Abstract
Background.
Despite an increasing number of O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) PET studies in supratentorial gliomas, studies regarding the usefulness of 18F-FET PET in brainstem and spinal cord gliomas to date remain scarce.
Methods.
Thirty-six 18F-FET PET scans were performed in 29 patients with brainstem (n = 29 scans) or spinal cord glioma (n = 7 scans). In 32 of 36 PET scans, a dynamic acquisition was performed. Fifteen scans in 15 patients were performed to assess newly diagnosed lesions, and 21 scans were obtained during follow-up: for diagnosing tumor progression (n = 15 scans in 14 patients) as well as for treatment monitoring (n = 6 scans in 3 patients). Four patients underwent additional serial scans (range, 1–2), and 3 of these 4 patients were examined for more than one indication. Maximum and mean tumor/brain ratios (TBRmax/mean) of 18F-FET uptake (20–40 min post injection) as well as kinetic 18F-FET uptake parameters were determined. Final diagnoses were confirmed histologically (54%) or by clinical follow-up (46%).
Results.
In all newly diagnosed high-grade (n = 3 patients) and in 5 of 11 patients with low-grade gliomas, 18F-FET uptake was increased (TBRmax ≥2.5 and/or TBRmean ≥1.9). In 2 patients with newly diagnosed gliomas without MR contrast enhancement, 18F-FET PET nevertheless showed increased metabolism. At suspected progression, the combination of TBRs with kinetic 18F-FET parameters correctly identified presence or absence of progressive disease in 9 of 11 patients (82%).
Conclusions.
This preliminary study suggests that 18F-FET PET adds valuable diagnostic information in brainstem and spinal cord glioma, particularly when the diagnostic information derived from MRI is equivocal.
Keywords: amino acid PET, brainstem glioma, dynamic acquisition, FET, infratentorial
Importance of the study
The brainstem and spinal cord can be affected by various pathologies, such as gliomas, demyelination, vascular lesions, and posttherapeutic effects, some of which may overlap clinico-radiologically, thereby constituting a diagnostic challenge to date. Although conventional MRI remains the standard neuroimaging method for diagnostic purposes as well as for the planning of stereotactic biopsies or neurosurgical resection, its lack of specificity imposes problems regarding treatment planning and patient counseling. O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) PET may help to overcome some of the diagnostic uncertainties of conventional MRI in suspected gliomas of the brainstem and spinal cord, which are rare in adults. Our data suggest that the metabolic information derived from 18F-FET PET may improve diagnostic procedures, thereby helping to avoid unnecessary invasive diagnostic procedures with the potential risk of inducing morbidity, and may thus facilitate patient counseling and treatment decisions, including the termination of potentially harmful treatments.
Brainstem gliomas are located in the midbrain, pons, or medulla oblongata. Furthermore, gliomas may occur in the spinal cord. In the pediatric population, brainstem gliomas account for about 20% of all brain tumors. In contrast, in adults, brainstem gliomas are rare and account for less than 5% of all gliomas.1,2
In 5%–10% of all spinal tumors in adults and approximately 35% in children, intramedullary spinal tumors can be diagnosed. About 90% of the tumors of the spinal cord are glial tumors, with most of these being ependymomas (~60%) or astrocytomas (~30%).3,4
Evaluation of the brainstem and spinal cord with PET is, in part, hampered by spatial resolution, with compromised sensitivity for the detection of small lesions. Up-to-date PET scanners with an improved resolution may overcome this problem, which has led to the fact that brainstem and spinal PET have recently gained clinical interest.
The wealth of studies using amino acid PET in supratentorial gliomas is contrasted by the dearth of data regarding brainstem and spinal cord gliomas. In this field, most experience has been gained with the PET tracer 11C-methyl-L-methionine (11C-MET).5–1211C-MET is an essential amino acid labeled with carbon-11, a positron-emitting isotope with a half-life of 20 minutes.13 This relatively short half-life limits the use of 11C-MET to PET centers with a cyclotron. More recently, novel amino acid tracers labeled with positron emitters with longer half-lives have been developed, providing better clinical reach, increased efficiency, and improved cost-effectiveness. Particularly, O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET), developed in the late 1990s, has become a well-established 18F-labeled amino acid for PET (half-life, 110 min) that provides logistic advantages for clinical practice compared with 11C-MET,14,15 while clinical results in brain tumors are comparable.16–18 Consequently, the use of 18F-FET has rapidly increased in western Europe during the last years and as the first country, Switzerland, has approved 18F-FET PET as a medical drug for brain imaging in 2014.19
To date, however, experience with 18F-FET and other amino acid PET tracers in patients with gliomas located in the brainstem and spinal cord remains limited and is based on single patients only.20 To the best of our knowledge, dynamic 18F-FET scans in a group of patients with gliomas located in the brainstem and spinal cord have not yet been reported.
Patients and Methods
Patients
Twenty-nine patients with brainstem and spinal cord glioma who had received between 2007 and 2015 dynamic 18F-FET PET scans (n = 32) at the Forschungszentrum Jülich, Germany, or static 18F-FET PET scans (n = 4) at the Department of Nuclear Medicine, University of Freiburg, Germany, were identified retrospectively (Supplementary Table 1). The local ethics committee approved the evaluation of retrospectively collected patient data. Patients had been referred for 18F-FET PET imaging for assessment of newly diagnosed cerebral lesions (n = 15 scans in 15 patients) (Supplementary Table 2), and for imaging during the course of the disease (n = 21 scans), for instance, for diagnosing tumor progression or recurrence (n = 15 scans in 14 patients) or for treatment monitoring (n = 6 scans in 3 patients) (Supplementary Tables 3, 4). Four patients underwent serial 18F-FET PET imaging (range of additional scans, 1–2), and 3 of these 4 patients were examined for more than one indication.
PET Imaging with 18F-FET
The amino acid 18F-FET was produced as described previously.15,21 According to the German guidelines for brain tumor imaging using labeled amino acid analogues, all patients fasted for at least 12 h before PET scanning.22 At the Research Center Jülich, dynamic PET studies were acquired up to 50 min after intravenous injection of 3 MBq of 18F-FET/kg of body weight on an ECAT EXACT HR+ scanner (CTI/Siemens Medical Systems) in 3-D mode (n = 22 patients) (axial field of view, 15.5 cm; image resolution, ~6 mm) or a hybrid 3T MR-PET scanner (n = 3 patients). The hybrid 3T MR-PET scanner used in the present study consisted of a 3T MR imaging system (Magnetom Tim Trio; Siemens Medical Systems) and a PET insert (BrainPET; Siemens Medical Systems).23–25 The BrainPET is a compact cylinder that fits in the bore of the Magnetom Trio MR scanner (axial field of view, 19.2 cm; optimum image resolution in the center of the field of view, ~3 mm).
The emission recording consisted of 16 time frames (time frames 1–5: 1 min, 6–10: 3 min, and 11–16: 5 min) covering the period up to 50 min post injection. After correction for random and scattered coincidences as well as dead time and attenuation, PET data were iteratively reconstructed.
At the Department of Nuclear Medicine, University of Freiburg, static PET scans (20–40 min post injection) were acquired on an ECAT 922/47 scanner (CTI/Siemens Medical Systems) (n = 2 patients) or on a PET/CT scanner (Gemini TF 64; Philips) (n = 2 patients). Images were iteratively reconstructed.
PET Data Analysis
18F-FET uptake in the tissue was expressed as standardized uptake value (SUV) by dividing the radioactivity concentration (kBq/mL) in the tissue by the radioactivity injected per gram of body weight. PET and MR images were co-registered using dedicated software (MPI tool v6.48, ATV). The fusion results were inspected and, if necessary, adapted based on anatomical landmarks. The regions of interest (ROI) analysis and the calculation of PET tumor volumes were based on the averaged PET data from 20–40 min post injection. The transaxial slices showing the highest tracer accumulation in the tumors were chosen for ROI analyses. The uptake in the unaffected brain tissue was determined by a larger ROI placed in an area of normal appearing supratentorial brain tissue including white and gray matter.22 The tumor area on 18F-FET PET scans was determined by a 3-D autocontouring process using a tumor–brain ratio ≥1.6 of 18F-FET uptake as cutoff. This cutoff is based on the results of a previous biopsy-controlled study in which the best lesion-to-brain ratio for differentiating tumor from peritumoral tissue was 1.6.26 When 18F-FET uptake in the lesions was similar to that in the normal brain tissue, a representative irregular ROI was placed manually on the area of signal abnormality in the T1- and T2-weighted transversal MRI scan and transferred to the coregistered 18F-FET PET scan in each case. Maximum and mean tumor–brain ratios (TBRmax, TBRmean) were calculated by dividing the maximum and mean SUVs of these tumor ROIs by the mean SUV of normal brain in the PET scan.
Furthermore, time-activity curves (TACs) of 18F-FET uptake in the tumor were generated by the application of a spherical volume-of-interest with a volume of 2 mL centered on maximal tumor uptake to the entire dynamic dataset.27,28 TAC of the brain tissue was generated by a reference ROI in the unaffected brain tissue (as described above). Time-to-peak (TTP; time in minutes from the beginning of the dynamic acquisition up to the maximum SUV of the lesion) was determined. As described previously,27–29 TACs of each lesion were assigned to one of the following curve patterns: (i) constantly increasing 18F-FET uptake without identifiable peak uptake during data acquisition; (ii) 18F-FET uptake peaking at a midway point (between 20 and 40 min) followed by a plateau; and (iii) 18F-FET uptake peaking early (≤20 min) followed by a constant descent.
Accuracy of 18F-FET PET in Newly Diagnosed Brainstem/Spinal Cord Lesions
Based on a previous study in a large series of untreated patients with suspected cerebral glioma on MR imaging, neoplastic tissue on 18F-FET PET scans was assumed when the TBRmax was ≥2.5 and/or the TBRmean was ≥1.9.30 For the evaluation of diagnostic accuracy of 18F-FET PET in newly diagnosed lesions, the histological diagnosis was used as reference. All tumors were histologically classified according to the World Health Organization (WHO) classification of tumors of the central nervous system.31
Accuracy of 18F-FET PET for Diagnosing Tumor Progression or Recurrence
Based on a previous study investigating the potential of 18F-FET PET to differentiate tumor recurrence or progression from treatment-induced changes in a large series of pretreated brain tumors,28 tumor progression or recurrence as evaluated by 18F-FET PET was assumed when a TBRmax ≥2.3 or a TBRmean ≥2.0 in combination with a curve pattern 2 or 3 was present. For the evaluation of diagnostic accuracy of 18F-FET PET for diagnosing tumor progression or recurrence, the histological diagnosis was used as reference (n = 4 patients). If histology was not available, diagnosis was based on the follow-up (ie, clinical course and results of follow-up MRI) (n = 10 patients). Absence of tumor progression or recurrence was assumed when the lesions showed spontaneous shrinkage or remained stable in size on contrast-enhanced MRI, and/or neurological deficits remained unchanged, ie, no new neurological symptoms occurred during follow-up (median follow-up, 11 mo; range, 8–29 mo). Presence of tumor progression or recurrence was assumed when clinical worsening prompted a change in treatment or palliative care had been initiated during follow-up/death occurred (median follow-up, 3 mo; range, 1–5 mo).
MR Imaging
All patients underwent routine MRI (1.5T or 3T) with standard coils before and after administration of a gadolinium-based contrast agent (T1- and T2-weighted and fluid attenuated inversion recovery [FLAIR] sequence). Diagnosis of tumor progression or recurrence was based on Response Assessment in Neuro-Oncology criteria.32
Statistical Analysis
Descriptive statistics are provided as mean and standard deviation and/or median and range. To compare 2 different groups, the Student t-test for independent samples was used. The Mann–Whitney rank sum test was used when variables were not normally distributed. P-values of .05 or less were considered significant. Statistical analyses were performed using SigmaPlot software (SigmaPlot v11.0, Systat Software) and SPSS Statistics software (Release 23.0.0).
Results
Patients with Newly Diagnosed Brainstem/Spinal Cord Lesions
In the 15 patients (mean age, 34 ± 16 y; range, 9–67 y) with newly diagnosed brainstem or spinal cord lesions, a total of 15 PET scans were performed (Supplementary Tables 1, 2). Diagnoses were distributed as follows (Supplementary Table 2): WHO grade I ganglioglioma (n =1), WHO grade I pilocytic astrocytoma (n =1), WHO grade II diffuse astrocytoma (n =6), WHO grade III anaplastic astrocytoma (n =2), WHO grade IV glioblastoma (n =1), low-grade glial tumor, WHO grade histologically not specified (n =3), and hyperintense MRI lesions on T2/FLAIR-weighted images without histological confirmation (n =1). Histology of the latter brainstem lesion could not be obtained due to the patient’s refusal (patient ID #24). In the other patients, histology was obtained by stereotactic biopsy (n =12) or neurosurgical resection (n =2).
18F-FET Uptake and Contrast Enhancement on MRI in Newly Diagnosed Brainstem/Spinal Cord Lesions
At initial diagnosis, in 8 of 14 histologically confirmed gliomas located in the brainstem or spinal cord, an increased 18F-FET uptake (57%) could be observed. All high-grade gliomas of WHO grade III (n = 2) (Fig. 1) and WHO grade IV (n = 1) as well as 5 of 11 low-grade gliomas exhibited a TBRmax ≥2.5 and/or a TBRmean ≥1.9 (Supplementary Tables 1, 2). In contrast, in 3 patients with a WHO grade II diffuse astrocytoma, in 2 patients with low-grade glial tumor in which the WHO grade could not be histologically specified, and in a patient with WHO grade I ganglioglioma, 18F-FET uptake was below these thresholds. Nevertheless, 3 of these 6 tumors showed 18F-FET uptake above background with a TBRmax ≥1.6 (range TBRmax, 1.6–2.0). The 18F-FET uptake in low-grade gliomas (median TBRmax, 2.2; range, 1.0–3.9; median TBRmean, 1.8; range, 0.7–2.6) was not significantly different compared with high-grade gliomas (median TBRmax, 3.0; range, 2.4–3.5; median TBRmean, 1.9; range, 1.9–2.1) (P > 0.05). In the patient (ID #24) without histological diagnosis of the brainstem lesion, no 18F-FET uptake could be observed. Static and dynamic data of 18F-FET uptake of each lesion are presented in Supplementary Table 2. Furthermore, in 2 patients with newly diagnosed gliomas without contrast enhancement on MRI, 18F-FET PET showed increased metabolism (TBRmax ≥2.5 and/or a TBRmean ≥1.9). 18F-FET uptake in non-enhancing gliomas (median TBRmax, 2.0; range, 1.2–3.3; median TBRmean, 1.7; range, 0.9–2.3) showed no significant difference compared with enhancing gliomas (median TBRmax, 2.4; range, 1.0–3.9; median TBRmean, 1.9; range, 0.7–2.6) (P > 0.05). In 2 patients with newly diagnosed glioma and contrast enhancement with a maximal diameter <5 mm, no FET uptake could be observed (Fig. 2) (Supplementary Table 2).
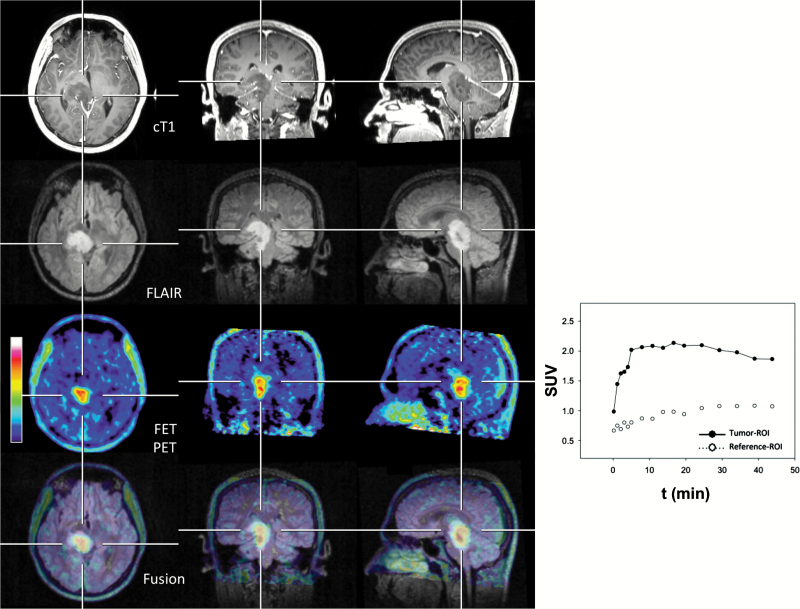
Fig. 1.
Twenty-five-year-old patient (patient ID #1) with a newly diagnosed anaplastic astrocytoma (WHO grade III) of the brainstem. The MRI shows only minimal contrast enhancement, whereas the increased 18F-FET uptake (TBRmax, 3.5) is located predominantly in spatial correspondence to the alterations of the FLAIR-weighted image (second row, bottom row). The time-activity curve shows an early 18F-FET uptake (17 min) followed by a constant descent.
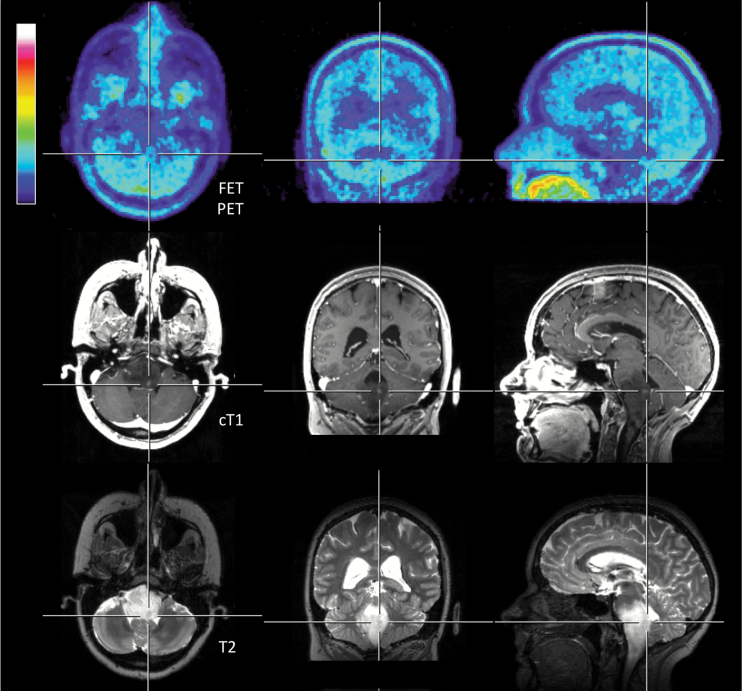
Fig. 2.
Thirty-six-year-old patient (patient ID #13) with a newly diagnosed diffuse astrocytoma (WHO grade II) of the brainstem. The lesion size of contrast enhancement is small (maximal diameter <5 mm) and the T2 signal alteration is considerably larger. However, no increased 18F-FET uptake can be observed.
Patients with Follow-up PET Imaging During the Course of the Disease
During follow-up, 15 patients (mean age, 46 ± 20 years; range, 7–73 years) underwent a total of 21 PET scans (Supplementary Table 3, 4). Four patients underwent serial 18F-FET PET imaging (range of additional scans, 1–2), and 3 of these 4 patients were examined for more than one indication. The histologically confirmed initial diagnoses in this group of patients were distributed as follows: WHO grade I pilocytic astrocytoma (n = 2), WHO grade II diffuse astrocytoma (n = 4), WHO grade II ependymoma (n = 1), WHO grade III secondary anaplastic astrocytoma after malignant progression (n = 1), WHO grade IV secondary glioblastoma after malignant progression (n = 3), WHO grade IV primary glioblastoma (n = 2), and contrast-enhancing or hyperintense MRI lesions on T2-/FLAIR-weighted images without histological confirmation due to the patient’s refusal (n = 2) (Fig. 3).
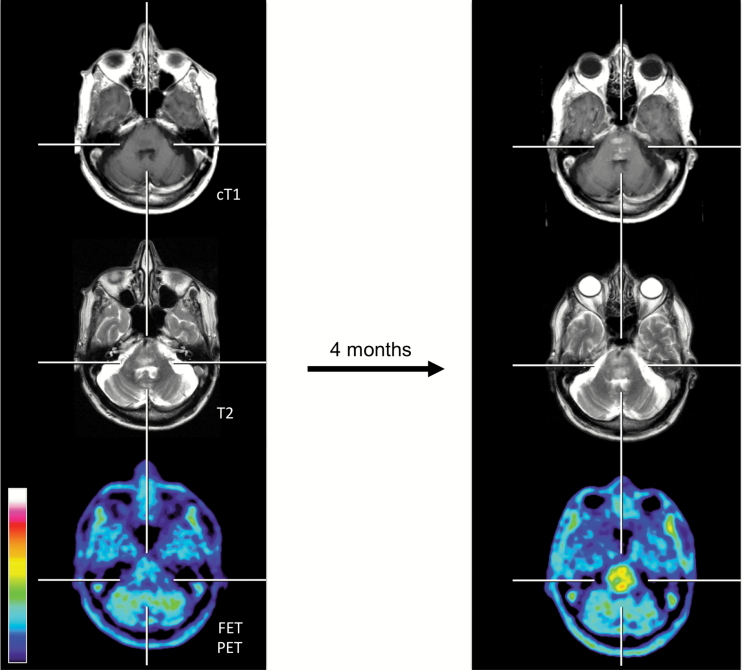
Fig. 3.
Seventy-three-year-old patient (patient ID #24) with a rapid progressive tumor of the brainstem (histology of this brainstem lesion could not be obtained due to the patient’s refusal). In correspondence to the clinical deterioration (ie, dysphagia, dysarthria) within 4 months, a contrast-enhancing lesion, a progression of the T2 signal, and an increase of the metabolic activity (TBRmax baseline, 1.6; TBRmax follow-up, 3.2) compared with baseline imaging (left column) are illustrated.
18F-FET PET for Diagnosing Tumor Progression or Recurrence
In patients in whom histological diagnosis confirmed tumor progression or recurrence (n = 4 patients), all corresponding 18F-FET PET scans (n = 4) showed increased uptake (TBRmax, 3.6 ± 0.8; range of TBRmax, 2.7–4.7; TBRmean, 2.7 ± 0.5; range of TBRmean, 2.0–3.1). Furthermore, when diagnosis was based on the follow-up, TBRs were significantly higher in patients with tumor progression or recurrence (n = 5 PET scans in 4 patients) than in nonprogressors (n = 4 PET scans in 4 patients) (TBRmax, 2.7 ± 0.7 vs 0.4 ± 0.8, P = .002; TBRmean, 1.9 ± 0.5 vs 0.3 ± 0.7, P = .004). However, 2 patients were lost to follow-up. Furthermore, all patients with confirmed tumor progression or recurrence showed a TBRmax ≥2.3 or a TBRmean ≥2.0. Using a threshold of TBRmax >2.3 or a TBRmean >2.0 in combination with presence of a curve pattern 2 or 3,28 presence or absence of tumor progression or recurrence was diagnosed correctly in 82% (9 of 11 patients; sensitivity, 71%; specificity, 100%). Static and dynamic data on 18F-FET uptake of each lesion are presented in Supplementary Table 3.
18F-FET PET for Treatment Monitoring
In a subgroup of patients (n = 3), serial 18F-FET PET imaging (at baseline and 2 mo later) was used to monitor chemotherapy effects of adjuvant temozolomide (5/28) in 2 patients with WHO grade IV glioblastoma33 of the brainstem (patient ID #15, #20) and of temozolomide in combination with bevacizumab in a patient with a WHO grade II diffuse astrocytoma of the spinal cord (patient ID #19) (Supplementary Table 4). Compared with baseline PET, changes of the TBRmax revealed a greater variability (range of TBRmax changes between −11% and 26%) than the TBRmean (range of TBRmean changes between −4% and 5%). After treatment, no changes of the TBRmean were observed,34 the metabolic activity remained largely unchanged. Clinically, this finding was associated with a stable clinical course for at least 6 months.
Discussion
In contrast to numerous studies that have used amino acid PET to image supratentorial brain tumors, reports using this technique to image brainstem gliomas and tumors of the spinal cord remain scarce, particularly using the tracer 18F-FET. The results of the present study suggest that 18F-FET PET may be helpful to detect metabolically active tumor in both the brainstem and in the spinal cord (Fig. 1, 3, 4) and may add valuable information to standard MRI for clinical decision making both at primary diagnosis and in the further course of the disease.
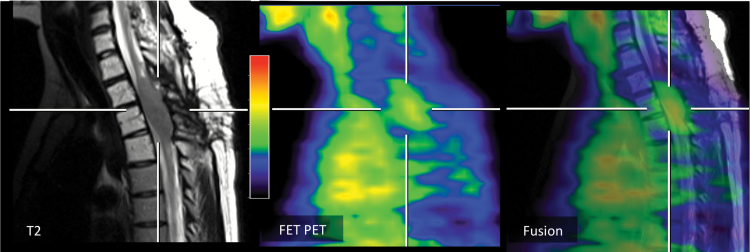
Fig. 4.
Twenty-five-year-old patient (patient ID #19) with a progressive diffuse astrocytoma (WHO grade II) of the thoracic spinal cord. In spatial correspondence to the MRI signal alterations (fusion image, right), the 18F-FET PET shows an increased metabolic activity (TBRmax, 2.7).
Since the number of patients in this study is too small to perform a receiver-operating-characteristic curve analysis, the cutoff values to determine the sensitivity of 18F-FET PET were based on threshold values determined in previous studies in larger series of brain tumors in the entire brain.28,30 This approach may not be appropriate, and the sensitivity of 18F-FET PET might be underestimated. Using a TBRmax threshold of 2.5, the sensitivity of 18F-FET PET for the detection of neoplastic tissue in the brainstem and spinal cord in newly diagnosed tumors achieved a sensitivity of 57 %, which is in line with previous results in brain tumors of the entire brain.30 Due to the lack of nonneoplastic lesions, the calculation of specificity was not possible in the present dataset. In low-grade gliomas, only 5 of 11 tumors exceeded the TBRmax threshold of 2.5. In contrast, in one of the largest datasets, with 136 low-grade gliomas of the entire brain, the sensitivity of 18F-FET PET was considerably higher (74%).35 However, in that study 18F-FET PET was evaluated only visually.36
Interestingly, in 2 newly diagnosed gliomas of the brainstem without contrast enhancement on MRI, 18F-FET PET showed increased metabolic activity, providing important additional diagnostic information for further clinical decision making, (ie, planning of stereotactic biopsy and definition of the target for biopsy). Accordingly, Massager and colleagues11 reported in a series of adult patients with predominantly brainstem mass lesions that in some cases the biopsy target was more accurately defined using PET and 11C-MET PET than MR imaging and that PET-guided stereotactic biopsy increased the diagnostic yield. Using 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG) PET as well as 11C-MET PET, similar findings have been reported in children with infiltrative brainstem lesions,8 resulting in an improved survival.9,12 However, the applicability of 11C-MET PET is restricted to centers with an on-site cyclotron unit, while the evaluation of 18F-FDG PET uptake of the brainstem may be impaired due to high physiological uptake of other infratentorial structures, such as gray matter of the cerebellum.13,37
Furthermore, our findings in pretreated patients with gliomas of the brainstem or the spinal cord suggest that static and dynamic imaging parameters derived from 18F-FET PET may be helpful for the diagnosis of tumor progression or recurrence. Using TBRs in combination with curve patterns of 18F-FET uptake, recurrent or progressive disease was identified correctly in 82%. Similar diagnostic performance was observed in previous studies in patients with high-grade glioma.27,28
Compared with the number of reports in patients with brainstem gliomas evaluated using amino acid PET, the number of patients with spinal cord gliomas examined with this technique is rather small5–7,38 and thus far predominantly focused on ependymomas. Again, primarily 11C-MET PET as well as 18F-FDG PET as non-amino acid PET tracers were used in these reports. In these studies, both 18F-FDG and 11C-MET accumulated to a large degree in ependymomas as well as in astrocytic gliomas. Correspondingly, in the present study we provide initial experience with 18F-FET PET in patients with both ependymoma and low-grade glioma histology of the spinal cord with a similar degree of tracer uptake.
Regarding treatment monitoring, in pretreated patients with malignant brainstem glioma, Reithmeier and coworkers20 demonstrated treatment response evaluation to anti-angiogenic therapy with bevacizumab using 18F-FET PET. Compared with the baseline scan, all patients of that case series (n = 3) revealed a decrease of metabolic activity of 25% or more. In our patients, however, no clear decrease of metabolic activity to the treatment as indicated by changes of the TBRmean34 could be observed. This finding was associated with a stable clinical course for at least 6 months, suggesting disease control without further tumor growth, but the dataset is too small to draw any conclusions.
There are limitations to the present study. It should be considered that particularly in infratentorial lesions smaller than 5 mm diameter, the evaluation of the brainstem and spinal cord with PET is likely to be compromised by the spatial resolution of the scanner. That is, sensitivity is likely to be reduced for the detection of hypermetabolic lesions smaller in size than the scanner spatial resolution, usually 5–6 mm. Furthermore, due to the retrospective study setting, only a few gliomas of the spinal cord were identified and other intramedullary tumors such as lymphomas, oligodendrogliomas, and subependymomas are not available in this series. Although this is the first study exploring the usefulness of static and dynamic 18F-FET PET in a series of patients with gliomas of the brainstem or the spinal cord, the number of patients in the different subgroups is small and the results should be considered with caution. Nevertheless, this study includes to date the largest sample of patients with this tumor localization studied using static and dynamic 18F-FET PET. The results are promising and further investigations in this field are recommendable.
With these caveats in mind, we recommend the use 18F-FET PET as an additional diagnostic tool in this group of patients, particularly when the diagnostic information derived from standard MR imaging is equivocal. Further investigation in a larger number of patients is warranted to determine the value of 18F-FET PET in newly diagnosed infratentorial brain lesions (ie, differential diagnosis of neoplastic from nonneoplastic lesions), for diagnosing tumor progression or recurrence, and for the evaluation of treatment response.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank Suzanne Schaden, Elisabeth Theelen, Silke Frensch, Kornelia Frey, and Lutz Tellmann for assistance in the patient studies; and Silke Grafmüller, Erika Wabbals, and Sascha Rehbein for radiosynthesis of 18F-FET.
Conflict of interest statement. The authors disclosed no potential conflicts of interest.
References
- 1. Purohit B, Kamli AA, Kollias SS. Imaging of adult brainstem gliomas. Eur J Radiol. 2015;84(4):709–720. [DOI] [PubMed] [Google Scholar]
- 2. Grimm SA, Chamberlain MC. Brainstem glioma: a review. Curr Neurol Neurosci Rep. 2013;13(5):346. [DOI] [PubMed] [Google Scholar]
- 3. Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11(3):320–328. [DOI] [PubMed] [Google Scholar]
- 4. Parsa AT, Chi JH, Acosta FL, Jr, et al. Intramedullary spinal cord tumors: molecular insights and surgical innovation. Clin Neurosurg. 2005;52:76–84. [PubMed] [Google Scholar]
- 5. Tomura N, Ito Y, Matsuoka H, et al. PET findings of intramedullary tumors of the spinal cord using [18F] FDG and [11C] methionine. AJNR Am J Neuroradiol. 2013;34(6):1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sasajima T, Mineura K, Itoh Y, et al. Spinal cord ependymoma: a positron emission tomographic study with (11C-methyl)-L-methionine. Neuroradiology. 1996;38(1):53–55. [DOI] [PubMed] [Google Scholar]
- 7. Higano S, Shishido F, Nagashima M, et al. PET evaluation of spinal cord tumor using 11C-methionine. J Comput Assist Tomogr. 1990;14(2):297–299. [DOI] [PubMed] [Google Scholar]
- 8. Pirotte BJ, Lubansu A, Massager N, et al. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. J Neurosurg. 2007;107(5 Suppl):392–399. [DOI] [PubMed] [Google Scholar]
- 9. Yamaguchi S, Terasaka S, Kobayashi H, et al. Indolent dorsal midbrain tumor: new findings based on positron emission tomography. J Neurosurg Pediatr. 2009;3(4):270–275. [DOI] [PubMed] [Google Scholar]
- 10. Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46(12):1948–1958. [PubMed] [Google Scholar]
- 11. Massager N, David P, Goldman S, et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg. 2000;93(6):951–957. [DOI] [PubMed] [Google Scholar]
- 12. Rosenfeld A, Etzl M, Bandy D, et al. Use of positron emission tomography in the evaluation of diffuse intrinsic brainstem gliomas in children. J Pediatr Hematol Oncol. 2011;33(5):369–373. [DOI] [PubMed] [Google Scholar]
- 13. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view - What is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langen KJ, Hamacher K, Weckesser M, et al. O-(2-[18F]fluoroethyl)-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol. 2006;33(3):287–294. [DOI] [PubMed] [Google Scholar]
- 15. Wester HJ, Herz M, Weber W, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40(1):205–212. [PubMed] [Google Scholar]
- 16. Langen KJ, Jarosch M, Mühlensiepen H, et al. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl Med Biol. 2003;30(5):501–508. [DOI] [PubMed] [Google Scholar]
- 17. Grosu AL, Astner ST, Riedel E, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–1058. [DOI] [PubMed] [Google Scholar]
- 18. Weber WA, Wester HJ, Grosu AL, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27(5):542–549. [DOI] [PubMed] [Google Scholar]
- 19. Swissmedic. Swiss agency for therapeutic products. Swissmedic Journal. 2014; 13(7):651. [Google Scholar]
- 20. Reithmeier T, Lopez WO, Spehl TS, et al. Bevacizumab as salvage therapy for progressive brain stem gliomas. Clin Neurol Neurosurg. 2013;115(2):165–169. [DOI] [PubMed] [Google Scholar]
- 21. Hamacher K, Coenen HH. Efficient routine production of the 18F-labelled amino acid O-2-18F fluoroethyl-L-tyrosine. Appl Radiat Isot. 2002;57(6):853–856. [DOI] [PubMed] [Google Scholar]
- 22. Langen KJ, Bartenstein P, Boecker H, et al. [German guidelines for brain tumour imaging by PET and SPECT using labelled amino acids]. Nuklearmedizin. 2011;50(4):167–173. [DOI] [PubMed] [Google Scholar]
- 23. Shah NJ, Oros-Peusquens AM, Arrubla J, et al. Advances in multimodal neuroimaging: hybrid MR-PET and MR-PET-EEG at 3 T and 9.4 T. J Magn Reson. 2013;229:101–115. [DOI] [PubMed] [Google Scholar]
- 24. Neuner I, Kaffanke JB, Langen KJ, et al. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur Radiol. 2012;22(12):2568–2580. [DOI] [PubMed] [Google Scholar]
- 25. Herzog H, Langen KJ, Weirich C, et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin. 2011;50(2):74–82. [DOI] [PubMed] [Google Scholar]
- 26. Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(Pt 3):678–687. [DOI] [PubMed] [Google Scholar]
- 27. Galldiks N, Dunkl V, Stoffels G, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. [DOI] [PubMed] [Google Scholar]
- 28. Galldiks N, Stoffels G, Filss C, et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calcagni ML, Galli G, Giordano A, et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin Nucl Med. 2011;36(10):841–847. [DOI] [PubMed] [Google Scholar]
- 30. Rapp M, Heinzel A, Galldiks N, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med. 2013;54(2):229–235. [DOI] [PubMed] [Google Scholar]
- 31. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 33. Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 34. Galldiks N, Langen KJ, Holy R, et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 35. Hutterer M, Nowosielski M, Putzer D, et al. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 2013;15(3):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langen KJ, Galldiks N. Reply to “[18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma” by Hutterer et al. Neuro Oncol. 2013;15(7):816–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am. 2002;12(4):615–626. [DOI] [PubMed] [Google Scholar]
- 38. Wilmshurst JM, Barrington SF, Pritchard D, et al. Positron emission tomography in imaging spinal cord tumors. J Child Neurol. 2000;15(7):465–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.