Abstract
Anthropometric and craniofacial profile patterns indicating the percent difference from the overall mean were developed on 34 physical parameters with 31 white, mentally retarded males (23 adults and 8 children) with the fra(X) syndrome matched for age with 31 white, mentally retarded males without a known cause of their retardation. The fra(X) syndrome males consistently showed larger dimensions for all anthropometric variables, with significant differences for height, sitting height, arm span, hand length, middle finger length, hand breadth, foot length, foot breadth, and testicular volume. A craniofacial pattern did emerge between the two groups of mentally retarded males, but with overlap of several variables. Significant differences were noted for head circumference, head breadth, lower face height, bizygomatic diameter, inner canthal distance, ear length and ear width, with the fra(X) syndrome males having larger head dimensions (head circumference, head breadth, head length, face height and lower face height), but smaller measurements for minimal frontal diameter, bizygomatic diameter, bigonial diameter, and inner canthal distance. Several significant correlations were found with the variables for both mentally retarded males with and without the fra(X) syndrome. In a combined anthropometric and craniofacial profile of 19 variables comparing 26 white fra(X) syndrome males (13 with high expression (>30%) and 13 with low expression (< 30%), but matched for age), a relatively flat profile was observed with no significant differences for any of the variables. Generally, fra(X) syndrome males with increased fragile X chromosome expression have larger amplifications of the CGG trinucleotide repeat of the FMR-1 gene. No physical differences were detectable in our study between fra(X) males with high expression and apparently larger amplifications of the CGG trinucleotide repeats compared with those patients with low expression. Our research illustrates the use of anthropometry in identifying differences between mentally retarded males with or without the fra(X) syndrome and offers a comprehensive approach for screening males for the fra(X) syndrome and selecting those individuals for cytogenetic and/or molecular genetic testing.
Keywords: anthropometry, fragile X chromosome expression, pattern profiles
Anthropometric methods have been applied increasingly in the clinical evaluation and diagnosis of individuals with genetic disorders and other health problems (Dequeker et al. 1983, Meaney & Farrer 1986, Meaney & Butler 1989, Butler et al. 1991a). One of these conditions is the fragile X (fra(X)) or Martin-Bell syndrome, the most common familial cause of mental retardation. This syndrome is characterized by mental retardation, macroorchidism, large or prominent ears, a long narrow face, hyperflexibility and a characteristic chromosome fragile site at Xq27.3 (Turner et al. 1986, Hagerman 1987, Butler 1988). The incidence of the fragile X syndrome is approximately one in 1000–2500 males (Herbst & Miller 1980, Turner et al. 1986, Webb et al. 1986). Unstable DNA sequence representing large increases in the number of CGG repeats have recently been located at the fragile site and characterization of the gene (FMR-1) implicated in the fra(X) syndrome is underway at several medical centers (Yu et al. 1991, Oberle et al. 1991, Rousseau et al. 1991, Sutherland et al. 1991, Verkerk et al. 1991, Pergolizzi et al. 1992). Alterations of the FMR-1 gene, detectable with several DNA probes with Southern blotting analysis and more recently with PCR methods, are gaining acceptance for diagnostic and clinical applications (Pieretti et al. 1991, Pergolizzi et al. 1992).
Phenotypic variability can make early recognition of fra(X) syndrome individuals difficult. Hagerman et al. (1988) reported finding positive fragile X chromosome results in 50% of mentally retarded males who were examined and found to have large ears, macroorchidims, and hand callouses. Recently, Butler et al. (1991a, 1991b) and Hagerman et al. (1991) reported that certain physical characteristics (e.g., ear width, testicular volume, bizygomatic diameter, head breadth, plantar crease, hyperflexibility) could be used to identify more than 90% of mentally retarded males with the fra(X) syndrome. Butler et al. (1992) also reported anthropometric standards for clinical use for weight, height, head circumference, ear length and testicular volume for fra(X) syndrome males. Until recently, these were only a few studies reporting on the linear growth patterns in fra(X) syndrome individuals (Partington 1984, Meryash et al. 1984, Sutherland & Hecht 1985, Locsch et al. 1988, Butler et al. 1988a, Butler et al. 1988b, Saul 1988). Our current research focuses on the development of anthropometric and craniofacial patterning profiles of mentally retarded males with and without the fra(X) syndrome through a comprehensive assessment of measurements at several body sites. The effects of fra(X) chromosome expression on anthropometric variables of affected males will also be presented.
Materials and methods
A cytogenetic and clinical survey of 236 mentally retarded males (39 with and 197 without the fra(X) syndrome or other recognizable cause of their retardation) was undertaken recently including a comprehensive assessment of anthropometric variables, and significant differences were reported for 18 measurements (weight, height, 7 linear, 2 breadth, 5 craniofacial, 1 skinfold and testicular volume) (Butler et al. 1991a, Butler & Singh 1993). A subset of these individuals was used in the current study to compare anthropometric and craniofacial profile patterns between mentally retarded males with and without the fra(X) syndrome and, in addition, affected males with either high or low fra(X) chromosome expression. Specially, 31 white, mentally retarded males (approximate average IQ = 50) diagnosed with the fra(X) syndrome with an average age of 29.5 years and a standard deviation of 16.0 with a range of 3.7 years to 75.6 years were matched for age with 31 white, mentally retarded males (approximate average IQ = 20) without a diagnosis or cause of their mental retardation. These males had an average age of 29.5 years and a standard deviation of 15.8 years, with an age range of 3.8 years to 74.7 years. Eight pairs of children (<18 years of age) were used in this study. The average age difference between these pairs was 0.22 years, with a range of 0.06 to 1.4 years. For the 23 pairs of adults the average age difference was 0.1 years between the pairs, with a range of 0.04 to 0.89 years. Thirteen white fra(X) syndrome males with high fragile X chromosome expression (>30% with an average of 43% and range of 32% to 58% in cells grown in folate deficient culture conditions (medium 199) were matched for age (within 1 year) with 13 white fra(X) syndrome males with low expression [< 30% with an average of 18% and a range of 3% to 28% in cells grown in folate deficient culture conditions (Butler et al. 1990)].
All measurements and physical examination were made by one of the authors (M.G.B. or M.S.W.), according to standard techniques as presented by Weiner & Lourie (1969), Snyder et al. (1977) and Farkas (1981). To monitor quality control and observer reliability, repeated measurements were obtaned on several individuals over a period of time by two observers. There was reasonable agreement (generally < 10% discrepancy) between the original and repeated measurements. Greater consistencies were found in intraobserver measurements than in the measurements obtained by two independent observers. Therefore, intraobserver consistency using comparable anthropometric equipment was in agreement with other anthropometric surveys (National Center for Health Statistics 1973, Brandt et al. 1990, Butler et al. 1992).
The anthropometric evaluation for this study consisted of 34 measurements including 19 anthropometric variables (weight, height, sitting height, arm span, shoulder-elbow length, elbow-wrist length, elbow-fingertip length, hand length, middle finger length, palm length, hand breadth, wrist breadth, knee-buttock length, foot length, foot breadth, ankle breadth, tricep skinfold, subscapular skinfold and testicular volume) and 15 craniofacial variables (head circumference, head breadth, head length, cephalic index (head breadth/head length × 100), face height (gnathion-trichion), lower face height (gnathion-nasion), minimal frontal diameter, bizygomatic diameter, bigonial diameter, inner canthal distance, outer canthal distance, nose length, nose breadth, ear length and ear width). Measurements were generally obtained on the left side, except for certain situations (e.g., a missing digit). Skinfold measurements were recorded to the nearest 0.5 mm with a Lange skinfold caliper. Arm span and head circumference were recorded to the nearest mm with a steel tape. Weight was measured with a balanced-beam scale, height and linear values were measured with an anthropometer or sliding calipers, breadth measurements were obtained with a sliding caliper (excluding cranial measurements which were obtained with a spreading caliper), and testicular volume was estimated by the use of a Prader orchiodometer (if greater than 25 ml, the length and width were measured with a sliding caliper and the volume was calculated by the use of the equation π/6 × L × W2 (Butler et al. 1992)).
The overall or combined mean is calculated for each anthropometric variable for the mentally retarded males with or without the fragile X syndrome matched for age and race. The mean of each variable for each group of matched individuals with or without the fragile X syndrome was also calculated. Finally, the percent change from the overall mean was calculated and the data plotted on a graph. Matched t-tests were also used to identify significant differences for each variable between age-matched mentally retarded males with or without the fra(X) syndrome and between fra(X) syndrome males with high fra(X) chromosome expression (> 30%) or low expression (< 30%). Scatterplots of the anthropometric measurements for each matched [fra(X) and non-fra(X)] subject pair were produced for the variables found to be significantly different. Pearson product moment correlation coefficients were calculated to compare the relationship of anthropometric variables in both fra(X) and non-fra(X) syndrome subjects.
Results
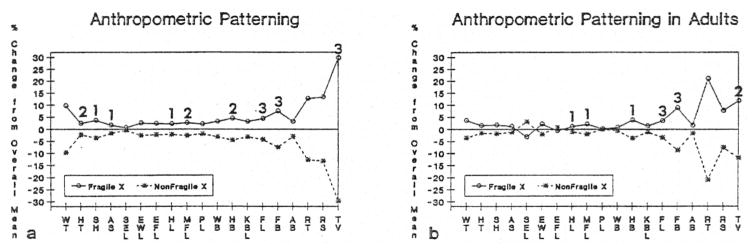
Fig. 1a displays an anthropometric profile indicating the percent difference or change from the overall mean comparing the 31 white mentally retarded males with the fra(X) syndrome matched for age with 31 white mentally retarded males without the fra(X) syndrome. The average age ± standard deviation for the fra(X) syndrome males was 29.5 + 16.0 years (age range was 3.7 to 75.6 years) with an average + standard deviation for fra(X) chromosome expression of 28% ± 14% in peripheral blood cells grown routinely in folate deficient culture conditions (Butler et al. 1990). The average age + standard deviation for the 31 non-fra(X) syndrome males was 29.5 + 15.9 years (age range of 3.8 to 74.7 years). An anthropometric pattern does emerge between the two groups of mentally retarded males with or without the fra(X) syndrome, with the fra(X) syndrome males consistently showing larger dimensions for all variables, with significant differences (matched t-test; p< 0.05) between the two groups for height, sitting height, arm span, hand length, middle finger length, hand breadth, foot length, foot breadth and testicular volume. The greatest differences were found for foot length, foot breadth and testicular volume. Fig. 1b displays an anthropometric profile of the adults only (>18 years), indicating the percent difference from the overall mean by excluding the eight pairs of children with or without the fra(X) syndrome.
Fig. 1.
Fig. 1a. Anthropometric profile indicating percent difference from overall mean for 31 (23 adults and 8 children) white mentally retarded males with the fra(X) syndrome matched for age with 31 white mentally retarded males with no known cause of their retardation. WT = weight, HT = height, SH = sitting height, AS = arm span, SEL = shoulder-elboe length, EWL = elbow-wrist length, EFL= elbow-fingertip length, HL = hand length, MFL = middle finger length, PL = palm length, WB = wrist breadth, HB = hand breadth, KBL = knee-buttock length, FL = foot length, FB = foot breadth, AB = ankle breadth, LT = left triceps skinfold, LS = left subscapular skinfold, TV = testicular volume. 1 = p< 0.05 (two-tailed matched t-test), 2=p < 0.01 (two-tailed matched t-test), 3 = p < 0.001 (two-tailed matched t-test), 1b. Anthropometric profile indicating percent difference from overall mean for 23 adult (>18 years) white mentally retarded males with the fra(X) syndrome matched for age with 23 white mentally retarded males with no known cause of their retardation.
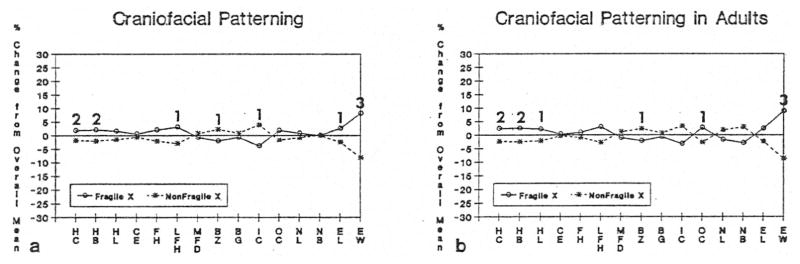
Fig. 2a displays a craniofacial profile indicating the percent difference from the overall mean comparing the 31 white, mentally retarded males with the fra(X) syndrome matched for age with 31 white, mentally retarded males without the fra(X) syndrome. A craniofacial pattern does emerge, with up and down deviation and overlap of several variables between the two groups of males with significant differences (matched t-test; p< 0.05) for head circumference, head breath, lower face height, bizygomatic diameter, inner canthal distance, ear length and ear width. The greatest difference was found for ear width. Fig. 2b displays a craniofacial profile of the adults only (>18 years) indicating the percent difference from the overall mean by excluding the eight pairs of children with or without the fra(X) syndrome. As indicated in Fig. 2a and 2b, the fra(X) syndrome males have larger head dimensions including head circumference, head breadth, head length, face height and lower face height, but smaller measurements for minimal frontal diameter, bizygomatic diameter, bigonial diameter, and inner canthal distance. In addition, outer canthal distance, nose length, ear length and ear width were greater in the fra(X) syndrome males. Significant differences (matched t-test; p< 0.05) were also found between the two groups of mentally retarded adults for head circumference, head length, bizygomatic diameter, ear width, outer canthal distance, hand breadth, middle finger length, hand length, foot length, foot breadth, and testicular volume.
Fig. 2.
Fig. 2a. Craniofacial profile indicating percent difference from overall mean for 31 (23 adults and 8 children) white mentally retarded males with the fragile X syndrome matched for age with 31 white mentally retarded males with no known cause of their retardation. HC = head circumference, HB = head breadth, HL = head length, CE = cephalic index, FH = face height, LFH = lower face height, MFD = minimal frontal diameter, BZ = bizygomatic diameter, BG = bigonial diameter, IC = inner canthal distance, OC = outer canthal distance, NL = nose length, NB = nose breadth, EL = ear length, EW = ear width. 1 = p < 0.05 (two-tailed matched t-test), 2=p < 0.01 (two-tailed matched t-test), 3= p < 0.001 (two-tailed matched t-test). 2b. Craniofacial profile indicating percent difference from overall mean for 23 adult (>18 years) white mentally retarded males with the fra(X) syndrome matched for age with 23 white mentally retarded males with no known cause of their retardation. HC = head circumference, HB = head breadth, HL = hcad length, CE = cephalic index, FH = face height, LFH = lower face height, MFD = minimal frontal diameter, BZ = bizygomatic diameter, BG = bigonial diameter, IC = inner canthal distance, OC = outer canthal distance, NL = nose length, NB = nose breadth, EL = ear length, EW = ear width. 1= p < 0.05 (two-tailed matched t-test), 2 = p < 0.01 (two-tailed matched t-test), 3 = p < 0.001 (two-tailed matched t-test).
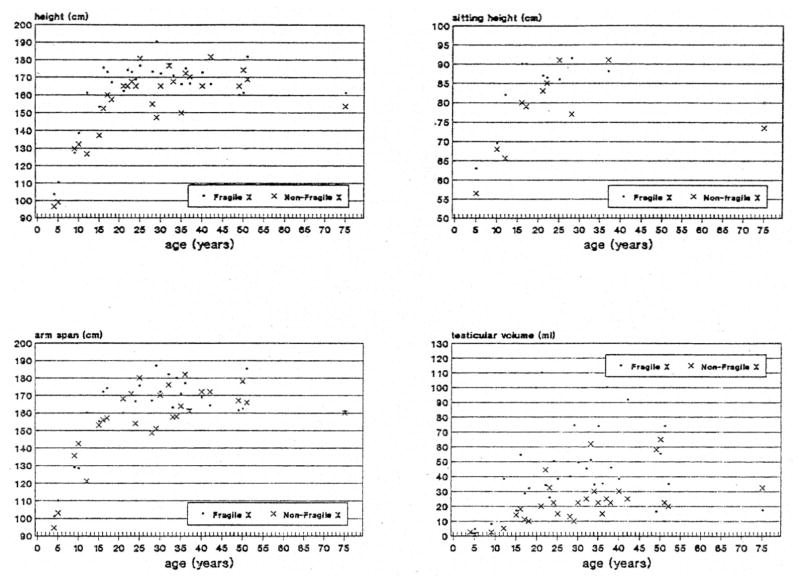
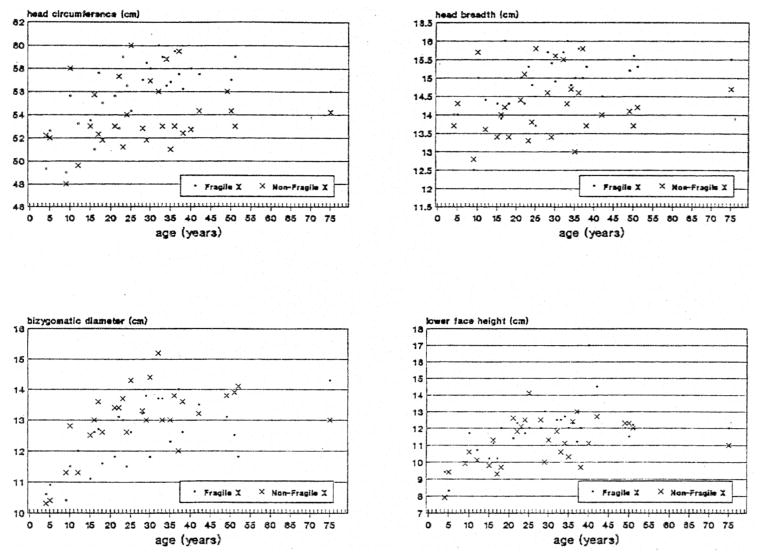
Table 1 shows the average + standard deviation and t-test value for each anthropometric and craniofacial variable for the two groups of 31 matched, mentally retarded males with or without the fra(X) syndrome. Fig. 3, 4, 5 & 6 show scatterplot data for 16 anthropometric and craniofacial variables that were found to be significantly different between the matched pairs of fra(X) and non-fra(X) subjects of all ages.
Table 1.
Anthropometric and craniofacial patterning data from the white fragile X syndrome males matched for age with the mentally retarded white males without a cause of their retardation utilized in this study
| Variables | Fragile X (mean±SD) (N=31) | Non-fragile X (mean±SD) (N=31) | Matched t-test value (N=31) | Fragile X (mean±SD) (N=31) Adults only | Non-fragile X (mean±SD) (N=31) Adults only | Matched t-test value Adults only |
|---|---|---|---|---|---|---|
| Weight (kg) | 54.5±25.2 | 44.9±23.0 | 1.57 | 66.6±11.2 | 62.0±18.1 | 0.59 |
| Height (cm) | 162.8 ±20.0 | 155.2±21.8 | 3.18b | 170.8±7.5 | 165.7±9.4 | 1.84 |
| Sitting height (cm) | 83.0±9.1 | 77.2±10.7 | 2.82a | 86.5±3.7 | 83.4±7.2 | 1.07 |
| Arm span (cm) | 161.7±2.1 | 156.2±21.5 | 2.07a | 170.3±8.6 | 166.1±9.7 | 1.45 |
| Head circumference (cm) | 55.9±2.9 | 53.9±2.9 | 2.95b | 57.1±1.8 | 54.4±2.7 | 3.43b |
| Head breatch (cm) | 14.9±0.8 | 14.3±0.8 | 3.19b | 15.1±0.6 | 14.4±0.8 | 3.06b |
| Head length (cm) | 19.2±1.2 | 18.6±1.1 | 2.04 | 19.6±0.7 | 18.8±1.1 | 2.40a |
| Face height (cm) | 18.2±1.5 | 17.5±2.2 | 1.34 | 19.0±1.0 | 18.7±1.5 | 0.36 |
| Lower face height (cm) | 11.8±1.4 | 11.5±1.4 | 2.42a | 12.4±1.2 | 11.7±1.1 | 1.79 |
| Min. frontal diameter (cm) | 10.5±0.6 | 10.7±0.7 | −0.89 | 10.8±0.5 | 11.1±0.5 | −0.75 |
| Bizygomatic diameter (cm) | 12.5±1.1 | 13.0±1.1 | −2.62a | 12.9±0.9 | 13.5±0.7 | −2.19a |
| Bigonial dia (cm) | 10.3±1.0 | 10.5±1.0 | −1.02 | 10.7±0.8 | 10.8±0.7 | −0.70 |
| Inner canthal distance (cm) | 2.9±0.3 | 3.1±0.3 | −2.06a | 2.9±0.3 | 3.1±0.3 | −1.78 |
| Outer canthal distance (cm) | 8.7±0.8 | 8.4±0.7 | 1.68 | 8.9±0.6 | 8.5±0.7 | 2.60a |
| Nose length (cm) | 4.5±0.7 | 4.5±0.9 | 0.40 | 5.0±0.5 | 5.2±0.7 | −0.53 |
| Nose breadth (cm) | 3.5±0.3 | 3.5±0.5 | 0.00 | 3.6±0.4 | 3.8±0.4 | −1.39 |
| Ear length (cm) | 7.0±0.7 | 6.6±0.8 | 2.38a | 7.2±0.6 | 6.9±0.7 | 1.88 |
| Ear width (cm) | 4.3±0.5 | 3.6±0.4 | 7.08c | 4.4±0.5 | 3.7±0.4 | 7.15c |
| Knee-buttock length (cm) | 51.8±9.8 | 48.7±10.0 | 1.77 | 56.1±2.2 | 54.8±5.9 | 0.50 |
| Shoulder-elbow length (cm) | 32.6±5.8 | 32.2±6.8 | 0.30 | 34.8±1.5 | 37.1±2.2 | −1.99 |
| Wrist breadth (cm) | 5.2±0.7 | 4.9±0.8 | 1.86 | 5.5±0.4 | 4.9±0.5 | 0.62 |
| Hand breadth (cm) | 7.8±1.1 | 7.1±1.1 | 3.70b | 8.3±0.7 | 7.8±0.7 | 2.42a |
| Middle finger length (cm) | 7.6±0.9 | 7.2±0.9 | 3.23b | 7.9±0.4 | 7.6±0.6 | 2.34a |
| Hand length (cm) | 17.5±2.1 | 16.9±2.2 | 2.07a | 18.3±1.0 | 17.9±1.2 | 1.16 |
| Palm length (cm) | 10.0±1.2 | 9.8±1.6 | 1.48 | 10.5±0.7 | 10.4±0.7 | 2.00 |
| Elbow-fingertip length (cm) | 41.3±7.0 | 39.4±7.9 | 1.80 | 44.8±1.7 | 45.4±2.5 | −1.06 |
| Elbow-wrist length (cm) | 25.8±5.8 | 24.5±5.0 | 0.91 | 28.9±4.6 | 27.7±2.5 | 0.45 |
| Ankle breadth (cm) | 6.8 ±0.7 | 6.4±1.0 | 1.98 | 7.0±0.3 | 6.8±0.7 | 0.67 |
| Foot length (cm) | 24.5±2.9 | 22.8±2.7 | 4.31c | 25.6±1.6 | 23.9±1.4 | 3.76c |
| Foot breadth (cm) | 9.3±1.4 | 8.0±1.1 | 5.39c | 9.8±1.0 | 8.3±0.9 | 5.46c |
| Triceps skinfold (cm) | 13.6±7.0 | 10.5±4.5 | 1.05 | 15.0±6.5 | 9.8±5.9 | 1.24 |
| Subscapular skinfold (cm) | 13.9±6.9 | 10.6±5.8 | 1.11 | 14.4±4.3 | 12.3±7.0 | 0.59 |
| Testicular volume (ml) | 44.1±26.8 | 23.3±16.3 | 3.90c | 50.8±25.2 | 28.0±15.5 | 3.39c |
| Cephalic index | 77.5±3.0 | 76.6±3.5 | 1.03 | 77.1±3.1 | 76.6±3.4 | 0.48 |
p<0.05 (two-tailed).
p<0.01 (two-tailed).
p< 0.001 (two-tailed).
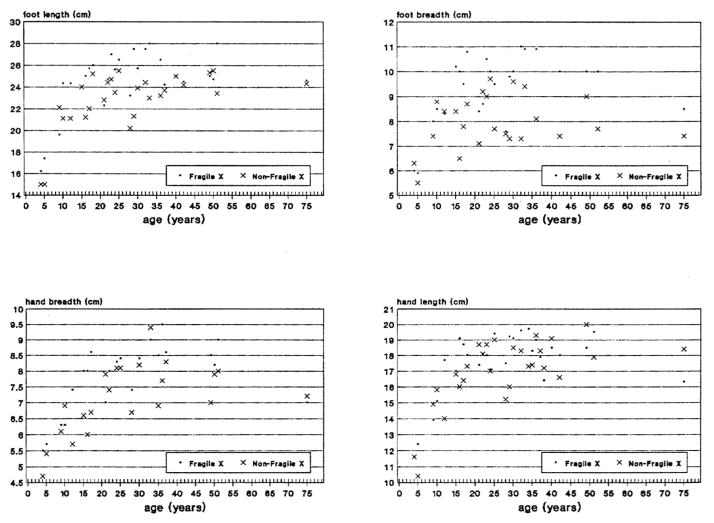
Fig. 3.
Scatterplot of measurements for height, sitting height, arm span and testicular volume obtained from mentally retarded white fra(X) and non-fra(X) males matched for age.
Fig. 4.
Scatterplot of measurements for head circumference, head breath, bizygomatic diameter and lower face height obtained from mentally retarded white fra(X) and non-fra(X) males matched for age.
Fig. 5.
Scatterplot of measurements for foot length, foot breadth, hand breadth and hand length obtained from mentally retarded white fra(X) and non-fra(X) males matched for age.
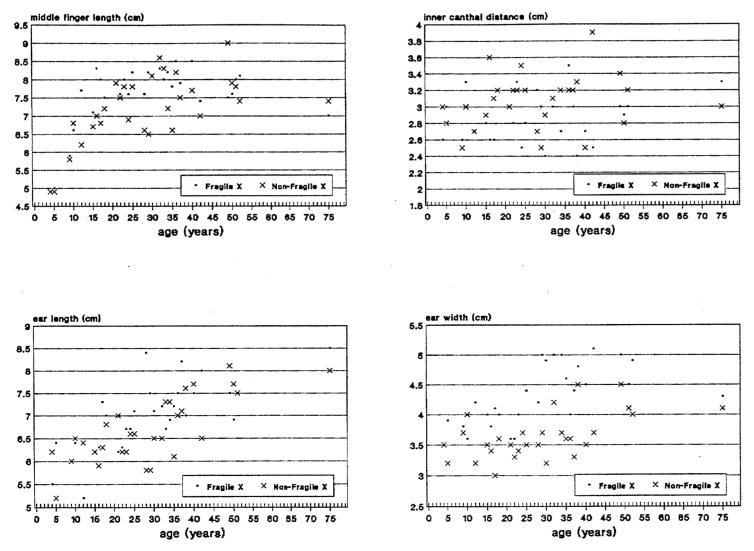
Fig. 6.
Scatterplot of measurements for middle finger length, inner canthal distance, ear length and ear width obtained from mentally retarded white fra(X) and non-fra(X) males matched for age.
A combined anthropometric and craniofacial profile was developed for 19 variables indicating the percent difference from the overall mean comparing 13 white fra(X) syndrome males with high fra(X) chromosome expression (>30%) with 13 age-matched white fra(X) syndrome males with low fra(X) expression (< 30%). A relatively flat profile was identified with overlapping of most variables. No significant differences (matched t-test; p> 0.05) were observed for any of 19 variables. Tables 2 and 3 show correlation matrices for weight, height, testicular volume, ear length and width, head circumference, head length and breadth, face height, lower face height, minimal frontal diameter, bizygomatic diameter, bigonial diameter, inner and outer canthal distances, and nose length and breadth for both fra(X) and non-fra(X) syndrome subjects of all ages. Sixty-eight significant correlations (p< 0.05) were seen in the comparison of 17 variables for the fra(X) males and 63 significant correlations identified with the non-fra(X) males.
Table 2.
Correlation matrix for testicular volume, weight, height, ear length and width, and craniofacial variables for all fragile X syndrome males used in this study
| WTFX | HTFX | TESFX | ELFX | EWFX | HCFX | HEBFX | HELFX | FHFX | LFHFX | MFDFX | BZFX | BGFX | ICDFX | OCDFX | NLFX | NBFX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WTFX | 1.00 | ||||||||||||||||
| HTFX | 0.77b | 1.00 | |||||||||||||||
| TESFX | 0.28 | 0.52b | 1.00 | ||||||||||||||
| ELFX | 0.61a | 0.54b | 0.32 | 1.00 | |||||||||||||
| EWFX | 0.60a | 0.48a | 0.25 | 0.59c | 1.00 | ||||||||||||
| HCFX | 0.62a | 0.68c | 0.45a | 0.65c | 0.57b | 1.00 | |||||||||||
| HEBFX | 0.48 | 0.52b | 0.28 | 0.54b | 0.35 | 0.85c | 1.00 | ||||||||||
| HELFX | 0.50 | 0.60b | 0.45a | 0.53b | 0.31 | 0.85c | 0.77c | 1.00 | |||||||||
| FHFX | 0.34 | 0.59 | 0.11 | 0.46 | 0.06 | 0.33 | 0.34 | 0.35 | 1.00 | ||||||||
| LEHFX | 0.38 | 0.57b | 0.34 | 0.50b | 0.49b | 0.56b | 0.41a | 0.50b | 0.94c | 1.00 | |||||||
| MFDFX | 0.58 | 0.68a | 0.33 | 0.75b | 0.46 | 0.60a | 0.56 | 0.52 | 0.56 | 0.58a | 1.00 | ||||||
| BZFX | 0.64a | 0.70c | 0.38 | 0.75c | 0.48a | 0.68c | 0.67c | 0.54b | 0.60 | 0.70c | 0.94c | 1.00 | |||||
| GBFX | 0.70a | 0.70c | 0.29 | 0.69c | 0.55b | 0.77c | 0.72c | 0.59b | 0.57 | 0.60b | 0.82b | 0.83c | 1.00 | ||||
| ICDFX | 0.11 | 0.10 | 0.06 | 0.29 | 0.18 | 0.32 | 0.39a | 0.41a | 0.43 | 0.04 | 0.28 | 0.35 | 0.50a | 1.00 | |||
| OCDFX | 0.33 | 0.52b | 0.44a | 0.53b | 0.57b | 0.76c | 0.59b | 0.55b | 0.45 | 0.53b | 0.61a | 0.47a | 0.60b | 0.30 | 1.00 | ||
| NLFX | 0.54 | 0.74b | 0.32 | 0.47 | 0.59a | 0.31 | 0.04 | 0.17 | 0.44 | 0.72b | 0.58a | 0.75c | 0.66a | 0.09 | 0.12 | 1.00 | |
| NBFX | 0.29 | 0.54 | 0.40 | 0.61a | 0.53 | 0.36 | 0.28 | 0.28 | 0.40 | 0.45 | 0.77b | 0.80c | 0.68a | 0.12 | 0.38 | 0.51 | 1.00 |
WTFX=weight; HTFX=height; TESFX-testicular volume; ELFX=ear length; EWFX=ear width; HCFX=head circumference; HEBFX=head breadth; HELFX=head length; FHFX=face height; LFHFX=lower face height; MFDFX=minimal frontal diameter; BZFX=bizygomatic diameter; BGFX=bigonial diameter; ICDFX=inner canthal distance; OCDFX=outer canthal distance; NLFX=nose length; NBFX=nose breadth.
p<0.05.
p<0.01.
p<0.001.
Table 3.
Correlation matrix for testicular volume, weight, height, ear length and width, and craniofacial variables for all fragile X syndrome males used in this study
| WTNX | HTNX | TESNX | ELNX | EWNX | HCNX | HEBNX | HELNX | FHNX | LFHNX | MFDNX | BZNX | BGNX | ICDNX | CDNX | NLNX | NBNX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WTNX | 1.00 | ||||||||||||||||
| HTNX | 0.98c | 1.00 | |||||||||||||||
| TESNX | 0.66a | 0.56b | 1.00 | ||||||||||||||
| ELNX | 0.52 | 0.55b | 0.65c | 1.00 | |||||||||||||
| EWNX | 0.03 | 0.20 | 0.43a | 0.62c | 1.00 | ||||||||||||
| HCNX | 0.71b | 0.43a | 0.36 | 0.32 | 0.08 | 1.00 | |||||||||||
| HEBNX | 0.63a | 0.32 | 0.16 | 0.20 | −0.02 | 0.84c | 1.00 | ||||||||||
| HELNX | 0.64a | 0.29 | 0.30 | 0.35 | 0.09 | 0.93c | 0.71c | 1.00 | |||||||||
| FHNX | 0.80b | 0.77b | 0.62 | 0.52 | 0.25 | 0.83c | 0.71a | 0.81b | 1.00 | ||||||||
| LFHNX | 0.87c | 0.83c | 0.42a | 0.35 | 0.12 | 0.53b | 0.46a | 0.44a | 0.89c | 1.00 | |||||||
| MFDNX | 0.64a | 0.84c | 0.51 | 0.22 | −0.13 | 0.41 | 0.42 | 0.24 | 0.72a | 0.81b | 1.00 | ||||||
| BZNX | 0.73b | 0.85c | 0.45a | 0.44a | 0.35 | 0.42a | 0.40a | 0.27 | 0.51 | 0.62c | 0.70a | 1.00 | |||||
| BGNX | 0.91c | 0.79c | 0.30 | 0.41a | 0.33 | 0.39a | 0.24 | 0.28 | 0.90c | 0.53b | 0.74b | 0.80c | 1.00 | ||||
| ICDNX | 0.53 | 0.44a | 0.26 | 0.23 | 0.23 | 0.41a | 0.16 | 0.48a | 0.55 | 0.39a | 0.28 | 0.34 | 0.34 | 1.00 | |||
| OCDNX | 0.50 | 0.52b | 0.16 | 0.21 | −0.07 | 0.47a | 0.33 | 0.45a | 0.60a | 0.42a | 0.66a | 0.35 | 0.56b | 0.62c | 1.00 | ||
| NLNX | 0.60a | 0.65a | 0.69a | 0.76b | 0.57 | 0.41 | 0.46 | 0.32 | 0.54 | 0.67a | 0.48 | 0.53 | 0.60a | 0.15 | 0.15 | 1.00 | |
| NBNX | 0.76b | 0.78b | 0.73a | 0.55 | 0.12 | 0.68a | 0.65a | 0.55 | 0.90c | 0.79b | 0.79b | 0.53 | 0.81b | 0.48 | 0.72b | 0.64a | 1.00 |
WTNX=weight; HTNX=height; TESNX=testicular volume; ELNX=ear length; EWNX=ear width; HCNX=head circumference; HEBNX=head breadth; HELNX=head length; FHNX=face height; LFHNX=lower face height; MFDNX=minimal frontal diameter; BZNX=bizygomatic diameter; BGNX=bigonial diameter; ICDNX=inner canthal distance; OCDNX=outer canthal distance; NLNX-nose length; NBNX=nose breadth.
p<0.05.
p<0.01.
p<0.001.
Discussion
Since Galton’s early attempts to explain human variation by careful observation and measurements (Galton 1876), several anthropometric studies have been undertaken on syndromes in order to describe their clinical entities better. Thus, we describe our attempt at anthropometric pattern analysis between mentally retarded males with or without the fra(X) syndrome. An anthropometric pattern profile did emerge between mentally retarded males with or without the fra(X) syndrome [or other recognizable cause of their retardation following a clinical evaluation by one of the authors (M.G.B.), a clinical geneticist] with the fra(X) syndrome males having greater dimensions for all anthropometric variables and significant differences for height, sitting height, arm span, hand length, middle finger length, hand breadth, foot length and foot breadth and testicular volume, with the greatest differences for foot length and foot breadth. Significant differences were identified with greater head circumference, head breadth, lower face height, ear length and ear width in the fra(X) syndrome males, supporting an elongated face, large ears and macroorchidism in fragile X syndrome males while bizygomatic diameter and inner canthal distance were significantly larger in the mentally retarded males without the fra(X) syndrome. Although the two groups of mentally retarded males with or without the fra(X) syndrome were matched for race and age, some of the differences in body size may be due to ethnic composition within a race. Similar anthropometric and craniofacial profiles were observed when comparing all fra(X) and non-fra(X) males in our study with or without the children, although significant difference for height, sitting height and arm span were only seen when the children were included in the anthropometric profile analysis. These data support accelerated growth or early maturation in fra(X) syndrome children, as suggested previously (Butler et al. 1992). There were more significant correlations found in comparing 17 anthropometric variables in the fra(X) males than in non-fra(X) males; thus, the suggestion of more homogeneity in fra(X) males. More significant positive correlations were found in fra(X) males than in non-fra(X) males for ear size (ear length and width) than in the comparison of the other variables. Specifically, eight variables were positively correlated with ear width for fra(X) males, while only two variables were correlated with ear width for the non-fra(X) males.
Better characterization of many features occurred through this analysis, and underrecognized physical parameters were also identified in fra(X) syndrome males when compared with mentally retarded males without the fra(X) syndrome. These included large head dimensions with an increased head circumference and head breath, but interestingly, a smaller minimal frontal diameter with relative hypotelorism (indicated by narrowing of the inner canthal distance and larger outer canthal distance), broad palpebral fissures, an increased ear width relative to ear length and larger feet were seen in the fra(X) syndrome males. A narrow face relative to face length in fra(X) syndrome patients is also supported by our data. Therefore, several head and face parameters (e.g., head breadth, inner and outer canthal distances, ear width) may be useful as previously under-recognized characteristics for screening individuals for the fra(X) syndrome.
In order to identify if physical parameters differ in fra(X) syndrome males with high fra(X) chromosome expression (>30%) compared with low expression (< 30%), combined anthropometric and craniofacial patterning analyses were performed on 19 variables in 26 fra(X) syndrome males matched for age and race. In our study, a flat profile (excluding testicular volume, which was not statistically significantly different) was observed for both the high-or low-expressing fra(X) syndrome males, and no significant differences were found.
Recent analysis with cytogenetic and DNA data indicates a significant positive correlation between high fragile X chromosome expression and large DNA alterations, i.e., increased number of CGG trinucleotide repeats (de Vries et al. 1992, Staley et al. 1992). Therefore, our fra(X) syndrome males with high expression probably have larger DNA alterations than those patients with low expression, even though our patients have not been analyzed at a molecular level. There appears to be no difference in physical parameters in our study in fra(X) syndrome males with or without high fra(X) chromosome expression. Additional studies are planned to characterize further the physical parameters and the degree of amplification of the CGG repeats.
The results of our research are encouraging in selecting variables useful for screening males for the fra(X) syndrome and in better characterizing the features of this syndrome. Additional research is needed with a larger sample of males with or without the fra(X) syndrome, not only for confirmation of our results, but also to correlate these physical parameters with alterations of the FMR-1 gene. Our research illustrates the utility of anthropometry in the evaluation of mentally retarded patients and offers a comprehensive approach for screening males for the fra(X) syndrome and selecting those individuals for cytogenetic and/ or molecular genetic testing.
Acknowledgments
We thank Pamela Grimm for her expert preparation of the manuscript; the Tennessee Department of Mental Health and Mental Retardation grant and Clinical Research Center grant, Meharry Medical College, Nashville, Tennessee (M.G.B., R.R, D.N.S.), Vanderbilt University Research Council grant (M.G.B.) & Department of Pathology (M.G.B.) and NICHD grant HD11624 (M.S.W., W.R.B.) for financial support. We also thank Dr. Ellen Roback, Karen King, Andy Allen, June Burns, Roberta Thomas and other personnel at Cloverbottom Developmental Center, Nashville for their assistance in the collection of data, and Lora Miller, Judy Haynes, Weitong Zhu and Dr. George Dahir for technical assistance.
References
- Brandt JM, Allen GA, Haynes JL, Butler MG. Normative standards and comparison of anthropometric data of white and black newborn infants. Dysmorph Clin Genet. 1990;4:121–137. [PMC free article] [PubMed] [Google Scholar]
- Butler MG. Fragile X syndrome: a major cause of X-linked mental retardation. Comp Ther. 1988;14:3–7. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Allen GA, Haynes JL, Clark SJ. Chromosome lesions which could be interpreted as “fragile sites” on the distal end of Xq. Am J Med Genet. 1990;37:250–253. doi: 10.1002/ajmg.1320370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Allen GA, Singh DN, Carpenter NJ, Hall BD. Preliminary communication: photoanthropometric analysis of individuals with the fragile X syndrome. Am J Med Genet. 1988a;30:165–168. doi: 10.1002/ajmg.1320300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Allen GA, Haynes JL, Singh DN, Watson MS, Breg WR. Anthropometric comparison of mentally retarded males with and without the fragile X syndrome. Am J Med Genet. 1991a;38:260–268. doi: 10.1002/ajmg.1320380220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Mangrum T, Gupta R, Singh D. A 15 item checklist for screening mentally retarded males for the fragile X syndrome. Clin Genet. 1991b;39:347–354. doi: 10.1111/j.1399-0004.1991.tb03041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Brunschwig A, Miller LK, Hagerman RJ. Standards for selected anthropometric measurements in males with the fragile X syndrome. Pediatrics. 1992;89:1059–1062. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Fletcher M, Gale DD, Meaney FJ, Mcleod DR, Fagan J, Carpenter NJ. Metacarpophalangeal pattern profile analysis in fragile X syndrome. Am J Med Genet. 1988b;31:767–773. doi: 10.1002/ajmg.1320310406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Singh DN. Clinical and cytogenetic survey of institutionalized mentally retarded patients with emphasis on the fragile X syndrome. J Intell Dis Res. 1993 doi: 10.1111/j.1365-2788.1993.tb00580.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequeker J, Goris P, Uytterhoeven R. Osteoporosis and osteoarthritis (osteoarthrosis) -anthropometric distinctions. JAMA. 1983;249:1448–1451. [PubMed] [Google Scholar]
- deVries LBA, Wiegers AM, Verkerk JMH, deGraaf E, Niermeijer MF, Halley DJJ, Curts LMG, Fryns JP, Oostra BA. FMR-1 gene mutation methylation, mental status and fragile X expression in 36 males fragile X patients. Third International Fragile X Conference; 1992, June 16–20; Snowmass, Colorado. [Google Scholar]
- Farkas LG. Anthropometry of the head and face in medicine. New York: Elsevier Publishing Company; 1981. [Google Scholar]
- Galton F. The history of twins as a criterion of the relative powers of nature and nurture. J Anthropol Inst. 1876;5:391. doi: 10.1093/ije/dys097. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Fragile X syndrome. Curr Probl Pediatr. 1987;17:621–674. doi: 10.1016/0045-9380(87)90011-9. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Berry R, Jackson AW, Campbell J, Smith ACM, McGavran L. Institutional screening for the fragile X syndrome. Am J Dis Child. 1988;142:1216–1221. doi: 10.1001/archpedi.1988.02150110094028. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Amir K, Cronister A. The fragile X checklist. Am J Med Genet. 1991;38:283–287. doi: 10.1002/ajmg.1320380223. [DOI] [PubMed] [Google Scholar]
- Herbst DS, Miller JR. Nonspecific X-linked mental retardation II: the frequency in British Columbia. Am J Med Genet. 1980;7:461–469. doi: 10.1002/ajmg.1320070407. [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Lafranchi M, Scott D. Anthropometry of Martin-Bell syndrome. Am J Med Genet. 1988;30:149–164. doi: 10.1002/ajmg.1320300113. [DOI] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG. Characterization of obesity in the Prader-Labhart-Willi syndrome: fatness patterning. Med Anthrop Quart. 1989;3:294–305. doi: 10.1525/maq.1989.3.3.02a00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney FJ, Farrer LA. Clinical anthropometry and medical genetics: a compilation of body measurements in genetic and congenital disorders. Am J Med Genet. 1986;25:343–359. doi: 10.1002/ajmg.1320250221. [DOI] [PubMed] [Google Scholar]
- Meryash DL, Cronk CE, Sachs B, Gerald PS. An anthropometric study of males with the fragile X syndrome. Am J Med Genet. 1984;17:159–174. doi: 10.1002/ajmg.1320170110. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Vital and Health Statistics. Washington, DC: US Government Printing Office; 1973. Selected body measurements for children 6–11 years, United States. Series 11, No. 123. US Department of Health, Education, and Welfare Publication 73–1605. [Google Scholar]
- Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boué J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Partington MW. The fragile X syndrome II: preliminary data on growth and development in males. Am J Med Genet. 1984;17:175–194. doi: 10.1002/ajmg.1320170111. [DOI] [PubMed] [Google Scholar]
- Pergolizzi RG, Erster SH, Goonewardena P, Brown WT. Detection of full fragile X mutation. Lancet. 1992;339:271–272. doi: 10.1016/0140-6736(92)91334-5. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang F, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Heitz D, Biancalana V. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. NEJM. 1991;325:1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Saul RA. Proceedings of the Greenwood Genetics Center. Vol. 7. Clinton, SC: Jacobs Press; 1988. [Google Scholar]
- Snyder RG, Schneider LW, Owings CL, Reynolds HM, Gollomb DH, Schork MA, editors. Anthropometry of infants, children and youths to age 18 for product safety design SP-450. Warrendale, PA: Society of Automobile Engineers Inc; 1977. [Google Scholar]
- Staley LW, Hull CE, Taylor A, Thibodeau S, Snow K, Schwabe S, McGavran L, Wilson V, Riddle JE, O’Connor R, Hagerman RJ. Molecular-clinical correlations and cognitive decline with age in children with the fragile X syndrome. Am J Hum Genet. 1992;51:A228. [Google Scholar]
- Sutherland GR, Gedeon A, Kornman L. Prenatal diagnosis of fragile X syndrome by direct detection of the unstable DNA sequence. NEJM. 1991;325:1702–1722. doi: 10.1056/NEJM199112123252407. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Hecht F. Oxford Monograph on Medical Genetics. Vol. 13. Oxford: Oxford University Press; 1985. Fragile sites on human chromosomes. [Google Scholar]
- Turner G, Opitz JM, Brown WT, Davies KE, Jacobs PA, Jenkins EC, Mikkelsen M, Partington MW, Sutherland GR. Conference report: Second international workshop on the fragile X and on X-linked mental retardation. Am J Med Genet. 1986;23:11–67. [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Webb TP, Bundey SE, Thake AI, Todd J. Population incidence and segregation ratios in the Martin-Bell syndrome. Am J Med Genet. 1986;23:573–580. doi: 10.1002/ajmg.1320230151. [DOI] [PubMed] [Google Scholar]
- Weiner JS, Lourie JA. International Biological Programme Handbook No. 9. Oxford: Blackwell Scientific Publications; 1969. Human biology: a guide to field methods. [Google Scholar]
- Yu S, Pritchard M, Kremer E, Lynch M, Nancarrow J, Baker E, Holman K, Mulley JC, Warren ST, Schlessinger D, Sutherland GR, Richards RI. Fragile X genotype characterized by an unstable region of DNA. Science. 1991;252:1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]