Abstract
Recently, our laboratory has demonstrated the technical feasibility of monitoring dopamine at one-min temporal resolution with microdialysis and online liquid chromatography. Here, we monitor dopamine in the rat striatum during local delivery of high potassium/low sodium or nomifensine in awake-behaving rats. Microdialysis probes were implanted and perfused continuously with or without dexamethasone in the perfusion fluid for four days. Dexamethasone is an anti-inflammatory agent that exhibits several positive effects on the apparent health of the brain tissue surrounding microdialysis probes. Dopamine was monitored one or four days after implantation under basal conditions, during 10-min applications of 60 mM or 100 mM K+, and during 15-min applications of 10 μM nomifensine. High K + or nomifensine were delivered locally by adding them to the microdialysis perfusion fluid using a computer-controlled, low-dead-volume six-port valve. Each day/K+/dexamethasone combination elicited specific dopamine responses. Dexamethasone treatment increased dopamine levels in basal dialysates (i.e., in the absence of K+ or nomifensine). Applications of 60 mM K+ evoked distinct responses on days one and four after probe implantation, depending upon the presence or absence of dexamethasone, consistent with dexamethasone’s ability to mitigate the traumatic effect of probe implantation. Applications of 100 mM K+ evoked dramatic oscillations in dopamine levels that correlated with changes in the field potential at a metal electrode implanted adjacent to the microdialysis probe. This combination of results indicates the role of spreading depolarization in response to 100 mM K+. With one-min temporal resolution we find that it is possible to characterize the pharmacokinetics of the response to the local delivery of nomifensine. Overall, the findings reported here confirm the benefits arising from the ability to monitor dopamine via microdialysis at high sensitivity and at high temporal resolution.
Keywords: capillary liquid chromatography, electrochemical detection, principal component analysis, dexamethasone, chronic implantation
Graphical abstract
Introduction
Microdialysis has been widely employed for intracranial chemical monitoring.1–5 Microdialysis probes are robust and can be used in both anesthetized and awake animals ranging from rodents to primates, including human patients. They collect a broad array of small molecules below the molecular weight cutoff of the dialysis membrane, several varieties of which are available in the required hollow-fiber format. The dialysis process produces samples that are free of tissue fragments, proteins, blood, and other forms of contamination. The dialysate samples can either be collected, stored, and analyzed later,6–8 or analyzed in near-real time by online methods.9, 10 Online analysis decreases the chance of sample degradation during storage and eliminates delays in obtaining the results. Online analysis is often performed with liquid chromatography (LC)11–13 or capillary electrophoresis14, 15 coupled to detectors employing laser-induced fluorescence,16, 17 mass spectrometry,18, 19 or electrochemistry.20–23
Recent progress in improving the time resolution of microdialysis sampling of neurotransmitters builds on earlier work.11 Developments in LC separation speed have enabled online collection and analysis of serotonin (5-HT) at three-24 and later two-min intervals25 on commercial instruments. We have used capillary LC with electrochemical detection to determine both dialysate DA and 5-HT (separately) in near real time at one minute intervals.26–28 Electrical stimulation to induce DA transients demonstrated that our approach yields an overall time resolution of at least one-minute.28
While there are many studies of DA release induced by retrodialysis of high K+/low Na+ at modest time resolution, we are not aware of any at one-minute time resolution. It would be of interest to learn what information is gained by measuring at this time resolution. Thus, in this study, we describe the remarkable variety of dialysate dopamine transients elicited by a ten-minute high K+/low Na+ stimulation29 (60 mM or 100 mM K+; we denote these as “high K+” below). Having seen oscillations in 5-HT concentrations resulting from high K+ stimulation, we anticipated that DA may respond similarly. Thus, we used a 10-min stimulation to accommodate the 2–3 min period of the oscillations.27 Responses in awake rats were measured one- and four days after probe implantation. One group of animals had microdialysis probes perfused with artificial cerebrospinal fluid only (aCSF). A second group had microdialysis probes perfused continuously with aCSF containing dexamethasone30–33 via retrodialysis. We refer to the latter set of probes as “local dexamethasone by retrodialysis” probes (LDR probes), and the former as “control” probes.
Results and Discussion
One-minute resolution online measurement of dopamine
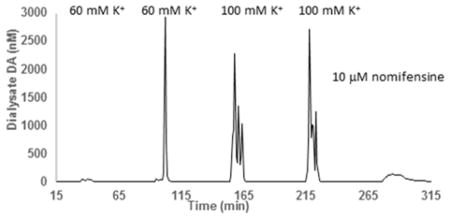
Separation of dialysate using the online system under conditions described in the experimental section result in completely resolved DA peaks, with DA retention times of approximately 44 s (Figure 1). Chromatograms were obtained continuously, with injections occurring once every minute for the entire six- to eight-hour duration of the experiment. For all conditions, there are no other peaks in the chromatogram near the DA peak. The DA retention time when measured during an online experiment is within 3% of the retention time measured from aqueous DA standards, as well as DA-spiked dialysate. Dialysate DA concentrations are calculated from average slopes and intercepts from a pair of linear calibration curves obtained prior to and after each day-long experiment (Figure S1). The slopes and intercepts from the two calibration curves were typically within 5% of each other. Figure 2 shows a DA dialysate concentrations obtained one day after implantation of an LDR probe. The five transients are (from left to right) the dialysate dopamine responses to 10-min stimulations with 60 mM K+ (twice), 100 mM K+ (twice), and a 15-min stimulation with 10 μM nomifensine. Both K+ and nomifensine were, like the dexamethasone, delivered through the probe.
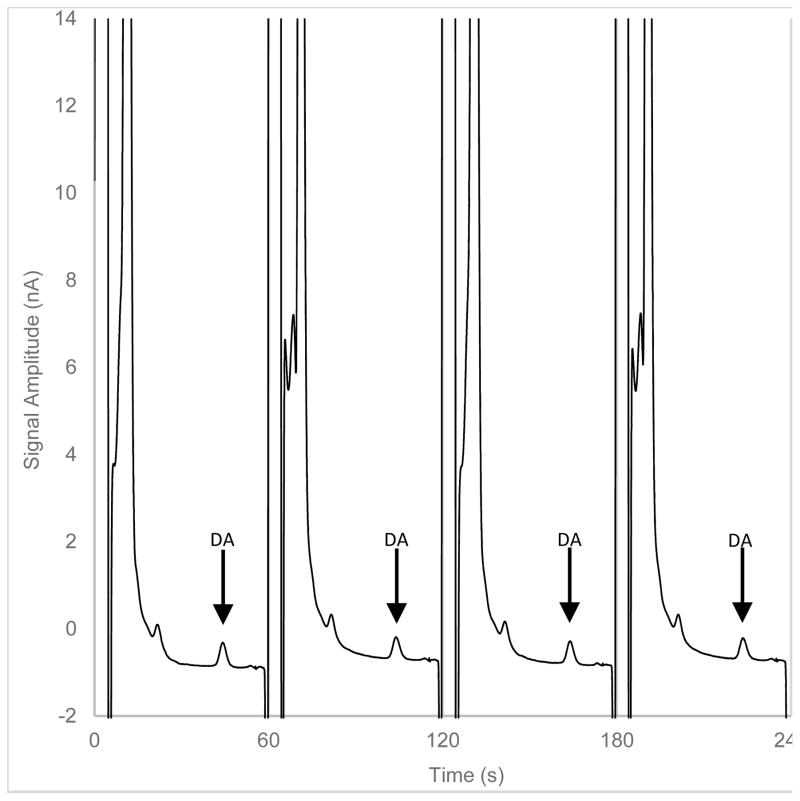
Figure 1.
Four chromatograms of striatal dialysate by online LC-EC from a longer sequence of injections. Consecutive 500 nL samples of dialysate were collected under basal conditions and analyzed online at one-min intervals. The dopamine peaks appear 44 s after each sample is injected onto the capillary column.
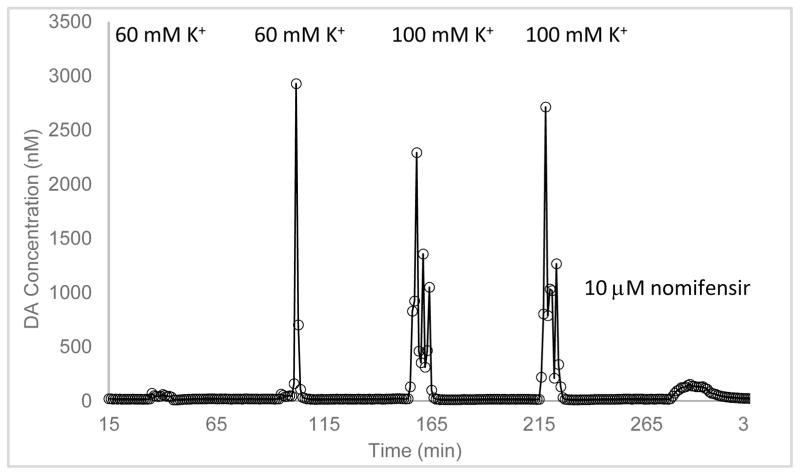
Figure 2.
DA dialysate concentration measured during an experimental run (day 1, [K+] = 60 or 100 mM, with an LDR probe). The five transients seen are dopamine responses to 10-min retrodialysis of 60 mM K+ (twice), 100 mM K+ (twice) and 15-min retrodialysis of 10 μM nomifensine.
Previous studies with K+ stimulated 5-HT25 and DA,34 using a range of K+ concentrations and stimulation times (25 – 120 mM K+ for 1–10 min) found that a 50–60 min recovery interval between stimulations is sufficient to avoid the influence of a prior stimulation on the effect of a following stimulation. The range of potassium concentrations and stimulation times encompasses our experimental conditions. Thus, during this work, we allowed a 50-min recovery time between each stimulus. Each of these data sets contains three measurable quantities: basal levels, transients caused by 10 min K+ stimulations, and the transient caused by the 15-min nomifensine stimulation. These will be assessed in that order below.
Basal
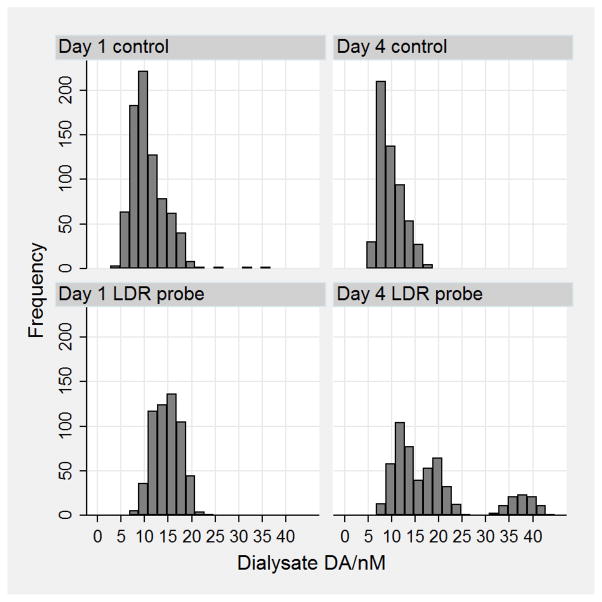
We define basal levels as DA levels in dialysate measured at least 10 minutes and at most 40 minutes from a transient. Figure 3 shows the frequency distributions of the one-minute measurements for each of the four conditions day = 1 and 4; with LDR or control probes. Consider first the data for control probes. These data have a skewed distribution indicating that there are more observations with basal levels below the mean than above the mean. The mean basal DA with control probes is comparable to published results from other groups as well as by our group.28, 35, 36 The distributions obtained with LDR probes show multiple maxima with a clearly separate distribution on day 4. This separate distribution is a single animal’s basal data. The basal levels also appear to be on average higher with LDR probes, thus we did a two-way (“day” and “dexamethasone”) analysis of variance on the mean basal DA with and without the aforementioned single animal’s high basal DA data (Full ANOVA and regression output are in Tables S1 and S2). “Day” is not significant, but “dexamethasone” is significant (p = 0.013, n = 22) when the aforementioned high basal DA data are included. The pattern is similar from ANOVA without the aforementioned high basal DA data, but the significance is higher (p = 0.0017, n = 21). A linear regression using the variable “dexamethasone” with values of 0 for control and 1 for LDR probes leads to the simple relationship: basal dialysate [DA]/nM = 10.4 + dexamethasone*4.4 (p = 0.0013, n = 21) without the single animal’s high basal DA data, and [DA]/nM = 10.4 + dexamethasone*6.7 (p = 0.0097, n = 22) with those data. We conclude that the basal dialysate DA level increases in the presence of dexamethasone retrodialysis.
Figure 3.
Frequency distributions of the basal concentrations measured each minute for the four conditions day = 1 and 4, probe = control and LDR. Note there are seven animals represented in day 1 control. There are five animals contributing data for the other three cases. The “frequency axis” represents single, one-minute measurements.
Potassium stimulations
As mentioned above, we anticipate oscillations in DA dialysate concentrations during a high K+ stimulation. It is therefore important to consider what the time resolution of the entire microdialysis/liquid chromatography system is. Previously,27 we determined the effect of the transport tubing and the microdialysis probe itself on the shape of a nominally instant change in 5-HT concentration in vitro. Importantly, we validated the use of a calculated Taylor dispersion standard deviation for the spreading induced by the inlet and outlet capillaries. The individual contributions in the current set up are approximately 8.2, 8.7, and 8.8 s in the inlet, probe, and outlet, respectively. The variances add, so the overall time resolution is eroded with a standard deviation of 14.8 s based on spreading of the stimulus pulse on passing through the inlet/probe and the DA response passing through the outlet/probe. The practical result is that a perfectly sharp concentration step of a stimulant would be “smoothed” slightly on its way to the probe. The resulting DA transient would also be smoothed slightly within the probe and on the way to the liquid chromatograph’s injection valve. As a result, we expect to see the results of a 10-min stimulation as an approximately 11-min response. We can categorize the DA responses to the 88 K+ stimulations that we observed as follows. Dialysate DA transients in response to K+ stimulations can be positive or negative, they can be small (less than 100 nM) with no spiking (n = 37), have one spike (n = 16) or have multiple spikes (n = 35). We can also compare the average of the DA transients over the entire 11 minutes from a single 100 mM K+ stimulation to the basal level. In doing so, we see results in accord with analogous experiments using 20-min resolution offline microdialysis,37, 38 namely dialysate DA transients of up to 50 – 60 times basal level.
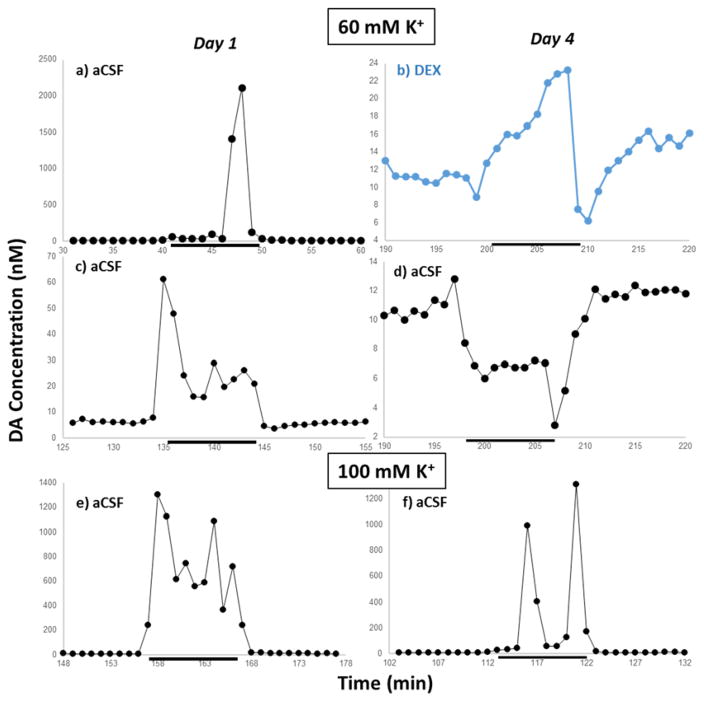
Figure 4 shows some of the dialysate DA transients we recorded from the experimental runs (all of them are in Figs. S2 – S23). While there is variability from stimulus to stimulus and from rat to rat, there are qualitative trends. The 100 mM K+ stimulations on day 1, control or LDR probe, yielded multiple (three or more) large spikes (200 nM or more, e.g., Figure 4e). Using similar equipment, we recently reported high-amplitude oscillations of serotonin.27 The same group on day 4 yields mostly transients with one or two spikes on top of a 50 – 100 nM base, e.g., Figure 4f but similar responses may be seen on day 1 (4a). The 60 mM K+ transients measured with control probes on day 1 exhibit either transients similar to those in Figure 4a or small spikes, with a larger spike (but small compared to those produced by 100 mM K+) at the leading edge (Figure 4c). On day 1, responses obtained with LDR probes are highly variable encompassing nearly all of the transients described above. Transients induced by 60-mM K+ on day 4 were typically small, although probes perfused with aCSF alone produced decreases in DA levels (Figure 4d) while LDR probes produced negligible to slightly positive responses with a negative transient after the cessation of the stimulations such as in Figure 4b. A reduction in dialysate DA in response to a 20 – 30 mM K+ stimulation 24 h post-striatal implantation has previously been reported in one study.39 It must be noted that, however, the measurements were done after 80 minutes of high K+ perfusion. Such prolonged perfusion of high K+ reduces DA extraction fraction40 which will alter dialysate DA concentration.
Figure 4.
Qualitatively distinct dialysate DA responses observed during the experiments. Left: day 1. Right: day 4. Top: (a–d) 60 mM K+. Bottom: (e–f) 100 mM K+. The example in blue is from an LDR probe. The group is selected to be representative to transient characteristics, not conditions. K+ stimulations are denoted by the black bar. Note the differing vertical scales. A complete set of images of all transients observed can be found in the Supporting Information, Figs. S2 – S23.
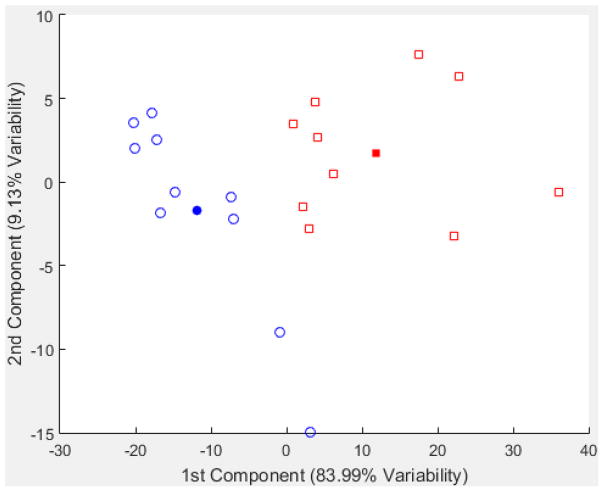
Principal component analysis of dopamine transients
There are clear qualitative differences between responses to 60 mM K+ and 100 mM K+ stimulations on both day 1 and day 4. However, there is also significant variability in the responses to each type of stimulation. Thus, we turned to principal component analysis (PCA) to help to classify the observed responses. All of the scatter plots of scores are in the Supporting Information. Figure 5 shows such a plot for the day 4, 60 mM K+ responses from both control and LDR probes. The first two principal components represent more than 95% of the variance in the data. Importantly, the PCA scores reveal a clear separation of the responses obtained with control from those of LDR probes. The solid symbols in the score plots represent the mean of each cluster of points. Converting those mean scores to hypothetical laboratory observations leads to Figure 6. The result is striking – responses with LDR probes tend to show positive amplitudes while responses with control probes show negative amplitudes. To confirm the PCA result, we also show the mean responses for each of the five animals in the two categories (ten transients in each). The correspondence of the average and the result from the first two principal components gives confidence in the observation. The PCA analysis of the analogous day 1 data shows that there is a greater variety of responses. There is a cluster of very similar responses (see score plot in Figure S24) with the same characteristics as Figure 4c, a small dialysate DA increase from basal level. Others are represented by Figure 4a and f, mostly small increases from basal levels with one or two high amplitude spikes. This level of response has been observed extensively in many of the microdialysis studies noted earlier (in the introduction). The short, one-min spike, on the other hand, would not have been observable without one-min time resolution.
Figure 5.
Scatterplot of scores of the first two principal components of all transients from day 4, 60 mM K+ stimulations. Blue circles represent data from animals with control probes, and red squares represent animals with LDR probes. The filled circle and square represent the centroid of respective types computed from the first two principal components.
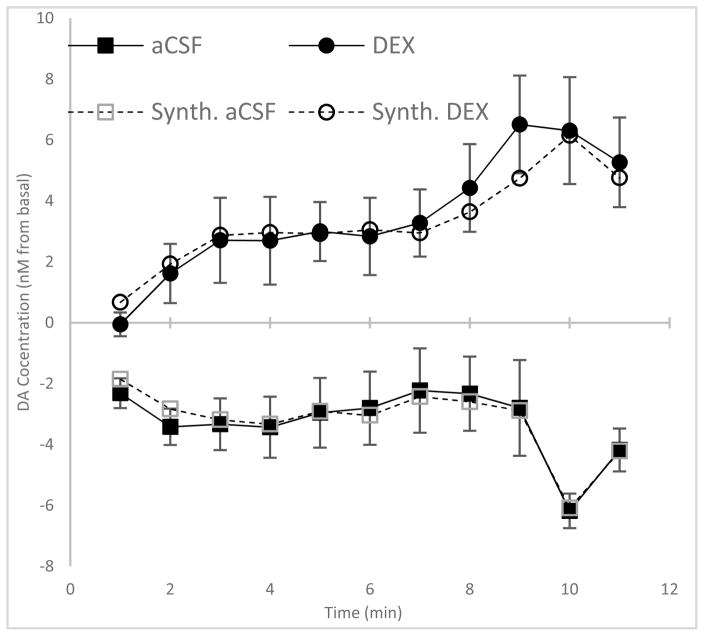
Figure 6.
Comparison of transients from LDR (top) and control (bottom) probes on day 4 with a 60 mM K+stimulation. Open symbols: Synthesized dopamine transients from the first two principal components. Solid symbols: experimental transients from averaging the responses at each minute.
Figure S26 is the scatter plot of scores from all transients from 100 mM K+ stimulations. Here, it is noteworthy that the score plot shows a clustering based on “day”, but not based on “dexamethasone”. We conclude from this classification that the more extreme perturbation of 100 mM K+ (compared to 60 mM) elicits a response mostly based on the time from implantation. The transients are for the most part oscillations of high magnitude.
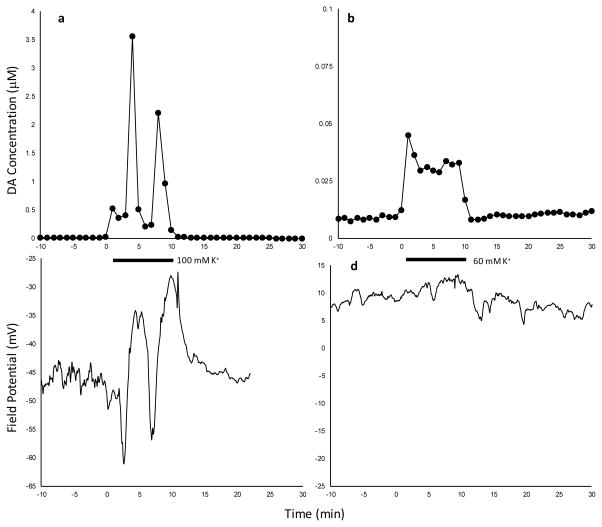
Simultaneous measurement of dialysate DA and field potential
In two separate animals, we implanted a microdialysis probe with an attached tungsten wire for field potential measurements (Figure S29). Figure 7 compares the simultaneously measured dialysate dopamine and field potential transients recorded on day 1 with LDR probes and 100 mM K+ (left) and 60 mM K+ (right) stimulations. High amplitude oscillations of dialysate dopamine levels during 100 mM K+ stimulations correlate to the oscillations in field potential with the same oscillation period of roughly 3-min, while the lack of dialysate dopamine oscillations during 60 mM K+ stimulations correlates to similarly insignificant change in field potential.
Figure 7.
Simultaneous measurement of dialysate DA and field potential in an awake animal. Left: 100 mM K+ stimulation. Right: 60 mM K+ stimulation. Measurements made on day 1 with LDR probes.
Such slow oscillations in field potential are an indication of spreading depolarization.41 which is a pathological event. Spreading depolarization and spreading depression comprise a set of complex processes that can originate from many irregular brain conditions and these manifest in several ways.41, 42 The role of microglia in spreading depression is, likewise, complicated. They were found to promote spreading depression in one polarization (M1 microglia), and increase the threshold for spreading depression in another polarization (M2a microglia).43 Their presence is strictly required for ischemia-induced, but not for high K+-induced, spreading depression.44 Although the effect of glia on spreading depolarization and depression is complicated, and not completely understood, it is nonetheless noteworthy that we see effects of dexamethasone both on gliosis33 and on the response in vivo to K+ stimulations.
With day 4 60 mM K+ experiments, we observed a small increase in dialysate DA level from LDR probes and a small decrease in dialysate DA level for control probes. While the small increase, again, is expected. The small decrease is unexpected and striking as it has been seen once but not reproduced.34, 39 We hypothesize that the decrease in dialysate DA level is a depression of neural electrical activity without accompanying spreading depolarization. The condition in these experiments, namely the proliferation of activated microglia and ischemia, is conducive41, 43, 44 to spreading depression, which can lead to reduced cerebral blood flow41 and silenced synaptic activity,42 possibly reducing spontaneous DA release to below basal levels.
Comparison to observations by fast-scan cyclic voltammetry
Fast-scan cyclic voltammetry (FSCV) in combination with carbon fiber microelectrodes is an alternative method for monitoring extracellular DA in the brain. A well-known application of FSCV is the monitoring of DA transients evoked by electrical stimulation of DA axons in the medial forebrain bundle (MFB). Previously, we monitored electrically evoked DA transients with carbon fiber microelectrodes placed in striatal tissues in close proximity to microdialysis probes and with carbon fiber electrodes positioned in the outlet of the probe.33 While a ten-minute, elevated K+ stimulation would not be expected to elicit the same response as a 25 s MFB stimulation, there are noteworthy contrasts between the responses observed during this work with K+ stimulation and that done previously with electrical MFB stimulation.
First, regardless of the time point after probe implantation (4 hr, 1 day, 5 days), electrically evoked DA responses were not observed by FSCV in the tissue adjacent to, or at the outlet of, probes perfused with aCSF without dexamethasone. These findings are difficult to interpret in isolation as they might either indicate an absence of DA terminals near the probe or the presence of DA terminals in some abnormal condition that suppresses electrically evoked DA release. Histology using two well-established markers for DA terminals, tyrosine hydroxylase and the dopamine transporter, identified DA terminals of a near-normal appearance in the tissues adjacent to the probes at time points beyond 4 hrs, suggesting the presence of DA terminals, albeit in some abnormal condition. The present results appear to support this conclusion, as 100 mM K+ stimulation evokes DA responses from animals with control probes on days 1 and 4. This result implies the presence of terminals near the probe capable of releasing DA upon direct K+-induced depolarization, even though electrically evoked DA release is suppressed in the absence of dexamethasone. This supports our prior conclusion that DA terminals survive the traumatic consequences of probe implantation, which we have called the traumatic penetration injury (TPI).
Second, during our work using FSCV to determine the effect of dexamethasone retrodialysis on dopamine measurements by microdialysis, we noticed dramatic differences between electrically evoked DA transients on Day 5 with control probes vs LDR probes.33 As just mentioned, with control probes, electrically evoked responses were not observed on day 5 either next to or at the outlet of probes. However, responses were normal next to LDR probes and were well-above detection limits at the probe outlet. In the current work, there is a similar dramatic difference in the responses from control vs. LDR probes with 60 mM K+ stimulation on day 4, namely there are decreases from basal with control probes, Figs. 4d, 6 and increases with LDR probes, Figs. 4b, 6. Again, these contrasting stimulation responses between control and LDR probes do not correlate with the histological appearance of DA terminals near the probes: The DA terminals appear normal on day 5 after probe implantation both with and without local dexamethasone via retrodialysis. However, these contrasting stimulation responses do correlate with the histological appearance of astrocytes and microglia on day 5, when glial activation is robust near control probes but nearly absent near LDR probes. Thus, our measurements of both electrically evoked and K+-evoked DA transients support the conclusion that dexamethasone facilitates the re-establishment of normal DA activity in the tissues affected by the TPI during probe implantation.
In contrast to the case for 60 mM-induced transients and as deduced from the principal components analysis, dialysate DA responses to stimulation with 100 mM K+ on days 1 and 4 did not depend on the presence of dexamethasone in the perfusion fluid. This is likely due to this high concentration of K+ being able to “force” the depolarization of DA terminals, possibly due to the induction of spreading depolarization, regardless of the presence or absence of activated glia. Thus, the responses to stimulation with 100-mM K+ are unique in that they appear to be the only ones we have recorded to date that are unaffected by activated glia.
Comments on the nature of the transients
Altering perfusate compositions can lead to changes in measured dialysate dopamine by at least three mechanisms. One mechanism is reverse transport via the dopamine transporter, DAT.45 Low Na+ perfusate, 50 mM, with choline replacement and normal K+, induces dopamine efflux into the extracellular space by this mechanism.46 Using low Na+ perfusate, but replacing Na+ with K+ rather than choline also evokes dopamine release47 even in the presence of nomifensine to block the first mechanism, reverse transport.46 Thus, high K+ elicits DA release by a second mechanism, depolarization. Finally, changes in the perfusate composition, including high K+ 40 can lead to changes in relative recovery/extraction fraction, e.g. by altering DA uptake rates.48–50 It is also possible for physical changes in the tissue to alter the effective diffusion coefficient, altering dialysis recovery.51 We have not attempted to unravel the contributions of each of these mechanisms to our observations. However, we have associated high amplitude oscillations during 100 mM K+ stimulation to spreading depolarization via simultaneous field potential measurements. It is interesting to speculate that some of the features that we see at high temporal resolution may be related to differences in the foregoing effects of high K+/low Na+ stimulation. Another intriguing possibility for future investigations is whether any of the variability that we see under the same stimulation conditions is related to the striatum’s physiological heterogeneity.52
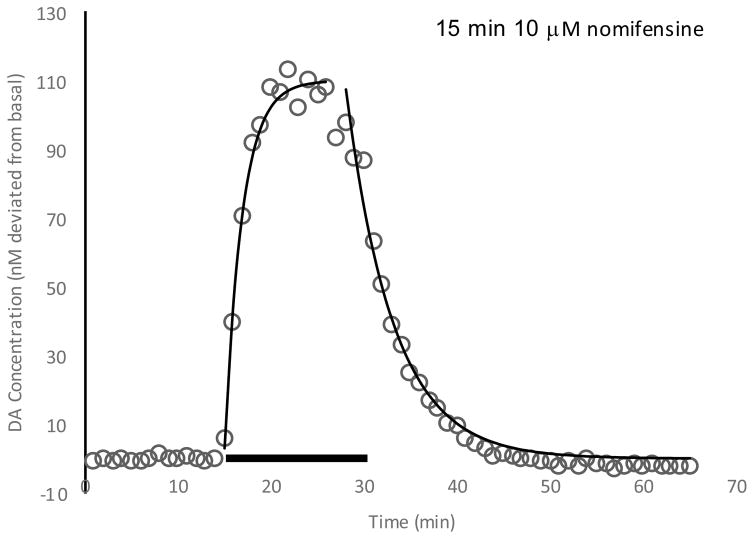
Pharmacokinetics of nomifensine
The DA responses to nomifensine stimulation can be modeled using a first-order model for rising and falling rates fitted with different time constants. Two-way ANOVA analysis of the time constants (Figure S28) show that neither the day of the experiment or dexamethasone treatment affects the nomifensine rising and falling characteristics. The maximum amplitude, however, is day-dependent, being lower on day 4 after implantation compared to day 1. Nomifensine is introduced by retrodialysis, so a significant contribution to the dynamics is the diffusion of nomifensine within the tissue. The results obtained indicate that the diffusion rates are not significantly different among the various conditions. The rising rate constant is found to be about two times higher than the falling rate.
Conclusions
In the present study, we have shown that in vivo monitoring of dopamine at one-minute time resolution using online microdialysis-LC-EC reveals patterns of responses to chemical stimulation or local drug treament by retrodialysis with considerably more information than can be obtained with lower resolution measurements. We find that using LDR probes increases basal dialysate DA levels. A high concentration of 100 mM K+ induces spreading depolarizations in the striatum with control or LDR probes. This is consistent with the presence of functional DA terminals near the probe in both cases. Remarkably, the 60 mM K+ transients are positive after four days with the LDR probe while they are negative (and of a similar magnitude) with control probes.
Given that spreading depolarization is a pathological condition, we are led to wonder whether the practice of stimulating release of neurotransmitters with K+ concentrations on the order of 100 mM in order to assess the experimental set up is wise. There are certainly legitimate reasons to use this method, but the lowest concentration of potassium ion that elicits a response is most likely preferred.
We note that the dexamethasone treatment had no significant effect on an essentially pathological response to 100 mM K+, while it had a significant and qualitatively obvious effect on exposure to a lower K+ concentration. This is interesting in that it implies that the effect of dexamethasone is more easily discerned with less extreme perturbations of the tissue. This is consistent with evidence from FSCV and immunohistochemistry experiments. We infer that dexamethasone-induced reduction of gliosis, in combination with the higher time resolution microdialysis, improves the ability to observe dopamine system function when microdialysis probes have been chronically implanted.
Methods
Chemicals and Materials
Chemicals (disodium EDTA, sodium acetate, sodium 1-octanesulfonate (SOS), acetonitrile, glacial acetic acid, NaCl, KCl, CaCl2, MgCl2, and NaH2PO4) were purchased from either Fisher Scientific (Fair Lawn, NJ) or Sigma (St. Louis, MO) and used as received. Dexamethasone sodium phosphate was from APP Fresenius Kabi USA, LLC, Lake Zurich IL. Ultra-pure water used was filtered using a Millipore Mili-Q Synthesis A10 system (Belford, MA).
Artificial cerebrospinal fluid (aCSF: 142 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, 1.0 mM MgCl2, and 2.0 mM NaH2PO4, pH 7.4) was used as the perfusion fluid for the microdialysis probes. The high K+ aCSF solutions were kept isotonic by lowering the Na+ concentration (60 mM K+ aCSF: 84.7 mM NaCl, 1.2 mM CaCl2, 60 mM KCl, 1.0 mM MgCl2, and 2.0 mM NaH2PO4. 100 mM K+ aCSF: 44.7 mM NaCl, 1.2 mM CaCl2, 100 mM KCl, 1.0 mM MgCl2, and 2.0 mM NaH2PO4). Dexamethasone sodium phosphate (APP Pharmaceuticals LLC, Schaumburg, IL) and nomifensine maleate (Sigma-Aldrich, St. Louis, MO) were diluted in aCSF. The microdialysis perfusion fluids were filtered with Nalgene sterile filter units (Fisher, Pittsburgh, PA; PES 0.2 μm pores).
Probe construction
Concentric-style microdialysis probes (4 mm membrane length) were built in-house (200 μm I.D, 280 μm O.D, 13 kDa MWCO Spectra/Por hollow fiber, Spectrum Laboratories Inc. Rancho, Dominquez, CA) see28 for details. The probe inlet consists of 100 cm of fused silica capillary (75 μm I.D., 150 μm O.D. Polymicro Technologies, Phoenix, AZ). The outlet capillary is of the same type but 115 cm in length. Each capillary is connected to 10 cm of 75 μm I.D., 360 μm O.D. fused silica to facilitate the connection of inlet and outlet lines to nanobore injection valves (described below). We estimate based on system volumes and perfusion flow rate that transport of a stimulant slug to the probe membrane takes 9.84 minutes, and a sample slug takes 9.05 minutes from the probe to the injection valve. The difference is mainly due to the internal volume of the probe which counts towards the inlet flow path. Prior to use, the probes were soaked in 70% ethanol and then immersed in and flushed with filtered perfusion fluid (aCSF or aCSF with dexamethasone) for several hours before implantation into the rat.
Surgical procedure and implantation
All use of animals was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Prior to surgery, rats (male Sprague–Dawley, 250–350g, Charles River, Raleigh, NC) were acclimated overnight to a Raturn Microdialysis Bowl (MD-1404, BASI, West Lafayette, IN). The next day, rats were anesthetized with isoflurane (5% v/v induction, 2.5% v/v maintenance) and implanted with microdialysis probes using aseptic stereotaxic surgical techniques. Using flat skull coordinates, each probe was slowly lowered into the striatum (1.6 mm anterior, 2.5 mm lateral from bregma, and 7.0 mm below the dura) at 5 μm/s using a micropositioner (David Kopf Instruments Model 2660, Tujunga, CA). The histology of the probe track in the striatum using these same coordinates has been documented numerous times by our group.33, 36 The probe was secured with bone screws and acrylic cement and the incision was closed with sutures. Anesthesia was removed and the animals were returned to the Raturn system and given free access to food and water for the duration of the experiment. Continuous perfusion was maintained for the entire duration of the experiment. During procedures involving dexamethasone, the probe was perfused with 10 μM dexamethasone for the first 24 hours and then with 2 μM dexamethasone for the remainder of the experiment.
Online microdialysis-LC-EC
The microdialysis/liquid chromatography system was similar to the one previously described.26 A schematic diagram is shown as Fig. S30. Perfusate was introduced using a syringe pump (Harvard Apparatus, Holliston, MA), running at 0.610 μL/min. The perfusate syringe contained 1.0 mL of perfusate and was refilled every 24 hours, at least 3 hours prior to any online measurement. To facilitate the introduction of a stimulant-containing solution, we used a 6-port nanobore injection valve (electrically actuated, Valco Instruments, Houston, TX) to introduce solution from a 9.5 μL fused silica loop (280 μm I.D., 360 μm O.D, Polymicro Technologies, Phoenix, AZ). The valve was configured so that the loop could be loaded with stimulant-containing solution while perfusate flow is maintained. To introduce the stimulating solution, the valve position was switched from load to inject position by computer control at predetermined times for durations equal to the stimulus duration. So-called timed injections suffer less spreading of the concentration profile at the trailing edge than injections that permit the entire loop to be pushed into the inlet capillary. During valve position switching, flow is interrupted for 105 milliseconds. The stimulus reaches the probe 9.8 minutes after switching. Therefore, any DA response due to switching the position of the valve would be seen approximately ten minutes prior to the response to the stimulus. We do not see any such response to the valve position switching itself in the data.
The outlet of the microdialysis probe carrying dialysate was connected directly to the inlet of the injection valve of the LC system (8-port nanobore, electrically actuated, Valco Instruments, Houston, TX) so that the dialysate is loaded into one of the two 600 nL, fused-silica sample loops (75 μm I.D., 360 μm O.D, Polymicro Technologies, Phoenix, AZ). While one sample loop is being loaded, the contents of other sample loop are injected into the column, separated, then detected at the end of the column using amperometric electrochemical detection. The dialysate flow from the brain to the detector is uninterrupted, except for during injection valve switching (230 milliseconds), thus achieving in vivo, online detection of dopamine.
For the chromatography, 4.5 cm long, 150 μm ID fused silica capillary columns were packed in-house at 20,000 psi with 1.7 μm BEH C18 reverse-phase particles (Waters, Milford, MA). Mobile phase was delivered using a Shimadzu LC-30DA pump with a maximum pressure of 18,900 psi (130 MPa) to achieve a flow rate of 7.5 μL/min during experiments. Column and injector were heated to 40 °C.
Separation of dopamine was achieved using ion-pairing reversed phased liquid chromatography with mobile phase containing 100 mM sodium acetate, 1.75 mM SOS, 0.150 mM EDTA, 3% v/v acetonitrile and 2% v/v acetic acid. The mobile phase was filtered and degassed with three passes of vacuum filtration using 0.22 μm nylon filter (Osminics, Minnetonka, MN). Analytes were detected at 400 mV (vs. Ag/AgCl 3M NaCl) using BASi radial-style flowcell, 3-mm glassy carbon electrode with 25 μm thick gaskets, and BASi Epsilon potentiostat (West Lafayette, IN).
Experimental design
Microdialysis probes were implanted and dialysate dopamine was measured in awake, freely moving male Sprague-Dawley rats. Each probe was either perfused continuously for four days with aCSF containing dexamethasone (via retrodialysis) or aCSF alone (control). On day 1 and day 4 after surgery (approximately 24 h and 96 h, respectively), once per hour, each rat was stimulated with 10-min retrodialysis of 60 mM K+ and 100 mM K+ (twice each), and 15-min retrodialysis of μM nomifensine (at the end of the experiment, when possible). Stimulant/drug retrodialysis times were achieved by controlling the valve position (see Fig. S30).
Dopamine transients as well as basal level dopamine in the dialysate were measured continuously at one-minute time resolution for the entire duration of the experiment, approximately six hours. Data were processed using an automated MATLAB script. For the purpose of principal component analysis, the 11 contiguous peaks that deviate the most from basal level during the K+ stimulation window were identified as the transient by the script. All MATLAB-identified peaks were confirmed by a human.
LC-EC and field potential simultaneous measurement
In a second set of experiments dopamine and the field potential were measured simultaneously during potassium stimulations. For the field potential measurements, a tungsten wire (50 μm diameter, 4 mm length) was glued next to the microdialysis probe so that the wire was parallel to the probe membrane, with approximately 0.5mm between the wire and the membrane (see Figure S29). A second tungsten wire was used as a reference and placed in the contralateral hemisphere of the brain. Both wires were attached to a larger nickel/chromium wire for electrical connection and protected with a plastic covering. Measurement were made using a Powerlab/4sp running LabChart Pro (AD Instruments). A 0.1 Hz low pass filter was used.
Supplementary Material
Figure 8.
Fitting of nomifensine response to exponential decay model. Time constants (min−1) Rising = 0.52 Falling = 0.20 Avg. Amplitude = 121
Acknowledgments
Funding for this work was provided by National Institutes of Health under award number R01 MH104386 and a Graduate Research Fellowship from the National Science Foundation, DGE-1247842 (ELV). We also thank Dr. Ed Bouvier and Dr. Moon Chul Jung of Waters Corporation for the generous gift of packing materials, and Dr. Chi Leng Leong and Professor Martyn Boutelle for the equipment necessary for the field potential measurements.
Footnotes
Additional information as noted in text. Supporting figures S1–23 contain all dialysate DA concentration plots measured during experimental runs, with S1 also includes pre- and post-calibration measurements. Figures S24–26 show scatter plots of scores of relevant PCA analyses. Figure S27 is a PCA comparison of 100 mM transients, including averaged and synthesized average from PCA analysis, of day 1 vs day 4. Figure S28 has the plot and ANOVA result for nomifensine fitting. Figure S29 shows the construction of microdialysis and field potential probes. Figure S30 contains a schematic diagram of the experimental setup. Tables S1–2 contain ANOVA analysis and regression of basal dialysate dopamine concentration.
References
- 1.Tossman U, Ungerstedt U. Neuroleptic action of putative amino acid neurotransmitters in brain studied with a new technique of brain dialysis. Neurosci Lett Suppl. 1981;7:S479. [Google Scholar]
- 2.Justice JB., Jr Quantitative microdialysis of neurotransmitters. J Neurosci Methods. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- 3.Davies MI. A review of microdialysis sampling for pharmacokinetic applications. Analytica Chimica Acta. 1999;379:227–249. [Google Scholar]
- 4.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of Brain Microdialysis. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy RT. Emerging trends in in vivo neurochemical monitoring by microdialysis. Curr Opin Chem Biol. 2013;17:860–867. doi: 10.1016/j.cbpa.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tossman U, Wieloch T, Ungerstedt U. gamma-Aminobutyric acid and taurine release in the striatum of the rat during hypoglycemic coma, studied by microdialysis. Neurosci Lett. 1985;62:231–235. doi: 10.1016/0304-3940(85)90360-x. [DOI] [PubMed] [Google Scholar]
- 7.Kehr J. Determination of glutamate and aspartate in microdialysis samples by reversed-phase column liquid chromatography with fluorescence and electrochemical detection. J Chromatogr B Biomed Sci Appl. 1998;708:27–38. doi: 10.1016/s0378-4347(97)00677-4. [DOI] [PubMed] [Google Scholar]
- 8.Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514(Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wages SA, Church WH, Justice JB., Jr Sampling considerations for on-line microbore liquid chromatography of brain dialysate. Anal Chem. 1986;58:1649–1656. doi: 10.1021/ac00121a012. [DOI] [PubMed] [Google Scholar]
- 10.Saigusa T, Fusa K, Okutsu H, Koshikawa N. Monitoring of extracellular dopamine levels in the dorsal striatum and the nucleus accumbens with 5-minute on-line microdialysis in freely moving rats. J Oral Sci. 2001;43:129–134. doi: 10.2334/josnusd.43.129. [DOI] [PubMed] [Google Scholar]
- 11.Church WH, Justice JB., Jr On-line small-bore chromatography for neurochemical analysis in the brain. Adv Chromatogr. 1989;28:165–194. [PubMed] [Google Scholar]
- 12.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 13.Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: a review. Anal Chim Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tellez S, Forges N, Roussin A, Hernandez L. Coupling of microdialysis with capillary electrophoresis: a new approach to the study of drug transfer between two compartments of the body in freely moving rats. J Chromatogr B Biomed Sci Appl. 1992;581:257–266. doi: 10.1016/0378-4347(92)80279-y. [DOI] [PubMed] [Google Scholar]
- 15.Saylor RA, Lunte SM. A review of microdialysis coupled to microchip electrophoresis for monitoring biological events. J Chromatogr A. 2015;1382:48–64. doi: 10.1016/j.chroma.2014.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshitake T, Kehr J, Todoroki K, Nohta H, Yamaguchi M. Derivatization chemistries for determination of serotonin, norepinephrine and dopamine in brain microdialysis samples by liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006;20:267–281. doi: 10.1002/bmc.560. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitake T, Fujino K, Kehr J, Ishida J, Nohta H, Yamaguchi M. Simultaneous determination of norepinephrine, serotonin, and 5-hydroxyindole-3-acetic acid in microdialysis samples from rat brain by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal Biochem. 2003;312:125–133. doi: 10.1016/s0003-2697(02)00435-9. [DOI] [PubMed] [Google Scholar]
- 18.Hows ME, Lacroix L, Heidbreder C, Organ AJ, Shah AJ. High-performance liquid chromatography/tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples. J Neurosci Methods. 2004;138:123–132. doi: 10.1016/j.jneumeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Shackman HM, Shou M, Cellar NA, Watson CJ, Kennedy RT. Microdialysis coupled on-line to capillary liquid chromatography with tandem mass spectrometry for monitoring acetylcholine in vivo. J Neurosci Methods. 2007;159:86–92. doi: 10.1016/j.jneumeth.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 21.Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- 22.Reinhoud NJ, Brouwer HJ, van Heerwaarden LM, Korte-Bouws GA. Analysis of glutamate, GABA, noradrenaline, dopamine, serotonin, and metabolites using microbore UHPLC with electrochemical detection. ACS Chem Neurosci. 2013;4:888–894. doi: 10.1021/cn400044s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry M, Li Q, Kennedy RT. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal Chim Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM. Physiologically relevant changes in serotonin resolved by fast microdialysis. ACS Chem Neurosci. 2013;4:790–798. doi: 10.1021/cn400072f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Sampson MM, Senturk D, Andrews AM. Sex- and SERT-mediated differences in stimulated serotonin revealed by fast microdialysis. ACS Chem Neurosci. 2015;6:1487–1501. doi: 10.1021/acschemneuro.5b00132. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Liu Y, Jaquins-Gerstl A, Shu Z, Michael AC, Weber SG. Optimization for speed and sensitivity in capillary high performance liquid chromatography. The importance of column diameter in online monitoring of serotonin by microdialysis. J Chromatogr A. 2012;1251:54–62. doi: 10.1016/j.chroma.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Jaquins-Gerstl A, Nesbitt KM, Rutan SC, Michael AC, Weber SG. In vivo monitoring of serotonin in the striatum of freely moving rats with one minute temporal resolution by online microdialysis-capillary high-performance liquid chromatography at elevated temperature and pressure. Anal Chem. 2013;85:9889–9897. doi: 10.1021/ac4023605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu H, Varner EL, Groskreutz SR, Michael AC, Weber SG. In Vivo Monitoring of Dopamine by Microdialysis with 1 min Temporal Resolution Using Online Capillary Liquid Chromatography with Electrochemical Detection. Anal Chem. 2015;87:6088–6094. doi: 10.1021/acs.analchem.5b00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerink BHC, Hofsteede RM, Tuntler J, Vries JB. Use of Calcium Antagonism for the Characterization of Drug-Evoked Dopamine Release from the Brain of Conscious Rats Determined by Microdialysis. J Neurochem. 1989;52:722–729. doi: 10.1111/j.1471-4159.1989.tb02514.x. [DOI] [PubMed] [Google Scholar]
- 30.Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Nesbitt KM, Jaquins-Gerstl A, Skoda EM, Wipf P, Michael AC. Pharmacological mitigation of tissue damage during brain microdialysis. Anal Chem. 2013;85:8173–8179. doi: 10.1021/ac401201x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nesbitt KM, Varner EL, Jaquins-Gerstl A, Michael AC. Microdialysis in the Rat Striatum: Effects of 24 h Dexamethasone Retrodialysis on Evoked Dopamine Release and Penetration Injury. ACS Chemical Neuroscience. 2015;6:163–173. doi: 10.1021/cn500257x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varner EL, Jaquins-Gerstl A, Michael AC. Enhanced Intracranial Microdialysis by Reduction of Traumatic Penetration Injury at the Probe Track. ACS Chem Neurosci. 2016;7:728–736. doi: 10.1021/acschemneuro.5b00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripley TL, Jaworski J, Randall PK, Gonzales RA. Repeated perfusion with elevated potassium in in vivo microdialysis--A method for detecting small changes in extracellular dopamine. J Neurosci Methods. 1997;78:7–14. doi: 10.1016/s0165-0270(97)00129-5. [DOI] [PubMed] [Google Scholar]
- 35.Tang A, Bungay PM, Gonzales RA. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J Neurosci Methods. 2003;126:1–11. doi: 10.1016/s0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 36.Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Effect of dexamethasone on gliosis, ischemia, and dopamine extraction during microdialysis sampling in brain tissue. Anal Chem. 2011;83:7662–7667. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kametani H, Iijima S, Spangler EL, Ingram DK, Joseph JA. In vivo assessment of striatal dopamine release in the aged male Fischer 344 rat. Neurobiol Aging. 1995;16:639–646. doi: 10.1016/0197-4580(95)00047-i. [DOI] [PubMed] [Google Scholar]
- 38.Oyamada T, Hayashi T, Kagaya A, Yokota N, Yamawaki S. Effect of dantrolene on K(+)- and caffeine-induced dopamine release in rat striatum assessed by in vivo microdialysis. Neurochem Int. 1998;32:171–176. doi: 10.1016/s0197-0186(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 39.Kihara T, Ikeda M, Miyazaki H, Matsushita A. Influence of potassium concentration in microdialysis perfusate on basal and stimulated striatal dopamine release: effect of ceruletide, a cholecystokinin-related peptide. J Neurochem. 1993;61:1859–1864. doi: 10.1111/j.1471-4159.1993.tb09827.x. [DOI] [PubMed] [Google Scholar]
- 40.Cosford RJ, Parsons LH, Justice JB., Jr Effect of tetrodotoxin and potassium infusion on microdialysis extraction fraction and extracellular dopamine in the nucleus accumbens. Neurosci Lett. 1994;178:175–178. doi: 10.1016/0304-3940(94)90753-6. [DOI] [PubMed] [Google Scholar]
- 41.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 42.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pusic KM, Pusic AD, Kemme J, Kraig RP. Spreading depression requires microglia and is decreased by their M2a polarization from environmental enrichment. Glia. 2014;62:1176–1194. doi: 10.1002/glia.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, Csaszar E, Fekete R, West BL, Katona G, Rozsa B, Denes A. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levi G, Raiteri M. Carrier-mediated release of neurotransmitters. Trends Neurosci. 1993;16:415–419. doi: 10.1016/0166-2236(93)90010-j. [DOI] [PubMed] [Google Scholar]
- 46.Hurd YL, Ungerstedt U. Influence of a carrier transport process on in vivo release and metabolism of dopamine: dependence on extracellular Na+ Life Sci. 1989;45:283–293. doi: 10.1016/0024-3205(89)90137-9. [DOI] [PubMed] [Google Scholar]
- 47.Hauber W, Fuchs H. Dopamine release in the rat globus pallidus characterised by in vivo microdialysis. Behav Brain Res. 2000;111:39–44. doi: 10.1016/s0166-4328(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 48.Chen KC. Evidence on extracellular dopamine level in rat striatum: Implications for the validity of quantitative microdialysis. J Neurochem. 2005;92:46–58. doi: 10.1111/j.1471-4159.2004.02848.x. [DOI] [PubMed] [Google Scholar]
- 49.Peters JL, Michael AC. Modeling voltammetry and microdialysis of striatal extracellular dopamine: the impact of dopamine uptake on extraction and recovery ratios. J Neurochem. 1998;70:594–603. doi: 10.1046/j.1471-4159.1998.70020594.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Peters JL, Allen C, Chern S-S, Coalson RD, Michael AC. A Theoretical Description of Microdialysis with Mass Transport Coupled to Chemical Events. Anal Chem. 2000;72:2042–2049. doi: 10.1021/ac991186r. [DOI] [PubMed] [Google Scholar]
- 51.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brimblecombe KR, Cragg SJ. The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chemical Neuroscience. 2016 doi: 10.1021/acschemneuro.6b00333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.