Abstract
Background.
Mibefradil (MIB), previously approved for treatment of hypertension, is a selective T-type calcium channel blocker with preclinical activity in high-grade gliomas (HGGs). To exploit its presumed mechanism of impacting cell cycle activity (G1 arrest), we designed a phase I study to determine safety and the maximum tolerated dose (MTD) of MIB when given sequentially with temozolomide (TMZ) in recurrent (r)HGG.
Methods.
Adult patients with rHGG ≥3 months from TMZ for initial therapy received MIB in 4 daily doses (q.i.d.) for 7 days followed by standard TMZ at 150–200 mg/m2 for 5 days per 28-day cycle. MIB dose escalation followed a modified 3 + 3 design, with an extension cohort of 10 patients at MTD who underwent 3’-deoxy-3’-18F-fluorothymidine (18F-FLT) PET imaging, to image proliferation before and after 7 days of MIB.
Results.
Twenty-seven patients were enrolled (20 World Health Organization grade IV, 7 grade III; median age 50 y; median KPS 90). The MTD of MIB was 87.5 mg p.o. q.i.d. Dose-limiting toxicities were elevation of alanine aminotransferase/aspartate aminotransferase (grade 3) and sinus bradycardia. The steady-state maximum plasma concentration of MIB at the MTD was 1693 ± 287 ng/mL (mean ± SD). 18F-FLT PET imaging showed a significant decline in standardized uptake value (SUV) signal in 2 of 10 patients after 7 days of treatment with MIB.
Conclusions.
MIB followed by TMZ was well tolerated in rHGG patients at the MTD. The lack of toxicity and presence of some responses in this selected patient population suggest that this regimen warrants further investigation.
Keywords: anaplastic glioma, glioblastoma, mibefradil, temozolomide, timed sequential therapy
Importance of the study
T-type calcium channel blockers are a promising class of antineoplastic drugs that inhibit calcium influx into tumor cells. Mibefradil, previously approved for treatment of hypertension, has preclinical activity in glioblastomas with ability to induce cell cycle arrest at G1/S. ABTC1101 was the first clinical study to use MIB as an anticancer drug in patients. This study determined the MTD of MIB, administered q.i.d. for 7 days and sequentially with TMZ. MIB showed convincing safety in patients with high-grade gliomas, and several responses to treatment with MIB followed by TMZ were observed. Exploratory 18F-FLT PET imaging, intended to assess proliferation, performed at baseline and after 7 days of MIB, was feasible in a multicenter setting and showed a significant decrease in the SUV signal in 2 of 10 patients.
High-grade gliomas (HGGs) are the most common primary brain cancers in adults.1 Despite recent advances in treatment of these diseases, they are still incurable and virtually all patients eventually die of their disease. New treatments and therapeutic concepts are urgently needed. There has been consideration that cell cycle arrest may potentiate the effect of chemotherapy in the treatment of cancers. This concept, also called timed sequential therapy, has been explored preclinically as well as in clinical trials.2–6 The basic concept of timed sequential therapy is to arrest cell cycle activity at the G1/S checkpoint of the cell cycle and to then simultaneously release cells into S phase, rendering them more vulnerable to cytotoxic therapy.
Mibefradil (MIB), a selective inhibitor of the T-type calcium channel Cav3, has preclinical evidence that it can interfere with cell cycle activity and sensitize tumor cells to chemotherapy, including in gliomas.5–9 The T-type calcium channel Cav3 is predominantly involved in calcium influx in most solid cancers, including in glioblastoma.7,10,11 It is ubiquitously expressed in fetal tissues but is downregulated in most adult tissues except in pathological conditions.10,12 Preclinical and clinical data demonstrate that MIB can cross the blood–brain barrier, which is a prerequisite for further development of this drug as an anticancer agent in humans13 (R. Bindra, personal communication).
MIB had previously received FDA approval for the treatment of hypertension and angina pectoris.14,15 Hence, the pharmacokinetics and side effects of this drug have been well studied. The clinical use of MIB for hypertension was eventually discontinued due to significant drug interactions, limiting the use of this drug in a larger patient population.15
This is the first study in humans to use MIB as an anticancer drug. Our aim was to determine safety and tolerability of the drug in sequential administration with standard temozolomide (TMZ) and 4-times-daily (q.i.d.) dosing and to define the maximum tolerated dose (MTD) for further clinical development. Historically, MIB was administered once daily when approved for use as an antihypertensive agent. Pharmacokinetic modeling and a pharmacokinetic healthy volunteer study showed that administering the drug q.i.d. rather than once daily enhances systemic exposure to near the maximum concentrations of MIB.
We performed 3’-deoxy-3’-18F-fluorothymidine (18F-FLT) PET imaging as an exploratory correlative imaging marker on patients at the MTD level, hypothesizing a potential correlation between the 18F-FLT PET signal within the tumor as a surrogate for cell cycle activity. 18F-FLT is a radiolabeled structural analog of thymidine that has been investigated for assessing cellular proliferation. The compound is phosphorylated by human thymidine kinase 1. The 3ʹ substitution, however, prevents incorporation into the replicating DNA, and the resulting phospho-FLT is trapped inside the cells. Due to its mechanism and potential dependence on cell cycle function, we chose 18F-FLT PET imaging as an exploratory correlative marker for this trial to assess MIB’s potential to induce cell cycle arrest.
Patients and Methods
This was an open label, multi-institutional phase Ib study, sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute (NCI) and conducted within the Adult Brain Tumor Consortium (ABTC). The protocol was approved by the respective institutional review boards of all participating institutions. Patients provided written informed consent prior to participating in this study. Eligibility criteria included: age ≥18 years, histologically proven HGG (World Health Organization [WHO] grade III or IV) that was progressive or recurrent following standard upfront radiation and TMZ. Patients needed evidence of measurable contrast-enhancing disease. Patients must have had an interval of at least 3 months after completion of their most recent therapy, including after the most recent TMZ treatment. No prior cytotoxic therapies other than TMZ and carmustine wafers were allowed. Additional requirements included an absolute neutrophil count of ≥1500/μL; platelet count of ≥100000/μL; bilirubin and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) of ≤3 times the upper institutional limit; creatinine clearance of ≥50 mL/min for subjects with creatinine levels above the institutional normal; and Karnofsky performance status (KPS) ≥60%.
Treatment Plan
This study consisted of 2 parts: (i) dose escalation of MIB in timed sequential administration with TMZ, (ii) dose expansion cohort with 10 patients at the MTD. Patients received MIB in q.i.d. dosing for a total of 7 days. After a 24-hour rest period, this was followed by standard 5-day treatment with TMZ at 150–200 mg/m2 per 28-day cycle (see Study Schema in the Supplementary materials, Supplement 1). The 24-hour rest period was chosen to allow for MIB washout prior to starting cytotoxic therapy with TMZ and to allow cells to reenter the cell cycle. Treatment was continued until unacceptable toxicities or disease progression, and patients had the option to discontinue treatment for any reason. MIB, provided by Cavion, was administered orally at predefined dose levels. Dose escalation of MIB followed a 3 + 3 design with dose levels of 25, 50, 75, 87.5, and 100 mg q.i.d. To assess for possible cardiac toxicity, all patients underwent continuous cardiac rhythm monitoring for the first 7 days of treatment with MIB. An additional 10 patients were enrolled at the MTD (dose expansion). These patients underwent 18F-FLT PET imaging as an exploratory quantitative imaging assessment prior to taking MIB (2 separate scans were performed as double baseline) and on day 7 of MIB. Treatment was then continued in the same fashion as during the dose escalation until disease progression or until unacceptable toxicities were observed. Standard contrast-enhanced MR imaging was performed every 2 cycles.
Response Assessment
Response assessment was performed on MRI using the Response Assessment in Neuro-Oncology (RANO) criteria.16 For patients with a reported partial or complete response, central review was performed by the ABTC central imaging core.
Pharmacokinetics
To facilitate pharmacokinetic sampling on an outpatient basis, patients were instructed to take the 4 daily doses of MIB on a 7:00 am, 12:00 pm, 5:00 pm, and 10:00 pm schedule, with the first dose taken at 5:00 pm on day 1. Pharmacokinetic sampling was performed for the 12:00 pm dose taken on days 2, 5, and 8 during the first cycle of therapy. On each of these days, blood samples were collected within 5 min prior to dosing and at 0.25, 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 h after dosing, with the last sample collected immediately before taking the 5:00 pm dose. Two additional samples were collected approximately 24 h and 48 h after the final dose on day 8 was taken. At each time point, 5 mL of peripheral venous blood was collected in tubes containing freeze-dried sodium heparin, which were placed on wet ice until centrifuged (1300 g, 10 min, 4°C) within 15 min. The plasma was removed and stored in cryovials maintained at −70°C or lower until assayed.
The concentration of MIB and its alcohol metabolite (methylazoxymethanol [MAM]) in the plasma samples were concurrently determined by reversed-phase high performance liquid chromatography with tandem mass spectrometric detection. The analytical method was extensively validated and applied to the analysis of study samples as recommended by the FDA Guidance for Industry: Bioanalytical Method Validation, May 2001 (http://www.fda.gov.cder/-guidance/index.htm). Briefly, plasma samples were prepared for analysis by precipitating proteins with acetonitrile after addition of NNC-55-0396 dihydrochloride (Tocris BioScience), a close structural analogue of MIB used as the internal standard. An aliquot of the supernatant afforded by centrifugation was injected directly onto a Phenomenex Luna 5 µm C8(2) high performance liquid chromatography column (150 mm × 4.6 mm i.d.) and separated by gradient elution using a mobile phase composed of acetonitrile and 25 mM ammonium formate. A triple quadrupole mass spectrometer with an electrospray ionization interface was operated in the selected-ion monitoring mode to detect the m/z 496.3→202.1, m/z 424.3→159.1, and m/z 492.3→159.1 transitions for MIB, MAM, and the internal standard, respectively. Calibration curves show excellent linearity at free base equivalent concentrations ranging from 0.21 to 84.2 ng/mL for MIB and 0.16 to 63.8 ng/mL for MAM. Samples with concentrations of either analyte that exceeded the upper range of the calibration curve were reassayed upon appropriate dilution with blank human plasma. At the lowest concentration included in the calibration curves, interday accuracy for both analytes was within 5.1% of the nominal concentration and the precision was ≤9.6%. The interday accuracy range was 98.6%–101.7% and the precision range was 1.9%–4.7% for all other calibration standards.
The plasma concentration–time data for MIB and MAM for single dosing intervals were analyzed by noncompartmental methods using Model 200 for extravascular input in WinNonlin Professional.17 Samples with concentrations below the assay limit of quantitation were excluded. The maximum concentration of each compound achieved in plasma (Cmax) and the time that it occurred following the immediately preceding dose (tmax) were based upon the observed values. Area under the plasma concentration–time curve for a single 5 h dosing interval (AUC5) was estimated using the log-linear trapezoidal algorithm. The apparent oral clearance (CL/F) of MIB was calculated as the dose (D) divided by the AUC5 for the final dose given on day 8, assuming that steady-state conditions for the multiple dosing regimen had been achieved. Values for the apparent biological half-life are not reported because the duration of sampling after the final dose was not sufficiently long to accurately define the terminal disposition phase. Routines provided in the Data Analysis ToolPak of Microsoft Excel 2003 (11.8231.8221) SP3, Professional Edition, were used for the descriptive statistics. Arithmetic averages and standard deviations were calculated for tmax, the ratio of the peak to trough drug concentrations in plasma, and the ratio of AUC5 values. Geometric means were calculated for all other pharmacokinetic variables.18,19
Correlative Imaging
The 10 patients making up the dose expansion cohort had 18F-FLT PET/CT imaging at baseline and again after treatment with MIB (day 7). In order to assess repeatability, each patient had two 18F-FLT PET/CT studies at the initial time point, each performed on consecutive days with no intervening treatment. Data were acquired on 5 different commercial PET/CT systems: Ingenuity TF (Philips Medical Systems), Discovery 710, Discovery ST, Discovery STE, and Discovery VCT (GE Healthcare). Quality assurance images from each system were acquired prior to patient imaging using standardized phantoms to ensure suitability of scanner performance. Although different scanners were involved, individual patients were studied using the same scanner system on each of their imaging days. Data acquisition on each day proceeded according to an identical imaging protocol that involved administration of 2.6 MBq/kg of 18F-FLT and a 10 minute static PET scan acquired in 3D mode at 1 hour post tracer injection. Digital images from all sites were transferred to a central laboratory and analyzed using an identical analysis protocol (XD3, Mirada Medical). Quantitative standardized uptake value (SUV) analysis (body weight normalization) employed volumes of interest (VOIs) defined in tumor and normal brain. Tumor VOIs were determined using isocontour segmentation (30% of the maximum tumor voxel). SUVpeak was determined from the 1 mL spherical volume with the highest tracer uptake. Additional SUV metrics were derived from the maximum voxel and the mean of all voxels within the isocontour VOI. Background SUV was determined by manually placing a 3-cm-diameter sphere in a normal brain region, approximately contralateral to the tumor site and recording the mean value (SUVnormal).
Statistical Considerations
The primary objective of this study was to define the MTD of MIB in timed sequential administration with TMZ in patients with recurrent (r)HGG who had completed initial radiation and TMZ treatment. The standard 3 + 3 design was used for the dose finding. The target rate of dose-limiting toxicity (DLT) was 33%. The MTD was defined at a dose yielding ≤33% DLT rate. The study also was designed to assess the overall safety of the treatment, to describe the pharmacokinetics of MIB in combination with TMZ, and to evaluate tumor characteristics as determined by 18F-FLT PET/CT imaging. All patients who had one dose of MIB were included in safety analysis. Descriptive statistics were used to summarize patient characteristics, toxicity data, pharmacokinetics, and the imaging outcomes. Survival probability was estimated using the Kaplan–Meier method.20 The confidence interval of median survival time was constructed by the Brookmeyer–Crowley method.21 All analyses were conducted using SAS software v9.2.
Results
Patient Characteristics
A total of 27 eligible patients with rHGG were enrolled in this study. These were 20 patients with glioblastoma, 5 with anaplastic astrocytoma, 1 with anaplastic oligodendroglioma, and 1 classified as malignant glioma. Median age of patients was 50 years, median KPS 90% (Table 1). All patients had received initial treatment with radiation and temozolomide. One additional patient had been enrolled who was later found to have a history of bradycardia (an exclusion criterion); the patient was withdrawn from the study and data were only included in the safety analysis (n = 28).
Table 1.
Patient baseline characteristics
| All Patients (N = 27) | |
|---|---|
| Age, y, median (range) | 50.3 (19.8–80.5) |
| Race | |
| White, n (%) | 25 (93) |
| Black or African American, n (%) | 2 (7) |
| Gender male, n (%) | 19 (70) |
| Anticonvulsant | |
| Yes, n (%) | 23 (85) |
| KPS | |
| 90–100, n (%) | 17 (63) |
| 70–80, n (%) | 10 (37) |
| Steroids | |
| Yes, n (%) | 9 (33) |
| No. of prior surgeries | |
| 1, n (%) | 11 (41) |
| 2–4, n (%) | 16 (59) |
| Histology prior study | |
| Glioblastoma, n (%) | 20 (74) |
| Anaplastic astrocytoma, n (%) | 5 (19) |
| Anaplastic oligodendroglioma, n (%) | 1 (4) |
| Malignant glioma, n (%) | 1 (4) |
| Surgery | |
| Biopsy, n (%) | 2 (7) |
| Subtotal resection, n (%) | 14 (52) |
| Total resection, n (%) | 11 (41) |
Dose Escalation and Determination of MTD
Dose escalation proceeded through 3 dose levels without occurrence of DLTs. Dose level 4, 87.5 mg p.o. q.i.d. was expanded by an additional 3 patients and was defined as the MTD. DLTs that were considered possibly related to MIB were grade 3 elevation of ALT/AST in one and grade 1 bradycardia in another patient.
Toxicities
Toxicities attributed to MIB and/or TMZ (Common Terminology Criteria for Adverse Events [CTCAE] grade ≥2) are shown in Table 2. A full description of all toxicities, including CTCAE grade 1 and separated by MIB dose level, is shown in the Supplementary material 2.
Table 2.
Adverse events (grade ≥2), all dose levels, among all patients (N = 28)
| CTCAE 4.2 Category | Adverse Event, N (%) | Grade 2 | Grade 3 |
|---|---|---|---|
| General disorders | Anorexia | 1 (4) | |
| Constipation | 4 (14) | ||
| Fatigue | 3 (11) | ||
| Nausea | 3 (11) | ||
| Mucositis (oral) | 1 (4) | ||
| Vomiting | 1 (4) | ||
| Musculoskeletal disorders | Generalized muscle weakness | 1 (4) | |
| Investigations | Aspartate aminotransferase increased | 1 (4)* | |
| Alanine aminotransferase increased | 1 (4)* | ||
| Neutrophil count decreased | 3 (11) | ||
| Platelet count decreased | 2 (7) | 1 (4) | |
| White blood cell count decreased | 4 (14) | ||
| Metabolic disorders | Hypophosphatemia | 3 (11) |
*Dose limiting.
Pharmacokinetics
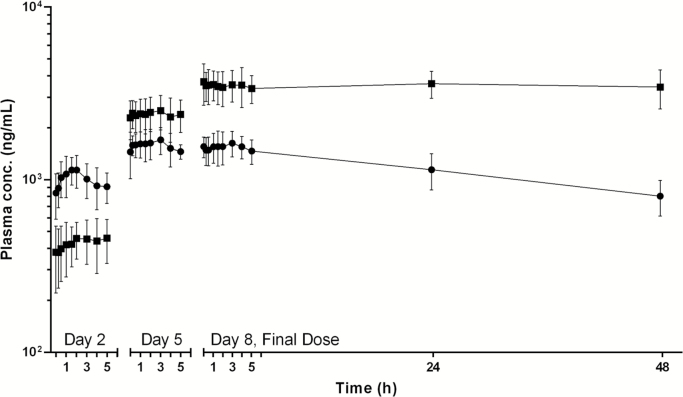
The mean plasma concentration–time profiles of MIB and MAM for the group of 7 patients treated with the 87.5 mg q.i.d. MTD are shown in Fig. 2. Mean values of the key pharmacokinetic parameters for MIB and MAM determined for the groups of patients at each dose level are presented in the Supplementary material 3. MIB was rapidly absorbed with a median tmax of 1.5 h for both the noon dose given on day 2 (range, 0.9–4.9 h) and the final dose on day 8 (0.25–4.0 h). Steady-state exposure to the parent drug for the repeated dosing schedule was achieved within 4 days of dosing based upon the close agreement between the AUC5 of MIB for the final dose given on day 8 (7797 ± 1323 ng h/mL for 87.5 mg q.i.d.) and that for dose 16 given at noon on day 5 (7520 ± 557 ng h/mL for 87.5 mg q.i.d.). The mean Cmin, Cmax, and AUC5 for the final dose given on day 8 exhibited good proportionality to the dose for the first 3 dose levels, which ranged from 25.0 to 75.0 mg q.i.d., with no further increase in the cohort of patients treated with 87.5 mg, although an insufficient number of patients were evaluated at the 2 highest dose levels for a meaningful statistical comparison of the data. The ratio of the peak-to-trough concentration of MIB in plasma at steady state was independent of the dose and had an average (±SD) of only 1.16 ± 0.18 for all patients (n = 15), indicating that the q.i.d. dosing schedule was very effective in minimizing the variations in the plasma concentration of the drug during treatment. The mean ± SD of the steady-state Cmax of MIB for the group of patients receiving the 87.5 mg q.i.d. was 1693 ± 287 ng/mL. The mean CL/F of MIB estimated from the AUC5 for the final dose was independent of the dose across all 4 dose levels, with a mean (±SD) of 10.5 ± 2.8 L/h for all patients (n = 16). The terminal phase half-life of the parent drug and MAM were both too long to enable them to be estimated with acceptable accuracy upon monitoring the decline in plasma levels for 48 h after administration of the final dose of the 7-day continuous dosing regimen. Estimates of the half-life for the parent drug based upon log-linear regression of the last 3 data points ranged from 37.6 to 73.8 h for patients receiving 8.5 mg q.i.d. in dose level 4. As illustrated in Fig. 1, MAM was eliminated much more slowly than the parent drug, and its concentration in plasma actually exceeded MIB on the fourth day of dosing. Accumulation of the metabolite in systemic circulation continued throughout the 7 days of treatment, with plasma levels achieved during the 5 h interval after administration of the final dose on day 8 being markedly greater than observed following the dose given on day 5.
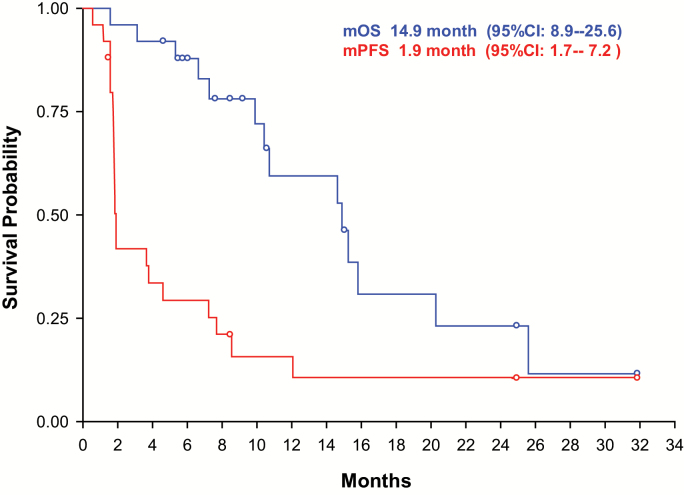
Fig. 2.
Progression-free and overall survival.
Fig. 1.
Mean plasma concentration–time profiles of mibefradil (solid circles) and its alcohol metabolite (solid squares) for the group of 6 patients receiving oral mibefradil 87.5 mg q.i.d. Pharmacokinetic sampling was performed over single dosing intervals on day 2 after administration of the fourth consecutive dose, on day 5 after the sixteenth dose, and for 48 h after the final dose on day 8. The error bars represent 1 SD of the mean concentrations.
Responses and Survival
Responses based upon site investigators’ assessment included 3 partial and 3 complete responses. Central review of these patients, however, confirmed only 1 partial and 1 complete response (Table 3). Interestingly, all patients with reported response had glioblastoma, and no responses were observed in WHO grade III tumors.
Table 3.
Patients with reported responses
| Mibefradil Dose Level (mg per day) | Age and Gender | Pathology | Review Assessment by Site Investigator | Central Review |
|---|---|---|---|---|
| 100 | 67 F | GBM | Partial response | Stable disease |
| 200 | 50 M | GBM | Partial response | Stable disease |
| 200 | 43 F | GBM | Partial response | Partial response |
| 300 | 33 M | GBM | Complete response | Complete response |
| 350 (MTD) | 71 M | GBM | Complete response | Stable disease |
| 350 (MTD) | 43 M | GBM | Complete response | Stable disease |
GBM = glioblastoma.
Median overall survival was 14.9 months (95% CI: 8.9–25.6). Median overall survival was 12.7 months (95% CI: 6.6–not reached) for patients with glioblastoma and 15.2 months (95% CI: 9.9–25.6) for patients with WHO grade III tumors (P = .88; not designed for comparison between the 2 groups). Median progression-free survival was 1.9 months (95% CI: 1.7–7.2) (Fig. 2).
18 F-FLT PET Imaging
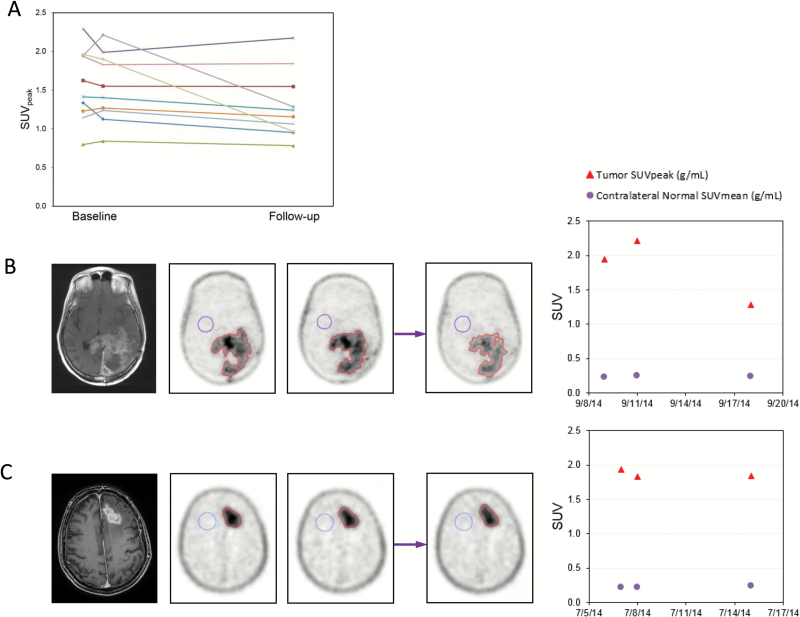
18F-FLT PET imaging was performed on 10 patients at the MTD with 2 baseline scans (double baseline) and 1 scan after 7 days of MIB. Six patients had glioblastoma (WHO grade IV) and 4 patients anaplastic gliomas (WHO grade III). Two of 10 patients had a statistically significant drop in SUVpeak, whereas the remaining 8 patients did not (Fig. 3). Repeatability of 18F-FLT PET was demonstrated by data from double-baseline imaging and data comparing baseline imaging between the different institutions, as reported previously.22
Fig. 3 .
18F-FLT PET imaging. (A) Synopsis of SUVpeak values of double-baseline and values after 7 days of treatment with mibefradil. (B and C) Examples of a patient with significant decline in SUV after 7 days of MIB (B) and a patient without a decline (C). MRI T1 contrast-enhanced image corresponding to the images of 18F-FLT PET imaging shown. Double baseline scans (left, 2 18F-FLT PET images), followed by 7 days on mibefradil and repeat imaging on day 7 (right, 18F-FLT PET images). Far right: changes in SUV values.
Discussion
This study demonstrates that sequential therapy of mibefradil in q.i.d. dosing for 7 days followed by standard treatment with temozolomide can be safely administered in patients with recurrent high-grade gliomas. The MTD of MIB in this clinical setting, as determined by this trial, is 87.5 mg p.o. q.i.d. Notably, we did not detect significant cardiac toxicity, including no symptomatic hypotension or symptomatic bradycardia, which was assessed by close cardiac monitoring during cycle 1 of MIB in all patients. In addition, we did not see enhanced myelotoxicity from this regimen beyond what is expected with treatment with TMZ. This finding is supported by previously published reports that did not find T-type calcium channel receptor expression in hematopoietic precursor cells.10 The regimen showed some clinical activity in this setting in this selected patient population as demonstrated by several reported and confirmed radiographic responses.
Central review of reported responses (partial or complete response) determined a lower response rate, compared with the site investigators’ assessment. Our study required “measurable disease” at time of enrollment for the dose-escalation part of the study. It turned out that not all cases had lesions that eventually qualified for the required 1 × 1 cm measurements that are considered the minimum for measurability per RANO. We are reporting both the investigators’ assessment as well as the data from central review, as we feel that both provide a reflection of activity of the MIB/TMZ regimen. The discrepancy between both reviews highlights several important points. Precise measurement criteria should be required from the outset when enrolling patients; in this trial it would have helped to clearly state that minimum measurement of contrast enhancement should have been 1 × 1 cm (this was explicitly stated and required only for the dose expansion cohort). This considered, we feel that listing the site investigators’ response assessment in addition to the central review data is still helpful. The site investigators, who follow the cases closely, may have information on the patients’ clinical status that is not available during remote central review, albeit the latter is more objective.
Assessment of MIB pharmacokinetics using q.i.d. dosing in this study led to several interesting observations:
In a prior clinical study involving healthy male volunteers, MIB was found to exhibit dose-dependent pharmacokinetics, with the mean CL/F for a single oral dose decreasing progressively from 71.4 ± 23.5 L/h in subjects receiving a single 10 mg dose to 9.7 ± 1.2 L/h in subjects given a single 320 mg dose.23 The dose-dependent CL/F was attributed to a reduction in first-pass hepatic metabolism as the oral dose was increased. The nonlinear pharmacokinetics of oral MIB were confirmed in a subsequent study undertaken in patients with hypertension who received MIB orally once a day for 8 days.24 The mean CL/F determined for the initial dose decreased from 26.7 ± 11.0 L/h to 10.4 ± 4.5 L/h as the daily dose was increased from 50 to 200 mg. The mean CL/F determined for the final dose of the repeated daily dosing regimen was independent of the dose, with values ranging from 7.1 ± 2.2 L/h to 10.4 ± 4.3 L/h. This was confirmed in a study in healthy male volunteers who received MIB at doses of 100, 150, or 250 mg once daily for 28 days for which the mean steady-state CL/F ranged from 8.3 ± 2.3 L/h to 10.8 ± 3.8 L/h.24 Consistent with these prior clinical investigations, the CL/F of MIB determined from the AUC for a single dosing interval after steady state was achieved did not show a dose-dependent trend over the relatively narrow range of doses evaluated in the present study. The overall mean steady-state CL/F of MIB when given q.i.d. to brain cancer patients, 10.5 ± 2.8 L/h, was in excellent agreement with the steady-state CL/F reported for once daily dosing.
As expected, the mean steady-state Cmax of MIB achieved with the q.i.d. administration schedule was lower than values reported in prior studies in which the same daily dose was given once a day.23 Administering 50 mg q.i.d. provided a mean steady-state Cmax of 873 ± 193 ng/mL compared with 1440 ± 364 ng/mL for 200 mg q.i.d. The mean steady-state Cmax achieved with doses of 75.0 and 87.5 mg q.i.d., 1798 ± 540 ng/mL and 1693 ± 287 ng/mL, respectively, were greater than 250 mg q.i.d. (1506 ± 163 ng/mL), the highest dose for the once daily administration schedule for which pharmacokinetic data have been reported. In addition, q.i.d. dosing resulted in a marked decrease in the steady-state peak-to-trough ratio for the concentration of MIB in plasma compared with q.i.d. dosing, from values ranging from 1.5–4.9 for q.i.d. dosing to only 1.16 ± 0.18 for q.i.d. dosing. Consequently, systemic exposure to near maximum concentrations of the drug is enhanced by q.i.d. dosing.
The relative generation of the alcohol metabolite observed in the present study is also entirely consistent with previously reported findings. After receiving multiple once a day doses of MIB, the ratio of the AUC for the metabolite-to–parent drug over a 24 h dosing interval increased as the dose was escalated from approximately 0.75 for a 50 mg dose to 2.0 for 150 mg doses.17 In the present study, the mean ratio of the AUC5 for the metabolite-to–parent drug for the final dose given on day 8 also exhibited a dose-dependent trend, increasing from 1.04 ± 0.32 for the 25 mg q.i.d. dose to 2.27 ± 0.94 for 87.5 mg q.i.d.
18F-FLT PET, which was used solely as an exploratory imaging component of this trial, showed statistically significant reductions in SUVpeak for 2 of the 10 patients in the expansion cohort. Interestingly, though, both of these patients had progressive disease after once cycle of MIB/TMZ per MRI-based assessment according to RANO. Further studies are required to explore the cause of the SUVpeak reductions. 18F-FLT uptake in brain tumors likely reflects a combination of tumor cell proliferation and tracer delivery (blood flow and permeability). As such, changes in SUVpeak cannot be directly interpreted as indicating a change in tumor cell proliferation but do support changes in tumor biology, and may nevertheless provide useful information.
This study had several limitations: As this was a phase I dose-escalation and safety study, we included patients with both WHO grades III and IV disease, adding heterogeneity to the overall survival secondary endpoint. In addition, this regimen included TMZ, which has known activity in gliomas. Observed responses may have been related to TMZ alone, and the degree of enhanced activity due to MIB remains uncertain. Additionally, promoter methylation status of O6-DNA methylguanine-methyltransferase, which is associated with better responses to TMZ, was not prospectively collected and not available for all patients. Full dynamic PET acquisitions with metabolite sampling and kinetic modeling could potentially have been of interest, as they might better isolate the proliferative component of the FLT signal. Such studies done on a multicenter basis were not feasible for our study.
Nonetheless, sequential treatment with MIB and TMZ was safe and met the criteria for further evaluation of this regimen. Safety was demonstrated, an MTD defined, and there have been documented responses. Prospective efficacy trials are needed to formally assess the potential role of this regimen in the management of patients with high-grade gliomas.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This study was supported by the NCI funded Adult Brain Tumor Consortium grant UM1CA137443, Sidney Kimmel Comprehensive Cancer Center core grant 5P30CA006973-52, NCI grant 5U01CA140204-05, and by a research grant from Cavion LLC. ClinicalTrials.gov identifier NCT01480050.
Conflict of interest statement. Matthias Holdhoff and David Schiff: Advisory role for Cavion; no personal compensation.
Supplementary Material
Acknowledgments
We thank Cavion for the general support and excellent collaboration for this clinical trial. Central review was performed through the ABTC Central Imaging Core (Dr Benjamin Ellingson).
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the united states in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9(1):307–315. [PubMed] [Google Scholar]
- 3. Geller RB, Burke PJ, Karp JE, et al. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989;74(5):1499–1506. [PubMed] [Google Scholar]
- 4. Burke PJ, Karp JE, Braine HG, Vaughan WP. Timed sequential therapy of human leukemia based upon the response of leukemic cells to humoral growth factors. Cancer Res. 1977;37(7 Pt 1):2138–2146. [PubMed] [Google Scholar]
- 5. Keir ST, Friedman HS, Reardon DA, Bigner DD, Gray LA. Mibefradil, a novel therapy for glioblastoma multiforme: cell cycle synchronization and interlaced therapy in a murine model. J Neurooncol. 2013;111(2):97–102. [DOI] [PubMed] [Google Scholar]
- 6. Dziegielewska B, Casarez EV, Yang WZ, Gray LS, Dziegielewski J, Slack-Davis JK. T-type Ca2+ channel inhibition sensitizes ovarian cancer to carboplatin. Mol Cancer Ther. 2016;15(3):460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dziegielewska B, Gray LS, Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflugers Arch. 2014;466(4):801–810. [DOI] [PubMed] [Google Scholar]
- 8. Valerie NC, Dziegielewska B, Hosing AS, et al. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem Pharmacol. 2013;85(7):888–897. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Cruickshanks N, Wulfkuhle J, et al. Inhibition of T-type calcium channels sensitizes glioblastoma stem cells to chemotherapy [abstract]. Neuro Oncol. 2015;17(suppl 5):v18. [Google Scholar]
- 10. Gray LS, Perez-Reyes E, Gomora JC, et al. The role of voltage gated T-type Ca2+ channel isoforms in mediating “capacitative” Ca2+ entry in cancer cells. Cell Calcium. 2004;36(6):489–497. [DOI] [PubMed] [Google Scholar]
- 11. Lory P, Bidaud I, Chemin J. T-type calcium channels in differentiation and proliferation. Cell Calcium. 2006;40(2):135–146. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Zhang J, Jiang D, et al. Inhibition of T-type Ca²⁺ channels by endostatin attenuates human glioblastoma cell proliferation and migration. Br J Pharmacol. 2012;166(4):1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lester-Coll NH, Kluytenaar J, Pavlik KF, et al. Mibefradil dihydrochloride with hypofractionated radiation for recurrent glioblastoma: preliminary results of a phase I dose expansion trial [abstract]. Neuro Oncol. 2015;17(suppl 5):v13. [Google Scholar]
- 14. Kobrin I, Charlon V, Lindberg E, Pordy R. Safety of mibefradil, a new once-a-day, selective T-type calcium channel antagonist. Am J Cardiol. 1997;80(4B):40C–46C. [DOI] [PubMed] [Google Scholar]
- 15. Po AL, Zhang WY. What lessons can be learnt from withdrawal of mibefradil from the market? Lancet. 1998;351(9119):1829–1830. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. Gabrielson J, Weiner D. Pharmacokinetic/Pharmacodynamic Data Analysis: Concepts and Applications. Stockholm: Swedish Pharmaceutical Press; 1994. [Google Scholar]
- 18. Lacey LF, Keene ON, Pritchard JF, Bye A. Common noncompartmental pharmacokinetic variables: are they normally or log-normally distributed? J Biopharm Stat. 1997;7(1):171–178. [DOI] [PubMed] [Google Scholar]
- 19. Mizuta E, Tsubotani A. Preparation of mean drug concentration–time curves in plasma. A study on the frequency distribution of pharmacokinetic parameters. Chem Pharm Bull (Tokyo). 1985;33(4):1620–1632. [DOI] [PubMed] [Google Scholar]
- 20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53(282):457–481. [Google Scholar]
- 21. Brookmeyer R, Crowley A. A confidence interval for the median survival time. Biometrics. 1982;38(1):29–41. [Google Scholar]
- 22. Lodge MA, Holdhoff M, Leal JP, et al. Repeatability of 18F-FLT PET in a multi-center study of patients with high grade glioma. J Nucl Med. 2016; doi:10.2967/jnumed.116.178434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Welker HA, Wiltshire H, Bullingham R. Clinical pharmacokinetics of mibefradil. Clin Pharmacokinet. 1998;35(6):405–423. [DOI] [PubMed] [Google Scholar]
- 24. Welker HA. Single- and multiple-dose mibefradil pharmacokinetics in normal and hypertensive subjects. J Pharm Pharmacol. 1998;50(9):983–987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.