmHg); and two depths of anesthesia (high and low), see figure 1. Measurements of spontaneous swallowing (see “measurements” below) were recorded under each condition. The initial measurements during wakefulness were made with no added carbon dioxide in the breathing circuit. Then carbon dioxide was introduced to obtain stable elevations of 4, then 8, mmHg in PETCO2. Subjects were then randomized to receive either propofol or sevoflurane first. The initial dose targets were the median concentrations necessary to prevent movement in response to a painful stimulus, established as a propofol predicted concentration of 3.7 μg/mL13 or sevoflurane end-tidal concentration of 1.5%.14 The anesthetic was administered for 30 minutes in order to ensure that the central nervous system reached approximate steady state. A 30mA tetanic nerve stimulus was applied to the forearm using a peripheral nerve stimulator (Life-Tech Inc, Stafford, TX) and presence or absence of a motor response was noted. If the subject moved the head or extremities (excluding the stimulated arm), the concentration was increased in 50% increments until a concentration was found that just produced absence of motor response. If the initial response was no movement, the concentration was decreased by 50% increments until the threshold for motor response to pain was reached. In this way, two levels of anesthesia could be defined for each subject, one corresponding to the level at the initial response and one that was higher or lower. There was no more than three pain stimuli applied to one subject to reach two distinct depths of anesthesia. The second level of anesthesia was also administered for 30 minutes in order to ensure that central nervous system reach steady state. At both levels of anesthesia, swallowing measurements were made without added carbon dioxide or with increases of 4mmHg and 8mmHg.
Figure 1.
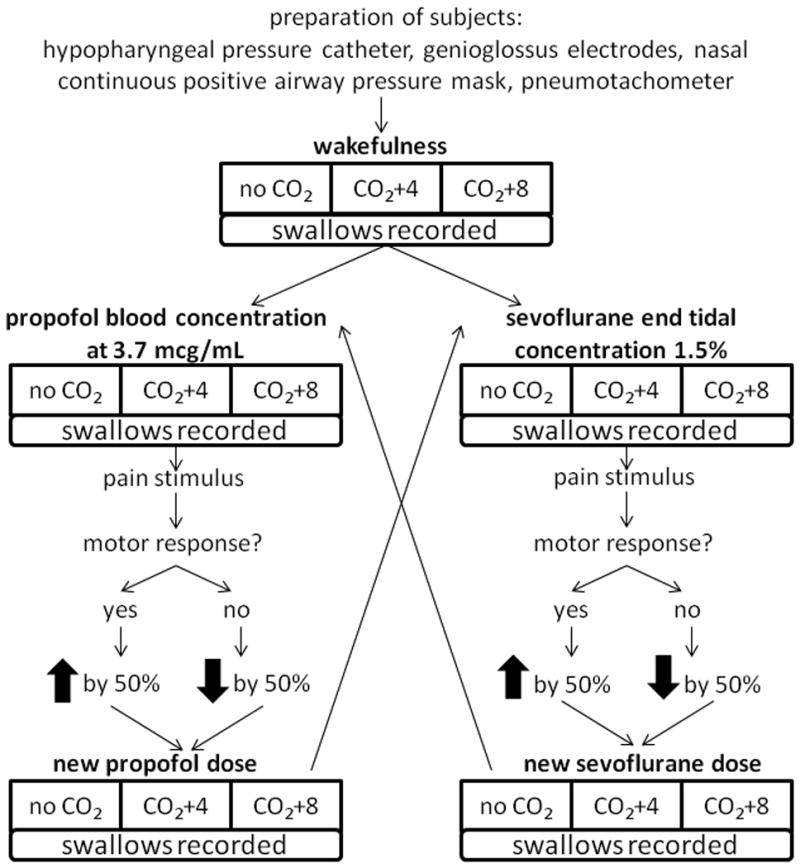
Protocol design. Baseline measurements were recorded during wakefulness and at three different levels of carbon dioxide (0, +4 and +8mmHg elevations above baseline). Subjects were then randomized for order to receive either propofol or sevoflurane first, starting at the same dose (estimated ED50) for each volunteer. To establish similar levels of shallow and deep anesthesia for each volunteer, a pain stimulus was applied to assess the depth of anesthesia. Depending on the response, the dosage was increased or decreased by 50% to arrive at a new dose of the drug. The same three levels of carbon dioxide (CO2, 0, +4, and +8mmHg) were applied during anesthesia. Spontaneous swallows were recorded during all conditions.
Measurements
The presence of a spontaneous swallow was identified by the all of following three criteria:
Rapid increase in the genioglossus electromyogram moving time average of at least 200% above tonic baseline
Rapid increase of hypopharyngeal pressure of 15cmH20 or more
Deglutition apnea
In a pilot study, the methodology was validated with electroglottography, a clinical tool used to detect the onset of swallowing.15 Electroglottography identifies the laryngeal excursion that occurs during swallowing.15 The timing of respiration and swallowing was determined as previously described.10 Expiratory swallows (E) were preceded and followed by expiratory flow, inspiratory-expiratory (I-E) swallows were preceded by inspiratory flow and followed by expiratory flow, inspiratory swallows (I) were preceded and followed by inspiratory flow, expiratory-inspiratory (E-I) swallows were preceded by expiratory flow and followed by inspiratory flow. We further categorized swallows into physiological or pathological. Physiological swallows were followed by expiratory flow (E or I-E). Pathological swallows were followed by inspiration (I and E-I) , and termed “pathological” because I and E-I swallows present a higher risk for aspiration7 (Fig. 2).
Figure 2.
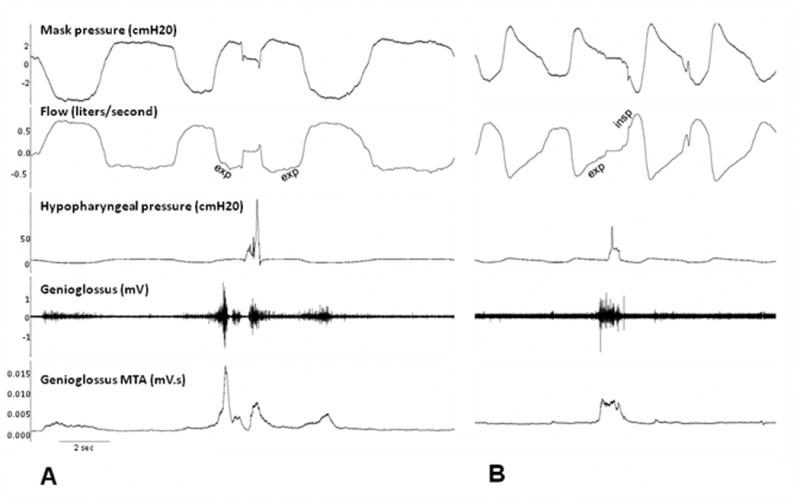
Experimental recordings of physiological and pathological swallow during wakefulness and anesthesia. The proportion of spontaneous swallows was indicated by an increase of genioglossus activity by at least 200%, along with an increase in hypopharyngeal pressure by at least 15cmH20, and deglutition apnea. A. Expiratory (E) swallow during wakefulness B. Pathological expiratory-inspiratory swallow during propofol anesthesia. Exp=expiratory flow, insp=inspiratory flow
To analyze the effects of anesthesia and hypercapnia on respiration, a breath-by-breath analysis was conducted on the five breaths before and after each swallow. Tidal volume was calculated as the integral of the flow signal. Respiratory rate and minute ventilation were calculated by averaging the values from the five breaths before and after each swallow. To examine the effect of carbon dioxide on the pharyngeal pressure generated during inspiration, we measured the maximum pharyngeal pressure during expiration and minimum pharyngeal pressure during inspiration and calculated the difference.
Statistical analysis
The primary aim of this study was to evaluate the effects of anesthesia (propofol and sevoflurane) on the proportion of pathological swallows. This was expressed as pathological swallows as a percentage of total spontaneous swallows. The secondary aim was to evaluate the effect of adding carbon dioxide during anesthesia on the proportion of pathological swallows. In order to test the hypothesis that anesthesia increases the proportion of pathological swallows, we included all measurements of swallows across anesthetic state (wakefulness vs. anesthesia), doses (awake, low, high) and carbon dioxide levels (0, 4, and 8mmHg above baseline). We created a mixed linear model (compound symmetry repeated covariance type) to test our hypothesis and modeled a binominal distribution with a logit link.
In order to evaluate the effect of general anesthesia on the proportion of pathological swallows, we modeled a binominal distribution with a logit link and included anesthetic state (anesthesia vs. wakefulness), anesthetic dose (shallow vs. deep), and carbon dioxide level (0, 4, and 8mmHg above baseline) as repeated independent variables. The type of swallow (pathological vs. physiological) was the dependent variable. In addition to testing the main effect of anesthesia on the proportion of pathological swallows, we also tested the main effect of bispectral index values. In order to address the secondary aim, we tested for an interaction between anesthesia and hypercapnia on the proportion of pathological swallows using the same mixed model modeled with a binominal distribution with logit link. For testing the secondary hypothesis, we adjusted the p-value (Bonferroni-Holm).
All other comparison were made with an exploratory intention. We used the same mixed model to evaluate the main effect of pharyngeal pressure generated during inspiration on type of swallow. To evaluate the effects of anesthesia and hypercapnia during anesthesia on respiratory rate, minute ventilation, and the frequency of spontaneous swallows (expressed as swallows per hour) we used the same linear mixed model but modeled a normal distribution with an identity link function. The statistical analyses for respiratory rate and minute ventilation were conducted as described for the primary outcome. To address the effect of carbon dioxide during anesthesia on swallows per hour, we weighted the residual for number of swallows. In order to examine the relationship between respiratory rate and pathological swallows, we used Spearman's non-parametric correlation analysis.
For our prospective power analysis, we used preliminary data to project a 10 percent difference in the fraction (in percent) of pathological swallows during anesthesia compared with wakefulness, with a standard deviation of 10 percent. Based on these assumptions, we calculated by using a paired t-test that 11 volunteers would provide us with a power >0.8 of 0.84 to identify a difference in pathological swallowing rate between wakefulness and anesthesia at alpha error p of five per cent. Data are presented as percentage of total for primary outcome and mean ± SD for other outcomes. Statistical analysis was performed using SPSS 22.0 (SPSS Inc. Chicago, IL).
Results
A total of 224 spontaneous swallows from 11 American Society of Anesthesiologists I healthy volunteers (age 24.3± 3.3, Body Mass Index 23.7 ± 1.8) were observed. Of the spontaneous swallows, 196 (87.5%) were physiological, and 28 (12.5%) were pathological. In this study, we titrated to two different levels of propofol and sevoflurane anesthesia, defined by a response vs. absence of response to a pain stimulus, and the corresponding level of consciousness was also evaluated by using bispectral index (Covidien, Boston, MA). There was no significant difference in BIS values between propofol and sevoflurane.
Primary aim: Effects of anesthesia on the proportion of pathological swallows
Anesthesia was associated with a significantly higher proportion and occurrence of pathological swallows compared to wakefulness (25.9% vs. 4.9%, p=0.001, Fig. 3A and 21 vs. 7 swallows, Table 1). Lower BIS levels indicating lower level of consciousness were associated with a higher proportion of pathological swallows (P=0.01, Fig. 3B). There was no difference in the proportion of pathological swallows between propofol and sevoflurane (31.1% vs. 10%, p=0.241). Pathological swallows occured with a slightly higher proportion at the low dose of anesthesia compared to high dose (28.4% vs. 14.3%, p=0.153), but this difference was not significant statistically. Under propofol anesthesia, the proportion of pathological swallows was slightly higher at lower doses compared to the high dose (36.2% vs. 14.3%, p=0.062), but this difference was not significant statistically. No swallows occurred during the deep level of sevoflurane. Respiratory rate was higher during anesthesia compared to wakefulness (19.8 ± 2.1 vs. 14.9 ± 3.6, p<0.001), although minute ventilation did not differ. This observed increased in respiratory rate was positively correlated with the proportion of pathological swallows (p=0.02).
Figure 3.
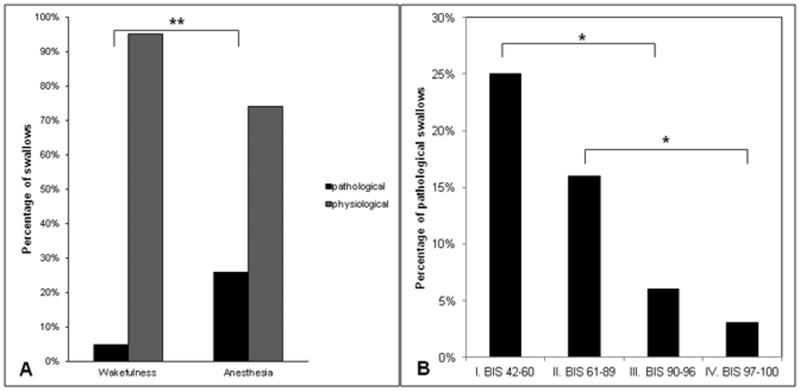
Impairing effect of anesthesia on coordination between breathing and swallowing. A. Significantly higher proportion of pathological swallows (inspiratory or expiratory-inspiratory swallows) defined as swallows followed by inspiration observed under anesthesia compared to wakefulness. B. Lower bispectral index (BIS) electroencephalogram values were associated with higher proportion of pathological swallows. * indicates p<.05, ** indicates p < 0.001.
Table 1. Swallow characteristics during wakefulness and anesthesia.
| Swallow Classification | Swallows under Wakefulness | Swallows under Anesthesia | Number |
|---|---|---|---|
| Physiological | 136 | 60 | 196 |
| Pathological | 7 | 21 | 28 |
| Total number | 143 | 81 | 224 |
Swallows are categorized as physiological (during expiration or followed by expiration), or pathological (during inspiration or followed by an inspiration) and displayed within conditions of wakefulness vs. anesthesia.
Secondary aim: Effects of induced hypercapnia on proportion of pathological swallows
The average baseline end-tidal carbon dioxide level during wakefulness was 39.4±5.36 mmHg, during propofol was 44.9±9.5 and 51.6±10.3 mmHg for the low and high dose, respectively, and during sevoflurane was 42.44±13.32 and 43.29±13.87mmHg for the low and high dose, respectively (p<0.05 for higher end-tidal carbon dioxide level during propofol compared with sevoflurane). The end tidal concentration was increased by 4 and 8mmHg at all of these levels. Hypercapnia (6 ± 2 mmHg above baseline) was associated with a higher proportion of pathological swallows compared to normocapnia (23.9% vs. 5.1%; p<0.001). The vulnerability to carbon dioxide induced pathological swallows was significantly higher during anesthesia compared to wakefulness (increase in rate of pathological swallows by 19.1% vs. 12.3%, respectively p<0.001, Fig. 4). Hypercapnia increased minute ventilation and respiratory rate both during wakefulness and anesthesia (Table 2). During wakefulness, minute ventilation increased from 9.1 ± 2.5 liters to 13.3 ± 3.3 liters during carbon dioxide insufflation (p<0.001), and respiratory rate increased from was 15.7 ± 5.3 breaths/min to 16.2 ± 3.9 breaths/min (p=0.048). During anesthesia, minute ventilation increased from 8.3 ± 3.0 liters to 12.9 ± 3.2 liters during hypercapnia (p<0.001), and respiratory rate increased from 19.8 ± 2.1 breaths/min to 22.3 ± 3.2 breaths/min (p=0.004), respectively. Hypercapnia during anesthesia also augmented the negative pharyngeal pressure generated during inspiration to more negative values (Figure 5A). Furthermore, pathological swallows were associated with greater pharyngeal pressure generated during inspiration compared to physiological swallows (Figure 5B).
Figure 4.
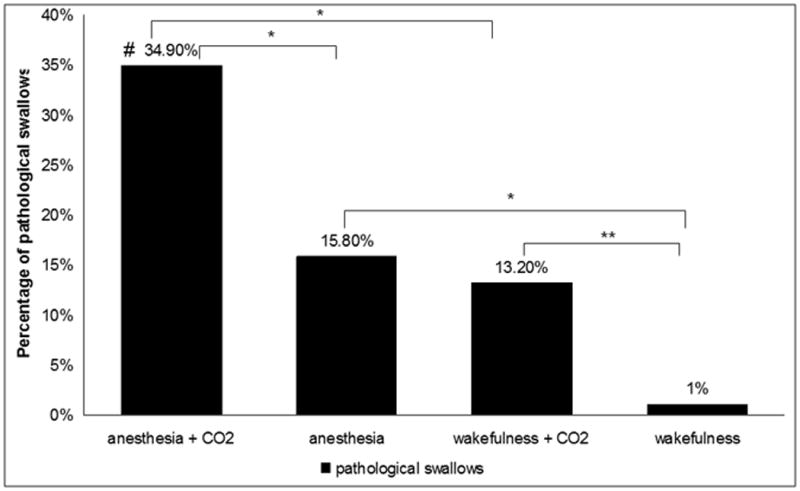
Proportion of pathological (inspiratory) swallows during wakefulness and anesthesia with and without evoked hypercapnia. Carbon dioxide (CO2) induces an increase in the proportion of pathological swallows during wakefulness and anesthesia. Anesthesia augmented the effects of carbon dioxide to impair breathing-swallowing coordination compared with wakefulness * indicates p<0.05, ** indicates p<0.001 # indicates interaction effect between anesthesia and carbon dioxide, p<0.05
Table 2. Effects of hypercapnia on respiratory rate and minute ventilation.
| Minute ventilation | Respiratory rate | |||
|---|---|---|---|---|
| Wakefulness | Anesthesia | Wakefulness | Anesthesia | |
| Normocarbia | 9.1 ± 2.5L | 8.3 ± 3.0L | 15.7 ± 5.3 | 19.8 ± 2.1 |
| Hypercarbia | 13.3 ± 3.3L | 12.9 ± 3.2L | 16.2 ± 3.9 | 22.3 ± 3.2 |
| p | <0.001 | <0.001 | 0.048 | 0.004 |
Effects of hypercapnia compared to normocapnia on both minute ventilation and respiratory rate are presented and categorized into wakefulness vs. anesthesia.
Figure 5.
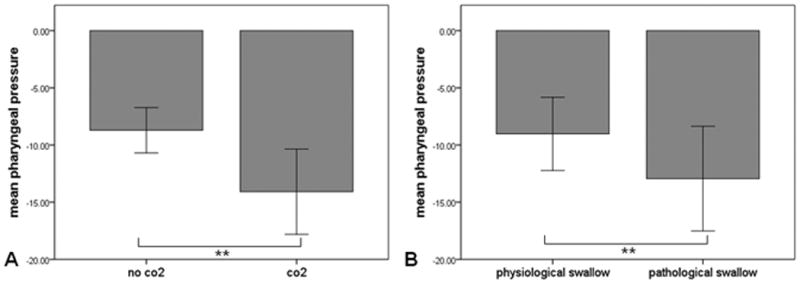
Association between hypercapnia, pharyngeal pressure during inspiration, and pathological swallows. A. Carbon dioxide (CO2 ) insufflation increases the pharyngeal pressure generated during inspiration. B. Increased pharyngeal pressure is associated with occurrence of pathological swallows. Error bars represent ± 1 standard deviation. ** p<0.001
Other outcome variables: frequency of spontaneous swallows during anesthesia vs. wakefulness, and during hypercapnia
The number of swallows per hour during anesthesia (1.7 ± 3.3) was significantly lower compared to wakefulness (28.0 ± 22.3, p<0.001, Fig. 6), with no difference between propofol (2.3 ± 4.1) and sevoflurane (0.93 ± 2.3) (p=0.38). Hypercapnia applied during anesthesia was associated with an increased number of swallows per hour compared to anesthesia alone (5.1 ± 17.0 1.3 ± 5.5 p=0.006, Fig. 7). We did not observe effects of carbon dioxide on the number of swallows per hour when hypercapnia was applied during wakefulness (22.3 ± 24.3 vs. 30.4 ± 23.6).
Figure 6.
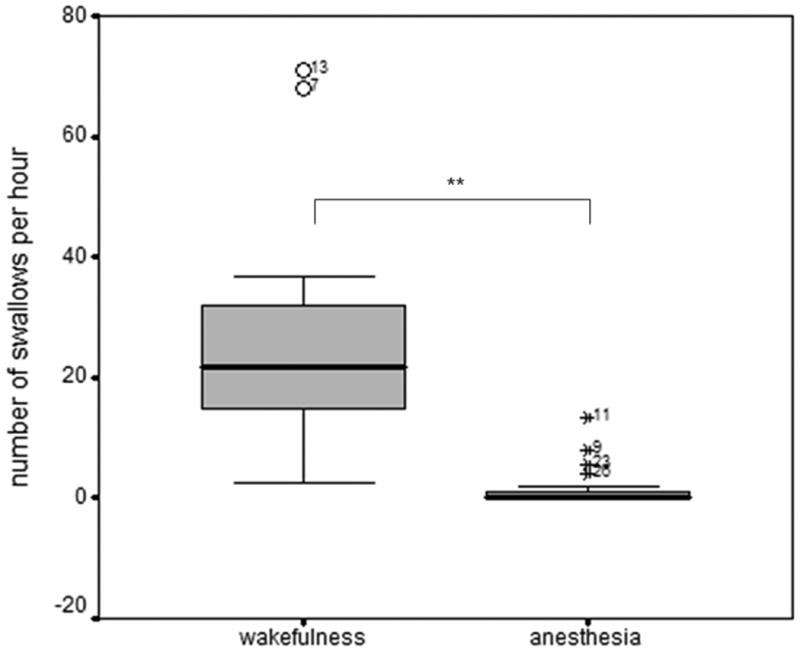
Box-plot (median, quartiles, 10/90 per cent percentile, and outer fence) of number of swallows per minute. The frequency of swallows was lower during anesthesia compared with wakefulness. ** p<0.001 for lower occurrence of swallows during anesthesia compared with wakefulness
Figure 7.
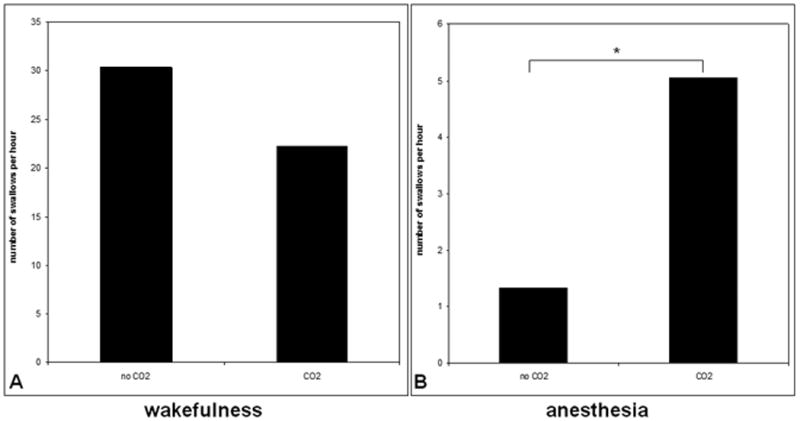
Swallows per hour with and without hypercapnia during wakefulness and anesthesia. A. The frequency of swallows was not significantly different with and without carbon dioxide (CO2 ) during wakefulness. B. Hypercapnia increased the number of swallows per hour during anesthesia. * indicates p< 0.05
Discussion
Sevoflurane and propofol increased the likelihood of pathological swallows, and decreased the frequency of swallowing. In addition, an increase in minute ventilation and an augmentation of the negative pharyngeal pressure generated during inspiration induced by carbon dioxide, increased both the frequency of swallowing and proportions of pathological swallows during anesthesia. These effects of hypercapnia during anesthesia may further increase the vulnerability to aspiration.
The most important finding of this study was that anesthesia impaired the coordination between breathing and swallowing, leading to a higher proportion of pathological swallows. Our data confirm the findings of several studies that physiological swallows during wakefulness typically occur either during expiration, or are followed immediately by the expiratory phase.16,3 There was no difference in the proportion of pathological swallows between propofol and sevoflurane. However, the absence of significant difference in pathological swallow proportion between sevoflurane and propofol does not indicate significance of absence, and further studies may be needed to address differential effects of GABAergic anesthetics on breathing swallowing coordination.
Sundman et. al demonstrated that propofol and sevoflurane increase the incidence of pharyngeal dysfunction measured by manometry, and the incidence of laryngeal penetration detected by fluoroscopy.17 While we did not formally quantify aspiration, our data taken together with other published findings7 suggest that impaired coordination between swallowing and breathing may a contributing mechanism of anesthesia-associated aspiration.
Nishino & Hiraga18 did not find a difference in the incidence of strictly inspiratory versus expiratory swallows in intubated, anesthetized patients following surgery, but in accordance with our finding, reported a higher proportion incidence of swallows occurring at the transition between inspiration and expiration. These investigators examined intubated subjects in the post-operative period, and measured volitional swallows after the presentation of a saline bolus. In contrast, our study examined reflexive swallowing, a scenario similar to that seen during procedural sedation in which a patient receives anesthetics without enteral fluid administration or an endotracheal tube, conditions that affect physiological airway reflexes. We believe that our methodology examined the interaction of breathing and swallowing in a more clinically relevant manner. The observed pattern of post-swallow inspiration in pathological swallowing has been reported in patients with high aspiration risk such as those with Parkinson's disease19,20 and chronic obstructive pulmonary disease.21,6 It has been demonstrated that swallows followed by inspiration in patients with Parkinson's disease are associated with a higher risk of aspiration indicated by higher penetration-aspiration scores.7
A decrease in the frequency of spontaneous swallows has been shown to be associated with a higher risk of aspiration in hospitalized patients.22 Our finding decreased frequency of swallows during anesthesia may also represent a contributing factor to the association between anesthesia and aspiration. However, we did not formally quantify the effectiveness of swallowing and therefore cannot draw any firm conclusions regarding the risk of aspiration.
Strengths and weaknesses of the study
Our study examined the coordination between breathing and reflexive swallows in volunteers who were spontaneously breathing through the regular anatomic route during anesthesia. The absence of an artificial bolus for induction of swallowing (i.e. water or other liquid administered) permitted our study to directly examine the breathing swallowing coordination under physiological conditions. Furthermore, the timing of an artificial bolus required to study volitional swallowing may influence the timing of respiration and swallowing (i.e. if the bolus was presented during inspiration vs. expiration). In addition, we administered carbon dioxide through the patent airway in a way that the laryngeal receptors could be directly exposed to carbon dioxide and the induced increase in negative pharyngeal pressure during inspiration23. This is important because laryngeal hypercapnia can directly activate superior laryngeal nerve fibers24 and the (carbon dioxide induced) augmentation of negative pharyngeal pressure during inspiration should activate the genioglossus muscle.23 These effects of hypercapnia on the genioglossus negative pressure reflex cannot be studied in endotracheally-intubated patients.
We were not able to visualize the pharynx to provide detailed information about the efficiency of the swallow. Further understanding of the quality of swallows in addition to its relation to respiratory timing is likely to provide important information about the effect of anesthesia and hypercapnia on aspiration rate. The use of any type of catheter in the hypopharynx could potentially lower the threshold for pharyngeal swallowing as it stimulates pharyngeal mechanoreceptors. In order to minimize this unwarranted stimulating effect, we used a very small catheter and secured the catheter with tape at the nostril to minimize any movement of the catheter and also kept the subject's head and neck position constant throughout the experiment.
Possible biological explanations
Several studies have reported that hypercapnia is associated with impaired coordination between breathing and swallowing, leading to a higher proportion of pathological (I and I-E) swallows.10,2 Our study confirms these observations and adds the information that hypercapnia has stronger effects on breathing-swallowing coordination during anesthesia compared to wakefulness.
Under the conditions in our study, carbon dioxide administered to the inspired air during anesthesia increased both the number of swallows per hour and the proportion of pathological swallows. In contrast, Nishino et. al found in anesthetized, endotracheally-intubated volunteers, airway reflexes were attenuated during administration of carbon dioxide .9 Reflexive carbon dioxide induced genioglossus activation via hypopharyngeal mechanoreceptors and laryngeal chemoreceptors cannot be studied in endotracheally-intubated patients.24
We speculate based on our data that carbon dioxide induced augmentation of the negative hypopharyngeal pressure (generated by respiratory pump muscles) contributes our finding of increased frequency of swallows during anesthesia23. Carbon dioxide and the negative hypopharynx pressure are sensed by chemoreceptors and mechanoreceptors activate the nucleus tractus solitarius via the superior laryngeal nerve and lowers the threshold for swallow initiation.25,26 Furthermore, the increased negative hypopharyngeal pressure also activates the genioglossus muscle via the premotoneurons in the periobex region.23 In our study, in parallel with carbon dioxide insufflation, we observed an augmentation of the negative pharyngeal pressure generated during inspiration, which was associated with high proportion of pathological swallows. This supports the view that hypercarbia induced augmentation of negative pharyngeal pressure during inspiration may lower the threshold for swallow initiation. In agreement with the observation of others, we did not find an increasing effect of hypercapnia on the number of swallows per hour during wakefulness.2,10 During wakefulness, reflexive swallows can be cortically modulated. It is possible that volunteers elected not to swallow in these short periods of evoked hypercapnia during which they perceived shortness of breath. Anesthesia allowed us to study the effect of hypercapnia on reflexive swallowing without cortical control.
Meaning of the study: implications for clinicians
Anesthesia impaired the coordination between swallowing and breathing, which has been demonstrated to increase the risk of aspiration.7 The intravenous anesthetic propofol and the volatile anesthetic sevoflurane had similar effects impairing the coordination of swallowing and breathing.
Our data indicate that anesthetized patients not only have less chances to clear the airway due to decrease in the number of swallows, but also have an increased chance of aspiration because of poor performance of the swallowing act (increased proportion of the pathological swallows). Clinically, one meaningful aspiration may be sufficient to translate into a bad respiratory outcome postoperatively. We therefore speculate based on our data that the combination of the decreased incidence of swallow and increased pathological swallow may contribute to an increased aspiration risk during anesthesia. 7,22
It is a common clinical observation that during the emergence from anesthesia, an increase in swallowing frequency is observed.18 An increased ventilatory drive during anesthesia was associated with increased risk of pathological swallows. In addition, our data support the view that conditions associated with increased ventilator drive (such as a pain stimulus) may impose a greater risk for pathological swallows during anesthesia. We speculate that in a setting of procedural sedation where the upper airway is not protected by a tracheal tube, shallow levels of anesthesia, also known to increase ventilatory drive, may increase the aspiration risk.
In summary, our data show that sevoflurane and propofol increase the likelihood of pathological swallows and decrease overall the frequency of swallowing. In addition, an increase in ventilatory drive induced by hypercapnia increases the vulnerability to pathological swallows.
MS #201403025 Final Box Summary.
What We Already Know about This Topic
Anesthesia is associated with a higher risk of aspiration compared to wakefulness.
The coordination between breathing and swallowing is important to prevent the aspiration of foreign material into the respiratory tract.
Hpercapnia disrupts the physiological coordination between swallowing and breathing.
What This Article Tells Us That Is New
In 11 healthy adult volunteers, this study demonstrated that compared to wakefulness, sevoflurane and propofol anesthesia decreased spontaneous swallowing frequency (28.0 ± 22.3 versus 1.7 ± 3.3 per hour) and increased proportion of pathological swallows (swallowing during inspiration or followed by an inspiration) (4.9% versus 25.9%).
Mild hypercapnia under general anesthesia augmented this pattern of swallowing impairment.
Acknowledgments
Funding: The Carl Rosow Clinical Research Center and this project were supported by an unrestricted research grant from the Buzen Fund, established by Jeffrey Buzen Ph.D. and Judith Buzen, as well as an unrestricted gift from Dr. Rosow, MD-PhD.
This project was also supported by Grant Number 8 UL1 TR000170-05, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Science, Bethesda, MD.
Footnotes
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Cedborg AI, Sundman E, Boden K, Hedstrom HW, Kuylenstierna R, Ekberg O, Eriksson LI. Pharyngeal Function and Breathing Pattern during Partial Neuromuscular Block in the Elderly: Effects on Airway Protection. Anesthesiology. 2014;120:312–25. doi: 10.1097/ALN.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 2.Sai T, Isono S, Nishino T. Effects of withdrawal of phasic lung inflation during normocapnia and hypercapnia on the swallowing reflex in humans. J Anesth. 2004;18:82–8. doi: 10.1007/s00540-004-0231-y. [DOI] [PubMed] [Google Scholar]
- 3.Hardemark Cedborg AI, Boden K, Witt Hedstrom H, Kuylenstierna R, Ekberg O, Eriksson LI, Sundman E. Breathing and swallowing in normal man--effects of changes in body position, bolus types, and respiratory drive. Neurogastroenterol Motil. 2010;22:1201–8. doi: 10.1111/j.1365-2982.2010.01551.x. e316. [DOI] [PubMed] [Google Scholar]
- 4.Butler SG, Stuart A, Pressman H, Poage G, Roche WJ. Preliminary investigation of swallowing apnea duration and swallow/respiratory phase relationships in individuals with cerebral vascular accident. Dysphagia. 2007;22:215–24. doi: 10.1007/s00455-007-9077-4. [DOI] [PubMed] [Google Scholar]
- 5.Terzi N, Orlikowski D, Aegerter P, Lejaille M, Ruquet M, Zalcman G, Fermanian C, Raphael JC, Lofaso F. Breathing-swallowing interaction in neuromuscular patients: A physiological evaluation. Am J Respir Crit Care Med. 2007;175:269–76. doi: 10.1164/rccm.200608-1067OC. [DOI] [PubMed] [Google Scholar]
- 6.Gross RD, Atwood CW, Jr, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:559–65. doi: 10.1164/rccm.200807-1139OC. [DOI] [PubMed] [Google Scholar]
- 7.Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM. Respiratory-swallowing coordination and swallowing safety in patients with Parkinson's disease. Dysphagia. 2011;26:218–24. doi: 10.1007/s00455-010-9289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock-Utne JG, Winning TJ, Rubin J, Kingston HG. Laryngeal incompetence during neuroleptanalgesia in combination with diazepam. Br J Anaesth. 1976;48:699–701. doi: 10.1093/bja/48.7.699. [DOI] [PubMed] [Google Scholar]
- 9.Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J Appl Physiol (1985) 1989;66:2642–6. doi: 10.1152/jappl.1989.66.6.2642. [DOI] [PubMed] [Google Scholar]
- 10.Nishino T, Hasegawa R, Ide T, Isono S. Hypercapnia enhances the development of coughing during continuous infusion of water into the pharynx. Am J Respir Crit Care Med. 1998;157:815–21. doi: 10.1164/ajrccm.157.3.9707158. [DOI] [PubMed] [Google Scholar]
- 11.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–9. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: Increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009;110:1253–60. doi: 10.1097/ALN.0b013e31819faa71. [DOI] [PubMed] [Google Scholar]
- 13.Baars JH, Tas S, Herold KF, Hadzidiakos DA, Rehberg B. The suppression of spinal F-waves by propofol does not predict immobility to painful stimuli in humans. Br J Anaesth. 2006;96:118–26. doi: 10.1093/bja/aei283. [DOI] [PubMed] [Google Scholar]
- 14.Baars JH, Kalisch D, Herold KF, Hadzidiakos DA, Rehberg B. Concentration-dependent suppression of F-waves by sevoflurane does not predict immobility to painful stimuli in humans. Br J Anaesth. 2005;95:789–97. doi: 10.1093/bja/aei252. [DOI] [PubMed] [Google Scholar]
- 15.Perlman AL, Grayhack JP. Use of the electroglottograph for measurement of temporal aspects of the swallow: Preliminary observations. Dysphagia. 1991;6:88–93. doi: 10.1007/BF02493485. [DOI] [PubMed] [Google Scholar]
- 16.Boden K, Cedborg AI, Eriksson LI, Hedstrom HW, Kuylenstierna R, Sundman E, Ekberg O. Swallowing and respiratory pattern in young healthy individuals recorded with high temporal resolution. Neurogastroenterol Motil. 2009;21:1163–e101. doi: 10.1111/j.1365-2982.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 17.Sundman E, Witt H, Sandin R, Kuylenstierna R, Boden K, Ekberg O, Eriksson LI. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: Volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anesthesiology. 2001;95:1125–32. doi: 10.1097/00000542-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol (1985) 1991;70:988–93. doi: 10.1152/jappl.1991.70.3.988. [DOI] [PubMed] [Google Scholar]
- 19.Pinnington LL, Muhiddin KA, Ellis RE, Playford ED. Non-invasive assessment of swallowing and respiration in Parkinson's disease. J Neurol. 2000;247:773–7. doi: 10.1007/s004150070091. [DOI] [PubMed] [Google Scholar]
- 20.Gross RD, Atwood CW, Jr, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The coordination of breathing and swallowing in Parkinson's disease. Dysphagia. 2008;23:136–45. doi: 10.1007/s00455-007-9113-4. [DOI] [PubMed] [Google Scholar]
- 21.Cvejic L, Harding R, Churchward T, Turton A, Finlay P, Massey D, Bardin PG, Guy P. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2011;16:269–75. doi: 10.1111/j.1440-1843.2010.01875.x. [DOI] [PubMed] [Google Scholar]
- 22.Murray J, Langmore SE, Ginsberg S, Dostie A. The significance of accumulated oropharyngeal secretions and swallowing frequency in predicting aspiration. Dysphagia. 1996;11:99–103. doi: 10.1007/BF00417898. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. 2007;579:515–26. doi: 10.1113/jphysiol.2006.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford A, Nolan P, O'Regan RG, McKeogh D. Carbon dioxide-sensitive superior laryngeal nerve afferents in the anaesthetized cat. Exp Physiol. 1993;78:787–98. doi: 10.1113/expphysiol.1993.sp003726. [DOI] [PubMed] [Google Scholar]
- 25.Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 26.Feindel W. The neural pattern of the epiglottis. J Comp Neurol. 1956;105:269–85. doi: 10.1002/cne.901050206. [DOI] [PubMed] [Google Scholar]


