Abstract
Background
This phase 1/2 study evaluated the dose-limiting toxicity and maximum tolerated dose of MLN2704, a humanized monoclonal antibody MLN591 targeting prostate-specific membrane antigen, linked to the maytansinoid DM1 in patients with progressive metastatic castration-resistant prostate cancer.
Patients and methods
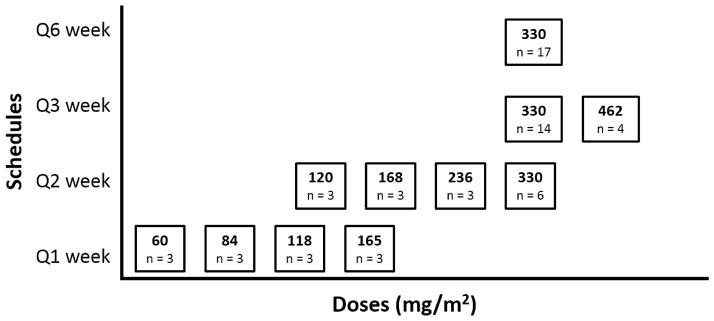
Sixty-two patients received MLN2704 at ascending doses on four schedules: weekly (60, 84, 118, and 165 mg/m2; 12 patients); every two weeks (120, 168, 236, and 330 mg/m2; 15 patients); every three weeks (330 and 426 mg/m2; 18 patients); and on days 1 and 15 of a six-week schedule (6 week cycle, 330 mg/m2; 17 patients). The primary efficacy endpoint was a sustained ≥ 50% decline from baseline prostate-specific antigen (PSA) without evidence of disease progression. Toxicity, pharmacokinetics, immunogenicity, and antitumor activity were assessed.
Results
Neurotoxicity was dose-limiting. 44 patients (71%) exhibited peripheral neuropathy: six (10%) had grade 3/4. Neurotoxicity rates remained high despite increasing the dosing interval to three- (13 of 14; one grade 3) and six-weeks (16 of 17; three grade 3). MLN2704 pharmacokinetics were dose-linear. Rapid deconjugation of DM1 from the conjugated antibody was seen. Five patients (8%) experienced ≥ 50% decline in PSA; five (8%) had PSA stabilization lasting ≥ 90 days. Only two of 35 patients on the three-week and six-week schedules achieved a PSA decline of ≥ 50%.
Conclusion
MLN2704 has limited activity in metastatic CRPC. Disulfide linker lability and rapid deconjugation lead to neurotoxicity and a narrow therapeutic window.
Keywords: Castration-resistant prostate cancer, DM1, Maytansinoid, MLN2704, Multiple ascending dose, PSMA
INTRODUCTION
Prostate-specific membrane antigen (PSMA) is an attractive therapeutic target for castration-resistant prostate cancer (CRPC). PSMA is a transmembrane protein expressed on virtually all tumor cells, is not secreted, and is upregulated after androgen depletion[1]. PSMA is also expressed on the vasculature of many solid tumors. MLN591 is a de-immunized anti-PSMAext monoclonal antibody with a high affinity (1 nM) to the external domain of the protein and is rapidly internalized upon binding. MLN2704 is an antibody drug conjugate (ADC) composed of MLN591 linked to the potent antimicrotubule chemotherapeutic drug maytansinoid-1 (DM1).
A first-in-man trial of MLN2704 studied doses of 18 to 343 mg/m2 administered at four-week intervals in 23 men with metastatic CRPC and demonstrated safety with repetitive dosing and antitumor effects[2]. A maximum tolerated dose was not defined because emerging data suggested improved efficacy with more frequent dosing. This phase 1/2 multiple ascending dose trial in metastatic CRPC was initiated to establish the optimal dose and schedule of the conjugated antibody MLN2704 and to evaluate its pharmacokinetics (PK), immunogenicity, and antitumor activity.
PATIENTS AND METHODS
Patient Population
Eligible patients were ≥ 18 yr old with a minimum Karnofsky Performance Status (KPS) of 60%, a life expectancy > 6 mo, and a diagnosis of progressive metastatic CRPC (testosterone < 50 ng/dL). Progression was defined as the presence of at least one of the following: 1) progressive soft-tissue disease; 2) new lesions by bone scan; or 3) rising prostate-specific antigen (PSA) levels. Eligibility required an absolute neutrophil count (ANC) > 1,500/mm4, hematocrit > 27%, and platelet count > 100,000/mm4. Patients were excluded if they had received corticosteroids or adrenal hormone inhibitors within 4 weeks of entering the trial or if they had received either cytotoxic chemotherapy or radiation therapy within 6 weeks. Patients were also excluded if they had received prior monoclonal antibodies. Other exclusions included: a serum creatinine > 2.0mg/dL, a serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 1.5 times the upper limit of normal (ULN), or a bilirubin > 1.25 times the ULN. Patients with peripheral neuropathy of ≥ grade 2 (NCI CTCAE version 3.0) were excluded. This study was approved by institutional review boards and all patients signed informed consent.
Treatment Plan and Toxicity Assessment
Pretreatment evaluation consisted of a complete history with examination and KPS, laboratory studies including PSA and testosterone levels, computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis, and bone scan. Patients were assigned sequentially to the currently accruing dose level.
The conjugated antibody was administered as an intravenous infusion in 0.9% saline over 2.5–3h. Doses ranged from 60 mg/m2 to 462mg/m2. Four dosing schedules were studied: once weekly, once every two weeks, once every three weeks, and a six week cycle in which doses were administered on days one and 15 followed by a four week off-period. (Figure 1)
Fig. 1.
Multiple ascending doses and schedules
Patients exhibiting a response and assigned to the weekly, every second week, and every third week schedules were eligible to receive up to six, three, and two additional doses, respectively. Responding patients assigned to the six week dosing schedule were eligible to receive MLN2704 until found to have progression of disease, up to a maximum of 18 mo.
Three patients were enrolled at the lowest dose in each cohort. If no dose-limiting toxicities (DLTs) were observed, dose escalation proceeded to the next higher dose level. DLT was defined as the occurrence of one or more NCI CTCAE toxicities and considered possibly related to the treatment. Hematologic toxicities included: platelet count < 10,000/mm4; platelet transfusion when platelet count < 20,000/mm4; febrile neutropenia; or grade 4 neutropenia (ANC < 500/mm4) of ≥ 7 d duration. Nonhematologic toxicities included any toxicity grade ≥ 3 considered possibly related to the drug. [NCI CTCAE version 3.0]
DLT cutoffs were: within one week after the fourth dose for patients on the weekly schedule; within two weeks after the second dose for patients on the two week schedule; and within three weeks after the first dose for patients on the three week schedule. For patients on the weekly schedule, a delay in administration of the second, third, or fourth dose was considered a DLT if the delay was greater than two days and due to toxicity; for patients on the two-week, three-week and six-week schedules, a delay in administration of the second dose was considered a DLT if the delay was greater than four days and due to toxicity.
If two DLTs were observed at the first dose level in a cohort, the dose for that cohort was de-escalated by 40% and three new patients were enrolled at the lower dose level. Assignment to the three-week schedule began when at least six patients had received 330 mg/m2 on the two-week schedule. No dose escalation/de-escalation was planned for subjects assigned to the six-week schedule.
Blood samples were collected at each study visit for the first three doses administered on the weekly, two-week, and three-week schedules. Safety was evaluated using laboratory assessments, the FACT/GOG NTX 11 questionnaire [3], and monitoring throughout treatment until 30 days afterward.
Antitumor activity
Antitumor activity was assessed by changes in PSA levels and/or measurable tumor lesions. The primary efficacy endpoint was a sustained ≥ 50% decline from baseline PSA, determined by two separate measurements taken no less than four weeks apart, and no evidence of disease progression by clinical evaluation and/or radiographic studies. Measurable soft-tissue lesions on CT or MRI at screening were reassessed using Response Evaluation Criteria in Solid Tumors (RECIST) criteria at 12-week intervals.
Pharmacokinetics and Immunogenicity
Serum concentrations of the conjugated antibody and its individual components were used to determine pharmacokinetics (PKs). Concentrations of conjugated antibody (MLN2704), deconjugated antibody (MLN591), deconjugated free DM1, and total antibody (sum of deconjugated and conjugated antibody) were assessed. The lower limit of DM1 quantitation was 0.1 ng/mL. Noncompartmental analysis was used to determine plasma PK parameters, including maximum serum concentration levels (Cmax), area under the concentration time curve from time zero to infinity (AUC0-∞), half-life (t1/2), and clearance (CL). AUC0-∞ data was calculated using a linear-up log-down approach. PK analyses were performed using Phoenix WinNonlin version 6.3 (Pharsight Corp, Mountain View, CA). Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). Samples from patients on the weekly and six-week dosing schedules were not included in the PK analyses due to insufficient data related to assay sensitivity and/or sampling time issues.
Immunogenicity was assessed by measuring serial serum levels of anti-MLN591 and anti-MLN2704 antibodies.
RESULTS
Patient Characteristics
Sixty-two patients with metastatic CRPC were enrolled at academic medical centers (Table 1). 27 (44%) had measurable disease and 35 (56%) received prior chemotherapy.
Table 1.
Patient and Disease Characteristics
| Characteristics | n = 62 |
|---|---|
| Age, median (range), y | 69 (52–84) |
| KPS (n = 57), median, range* | 90 (70–100) |
| Measurable disease, no. of patients (%) | 27 (44) |
| Prior therapies, no. of patients (%) | |
| Surgery | 33 (53) |
| Radiation | 37 (60) |
| Any chemotherapy | 35 (56) |
| Taxane-based chemotherapy | 33 (53) |
| Hormone therapy | 62 (100) |
| Baseline laboratory parameters | |
| Albumin (g/L), median (range) | 41 (31–48) |
| Alkaline phosphatase (units/L), median (range) | 121 (38–836) |
| Hemoglobin (g/dL), median (range) | 131 (111–144) |
| LDH (units/L), median (range) | 201.5 (108–700) |
| PSA (ng/mL), median (range) | 56.8 (3.7–5241) |
KPS = Karnofsky Performance Status; LDH = lactate dehydrogenase; PSA = prostate-specific antigen.
Dosing Schedules
The conjugated antibody dosing schedules and number of patients treated at each dose level are shown in Figure 1 and Supplemental Table 1.
Adverse Events
Adverse events observed in ≥ 15% of patients are presented in Table 2. Peripheral neuropathy occurred in 44 cases (71%), and was grade 3/4 in six (10%). Other common toxicities included nausea (61%), fatigue (60%), anorexia (39%), and diarrhea (39%). Fifteen patients (38%) discontinued treatment secondary to an adverse event (Supplemental Table 1).
Table 2.
Toxicities observed in >15% of patients, by schedule
| Toxicity | Q1Wk (n = 12) | Q2Wk (n = 15) | Q3Wk (n = 18) | Q6Wk (n = 17) | All schedules (n = 62) |
|---|---|---|---|---|---|
| Peripheral neuropathy* | 5 (42%) | 10 (67%) | 13 (72%) | 16 (94%) | 44 (71%) |
| NOS | |||||
| Nausea | 4 (33%) | 10 (67%) | 12 (67%) | 12 (71%) | 38 (61%) |
| Fatigue | 6 (50%) | 6 (40%) | 16 (89%) | 9 (53%) | 37 (60%) |
| Anorexia | 4 (33%) | 6 (40%) | 9 (50%) | 5 (29%) | 24 (39%) |
| Diarrhea | 6 (50%) | 4 (27%) | 6 (33%) | 8 (47%) | 24 (39%) |
| Constipation | 4 (33%) | 2 (13%) | 8 (44%) | 7 (41%) | 21 (34%) |
| AST/ALT elevation | 0 | 5 (33%) | 6 (33%) | 1 (6%) | 12 (19%) |
| Pyrexia | 1 (8%) | 2 (13%) | 5 (28%) | 3 (18%) | 11 (18%) |
| Vomiting | 1 (8%) | 4 (27%) | 6 (33%) | 0 | 11 (18%) |
| Rigors | 2 (17%) | 2 (13%) | 4 (22%) | 2 (12%) | 10 (16%) |
| Weight decrease | 0 | 1 (7%) | 3 (17%) | 6 (35%) | 10 (16%) |
| Bone pain | 3 (25%) | 2 (13%) | 1 (6%) | 3 (18%) | 9 (15%) |
| Musculoskeletal pain | 2 (17%) | 1 (7%) | 5 (28%) | 1 (6%) | 9 (15%) |
NOS = not otherwise specified.
Any grade
A protocol amendment increased the dosing interval and introduced the three-week schedule for patients receiving doses ≥ 330 mg/m2. 14 patients received 330mg/m2 every three weeks. In this cohort, 12 patients developed peripheral neuropathy: one developed grade 3 neuropathy while 11 developed less than grade 3 neuropathy. (Table 2)
Five of 17 (29%) patients on the six-week schedule (330 mg/m2) discontinued treatment due to at least one adverse event. (Supplemental Table 1) Grades 2 and 3 peripheral neuropathy developed in five and three patients, respectively. One patient in this cohort developed grade 3 AST/ALT elevation. (Table 2) In multivariate analysis, prior neuropathy, prior taxane therapy, and diabetes mellitus were not predictors for worsening neuropathy.
Pharmacokinetics and Immunogenicity
Dose-linear PKs were observed in the conjugated antibody across the range of doses analyzed in this study (Table 3). Conjugated antibody exposure, determined by Cmax and AUC0-∞, was dose-proportional at levels ranging from 120 to 462 mg/m2. Significant linear relationships between both Cmax and AUC0-∞ and conjugated antibody doses were observed, where dose escalations corresponded with proportional increases in Cmax (r2 = 0.95; p < 0.0001) and AUC0-∞ (r2 = 0.92; p <0.0001). At 330 mg/m2, significant differences in exposure between the two-week and three-week dose schedules were likely due to modest accumulation of the conjugated antibody. Mean Cmax and AUC0-∞inf levels of the conjugated antibody tended to increase during the second and third cycles across dose levels ranging from 120 to 462 mg/m2.
Table 3.
Mean Pharmacokinetic Parameters for All Cycles of MLN2704 on Two- and Three-Week Schedules
| Dose (mg/m2) | No. of Patients | Apparent half-life(h) | Cmax (μg/mL) | AUC0-∞ (μg*h/mL) | Apparent clearance mL/(h*m2) | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Two-week Schedule | |||||||||
| 120 | 3 | 55.7 | 4.9 | 52.4 | 12.3 | 2692 | 670 | 51.1* | 20.2** |
| 168 | 3 | 52.7 | 0.6 | 89.6 | 12.4 | 4858 | 671 | 35.6 | 5.4 |
| 236 | 3 | 59.2 | 9.9 | 138.1 | 16.3 | 9535 | 1542 | 26.5 | 6.4 |
| 330 | 6 | 59.3 | 8.0 | 214.9 | 13.3 | 13210 | 1364 | 26.1 | 2.8 |
| Three-week Schedule | |||||||||
| 330 | 14 | 59.9 | 0.7 | 163.6 | 8.6 | 10359 | 356 | 33.5 | 0.4 |
| 462 | 4 | 65.8 | 1.1 | 273.3 | 15.9 | 17658 | 2048 | 26.8 | 3.4 |
AUC0-∞ = area under the concentration time curve from time zero extrapolated to infinity; Cmax = maximum serum concentrations; SD = standard deviation.
Result driven by patient 001-001’s observed rate of clearance during cycle 1. When removed, the mean clearance for patients at the 120mg/m2 dose is 43.0 +/− 5.1 mL/(h*m2).
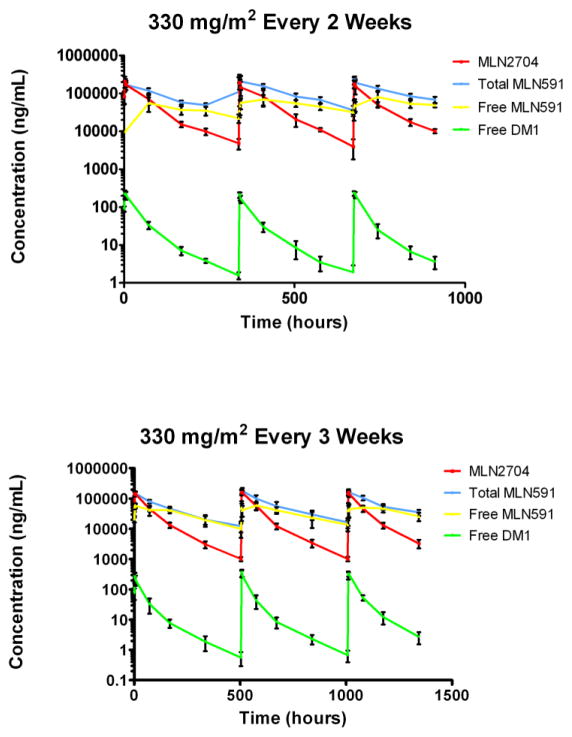
A significant inverse relationship between conjugated antibody (MLN2704) clearance and dose was detected across a range of dose levels (120–462 mg/m2): the higher the dose, the slower the clearance (r2 = 0.31; p = 0.0014), similar to the first-in-man study of MLN2704[2]. The relationship was no longer present when the 120 mg/m2 dose level was excluded (r2 = 0.17; p = 0.13). MLN2704 clearance was approximately 2 times and 2.5 times more rapid than the apparent clearance of the free antibody and the total antibody, respectively (Figure 2). The majority of conjugated antibody was cleared within seven days after each of the first three cycles although conjugated antibody levels remained detectable for up to 30 days.
Fig. 2.
Mean levels of MLN2704, total MLN591 antibody, free MLN 591 antibody, and free DM1 after three cycles at the maximum tolerated dose of 330mg/m2 administered every two (top) and every three (bottom) weeks. Error bars indicate the standard deviation.
A dose schedule–exposure relationship was also observed. Significant differences between mean Cmax (p = 0.0002) and AUC0-∞ (p = 0.01) were evident when the 330 mg/m2 conjugated antibody dose was administered every two weeks relative to every three weeks. However, a dose schedule–clearance relationship was not observed between patients on these two schedules (p = 0.1).
Free DM1 levels were measurable in serum samples from all patients treated with 120–462 mg/m2 during cycles 1–3 (Supplemental Table 2). After a dose of 330 mg/m2, peak plasma levels of free DM1 were >200 ng/mL throughout all three cycles for patients on the two- and three-week schedules. Similar to findings for the conjugated antibody, pretreatment serum samples from subjects receiving 330 mg/m2 revealed a detectable greater accumulation of free DM1 among patients on the two-week schedule when compared to those on the three-week schedule. Also similar to the conjugated antibody findings, the apparent clearance of free DM1 was more rapid than the apparent clearance of both free antibody and total antibody (Figure 2).
No antibodies were detected to MLN2704, MLN591, or free DM1 at any time point.
Antitumor Effects
PSA response
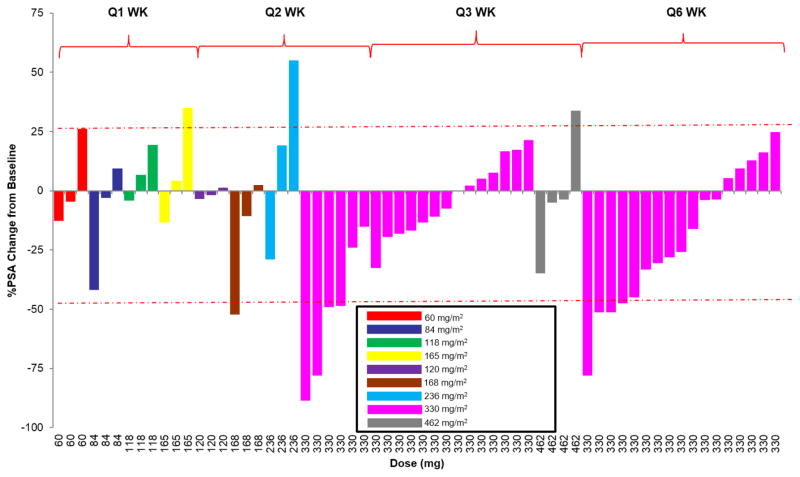
Figure 3 shows a waterfall plot of the maximal PSA decline by dose level. Overall PSA response rate is summarized in Supplemental Table 3. Five patients (8%) experienced a ≥ 50% PSA decline for a median duration of 86 days (range, 56–520 days) and five patients (8%) experienced PSA stabilization (PSA ≤ 25% above baseline for ≥ 90 days). Activity was seen in both chemotherapy-naïve patients and those with prior chemotherapy. One instance of ≥ 50% PSA decline was observed at a 168mg/m2 dose level and the remaining four were seen at a 330 mg/m2 dose level: of these, two patients received 330mg/m2 on the two-week dosing schedule and two received 330mg/m2 on the six-week schedule. Two patients with PSA stabilization received 330 mg/m2 on the two-week schedule. These findings supported evaluating a less frequent dosing schedule for 330 mg/m2 and above to further determine activity and decrease potential neurotoxicity. However, no PSA responses were seen on the three-week dosing schedule and only two of 17 patients (12%) treated on the six-week schedule had PSA responses.
Fig. 3.
Maximal PSA decline in all patients
Measurable disease
No tumor regressions were seen in measurable soft-tissue lesions, although 35% remained stable.
DISCUSSION
The first-in-man study of MLN2704 showed dose-dependent antitumor effects without significant peripheral neuropathy, but further dose escalations performed in the current study along with repetitive dosing proved to be limited by DLTs[2]. The toxicities occurred most commonly after the second or third dose. Overall efficacy was also limited, as only five patients (8%) experienced a ≥ 50% PSA decline and five had PSA stabilization lasting ≥90 days; four of those showing a PSA response received 330 mg/m2 every two weeks. Less frequent dosing did not maintain efficacy or reduce neurotoxicity.
The etiology of the peripheral neuropathy associated with MLN2704 is likely due to deconjugation in plasma of the small-molecule antimicrotubule, DM1, from the large-molecule antibody, MLN591. Similar PKs, deconjugation rates, and toxicity patterns (including peripheral neuropathy) were observed in a phase I trial of an immunoconjugate targeting MUC1, cantuzumab mertansine [4]. In this study, both free DM1 and free antibody were detectable in a time-dependent fashion shortly after catabolism of MLN2704 and plasma clearance. Free DM1 was detectable for up to 30 days after the third treatment cycle, providing evidence that free DM1 is not immediately metabolized and eliminated after rapid deconjugation from MLN591.
The PKs of MLN2704 are complex because it has been formulated with a small molecule (DM1) attached to a large molecule (MLN591 antibody) at lysine residues by a thiopentanoate linker. Intracellular reduction of a disulfide bond within the thiopentanoate linker allows DM1 to be released at the site of action within prostate cancer cells expressing PSMA. ADC disulfide linkers have been shown to be significantly more labile than other types of linkers: this results in reduction of the disulfide bond in the plasma rather than within cancer cells as intended[5]. Phase 1 and phase 2 clinical trials involving the ADC trastuzumab emtansine (T-DM1) have demonstrated that immunoconjugates formulated with a less labile thioether linker are sufficiently potent and even at high doses do not have the same toxicity profile as MLN2704[6,7]. An average of three to five molecules of DM1 are attached to every antibody molecule of both the MLN591 antibody and trastuzumab: the differences in pharmacodynamic profiles between the two agents are likely due to formulation differences at the linker[6,8].
MLN2704 linker instability is exemplified by comparing peak plasma levels from this phase 1/2 trial to those from the phase 1 T-DM1 trial. In the T-DM1 trial, peak plasma levels of free DM1 were < 10 ng/mL for all dose levels[6]. However, we observed > 20-fold higher levels of free DM1 at the 330 mg/m2 dose in patients on the two-week and three-week schedules. A > 20-fold increase of circulating DM1 in the periphery could plausibly explain both the lower than expected PSA response and the high incidence of peripheral neuropathy.
Radiolabeled anti-PSMA based therapy is an alternative strategy to a toxin conjugate approach. Since October 2000, five studies have evaluated Lutetium-177 (177Lu) radiolabeled anti-PSMA monoclonal antibody J591 (177Lu-J591) in patients with metastatic CRPC[9]. In a phase II study in patients with progressive metastatic CRPC at a dose of 65–70 mCi/m2, significant PSA declines were seen in 60% of patients. Myelosuppression was the dose limiting toxicity. Dose fractionation alone or in combination with docetaxel chemotherapy has also resulted in significant PSA declines. 177Lu imaging studies have demonstrated accurate targeting of metastatic sites in > 90% of patients. Alternative PSMA targeting with radiolabeled small molecules involving radioligand therapy rather than an antibody based approach have been investigated with interesting results. The small molecule approach circumvents issues with antibody based approaches including the slow diffusion of antibodies into solid lesions and the prolonged circulation time of antibodies associated with an increased risk for toxicity. The DOTA-conjugated ligand PSMA-617 with high binding affinity and long tumor retention has been evaluated in metastatic CRPC with promising results[10,11]. In a retrospective experience with 74 consecutive analyzable patients with metastatic CRPC selected for PSMA expression by 68Ga-PSMA-11 PET/CT who received a single dose of 177Lu-PSMA-617, a PSA decline was seen in 47 (63%) with 23 (31%) having a > 50% decline with minimal toxicity. Another experience with 177Lu-PSMA radioligand therapy in 56 patients with metastatic CRPC revealed PSA responses in 45 of 56 (80.4%) patients, radiologic responses and excellent tolerability. Hematologic toxicity was minimal and significantly less than what is seen with 177Lu-J591 where the mean absorbed dose of 177Lu-J591 delivered to the red marrow is 20-fold higher than what was seen with 177Lu-PSMA radioligand therapy[12]. Radioimmunotherapy for metastatic CRPC has demonstrated promising results with several ongoing trials in patients with advanced prostate cancer.
Overall, the results provide further validation of PSMA as a therapeutic target for immunoconjugates with more favorable PK and pharmacodynamic profiles. Here, linker instability, increased dose intensity relative to the previous phase 1 clinical trial, and the detection of free DM1 with a long-terminal elimination led to an unfavorable safety profile that precluded further study.
CONCLUSIONS
While there is proof-of-concept evidence supporting MLN2704 as a potentially effective agent for patients with CRPC, it possesses an extremely narrow therapeutic window. Thiopentoate linker lability leads to rapid catabolism and deconjugation of DM1 from the conjugated antibody. Increased DM1 levels are the likely etiology for the toxicity leading to the narrow therapeutic window. This study provides a rationale for developing anti-PSMA ADCs for CRPC treatment utilizing novel linker strategies to avert toxicity.
Supplementary Material
Highlights.
MLN2704, an antibody drug conjugate targeting PSMA was evaluated in metastatic CRPC.
MLN2704 at multiple ascending doses had limited activity in metastatic CRPC.
Neurotoxicity with peripheral neuropathy was dose-limiting.
Disulfide linker lability and rapid deconjugation led to a narrow therapeutic window.
Footnotes
Conflict of Interest
Dreicer – Research support: Millenium/Takeda
George –Research support: Millenium/Takeda
Galsky - Research support: Millenium/Takeda
Crona – None
Nanus – Research support: Millenium/Takeda
Webb - Employee of Millennium Pharmaceuticals, a fully owned subsidiary of Takeda Pharmaceuticals International
Petruck - Employee of Millennium Pharmaceuticals, a fully owned subsidiary of Takeda Pharmaceuticals International
Bander - an inventor on patents that are assigned to Cornell Research Foundation (“CRF”) for anti-PSMA antibody technology. Dr. Bander is a paid consultant to and holds equity in BZL Biologics, the company to which the patents were licensed by CRF for further research and development.
Scher – Research support: Millenium/Takeda
Milowsky - Research support: Millenium/Takeda
Disclosures
The study was sponsored by Millennium Pharmaceuticals, Inc., Cambridge, MA, and supported by a grant from the NIH/NCI Cancer Center Support Grant (P30-CA008748).
References
- 1.Tagawa ST, Beltran H, Vallabhajosula S, et al. Anti-prostate-specific membrane antigen- based radioimmunotherapy for prostate cancer. Cancer. 2010;116:1075–83. doi: 10.1002/cncr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galsky MD, Eisenberger M, Moore-Cooper S, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol. 2008;26:2147–54. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13:741–8. doi: 10.1111/j.1525-1438.2003.13603.x. [DOI] [PubMed] [Google Scholar]
- 4.Tolcher AW, Ochoa L, Hammond LA, et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21:211–22. doi: 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- 5.Kellogg BA, Garrett L, Kovtun Y, et al. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem. 2011;22:717–27. doi: 10.1021/bc100480a. [DOI] [PubMed] [Google Scholar]
- 6.Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 7.Burris HA, 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 8.Henry MD, Wen S, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64:7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhajosula S, Nikolopoulou A, Jhanwar YS, et al. Radioimmunotherapy of Metastatic Prostate Cancer with 177Lu-DOTAhuJ591 Anti Prostate Specific Membrane Antigen Specific Monoclonal Antibody. Curr Radiopharm. 2016;9:44–53. doi: 10.2174/1874471008666150313114005. [DOI] [PubMed] [Google Scholar]
- 10.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016 Mar 16; doi: 10.2967/jnumed.115.171397. pii: jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt RK, Heinzel A, Eppard E, et al. Response and tolerability of a single dose of 177Lu-PSMA-DKFZ-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016 Apr 7; doi: 10.2967/jnumed.116.173757. pii: jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 12.Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med. 2016 Jan 21; doi: 10.2967/jnumed.115.168443. pii: jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.