Abstract
Background.
Medulloblastoma is the most common malignant childhood brain tumor, although long-term risks for chronic neurologic health and psychosocial functioning in aging adult survivors are incompletely characterized.
Methods.
The Childhood Cancer Survivor Study (CCSS) includes 380 five-year survivors of medulloblastoma/primitive neuroectodermal tumor (PNET; median age at follow-up: 30 y, interquartile range 24–36) and sibling comparison (n = 4031). Cumulative incidence of neurologic health conditions was reported. Cox regression models provided hazard ratios (HRs) and 95% CIs. Cross-sectional outcomes were assessed using generalized linear models.
Results.
Compared with siblings, survivors were at increased risk of late-onset hearing loss (HR: 36.0, 95% CI: 23.6–54.9), stroke (HR: 33.9, 95% CI: 17.8–64.7), seizure (HR: 12.8, 95% CI: 9.0–18.1), poor balance (HR: 10.4, 95% CI: 6.7–15.9), tinnitus (HR: 4.8, 95% CI: 3.5–6.8), and cataracts (HR: 31.8, 95% CI: 16.7–60.5). Temporal/frontal lobe radiotherapy of 50 Gy or more increased risk for hearing loss (HR: 1.9, 95% CI: 1.1–1.3), seizure (HR: 2.1, 95% CI: 1.1–3.9), stroke (HR: 3.5, 95% CI: 1.3–9.1), and tinnitus (HR: 2.0, 95% CI: 1.0–3.9). Survivors were less likely than siblings to earn a college degree (relative risk [RR]: 0.49, 95% CI: 0.39–0.60), marry (RR: 0.35, 95% CI: 0.29–0.42), and live independently (RR: 0.58, 95% CI: 0.52–0.66).
Conclusions.
Adult survivors of childhood medulloblastoma/PNET demonstrate pronounced risk for hearing impairment, stroke, lower educational attainment, and social independence. Interventions to support survivors should be a high priority.
Keywords: late effects, medulloblastoma, neurologic outcomes, psychosocial outcomes
Importance of the study
The current study estimates the risk for late-onset neurologic and psychosocial outcomes in one of the largest cohorts to date of adult survivors of medulloblastoma/PNET. Chronic neurosensory late effects were common among survivors and continued to increase in incidence with age. In addition, lower levels of educational attainment and social independence were observed relative to siblings. Lessons learned are twofold. For practitioners providing long-term follow-up care of survivors, lifelong surveillance for conditions such as hearing loss, cataracts, and cerebrovascular disease is needed. Second, given the high burden of morbidity among these survivors, multidisciplinary support in medical, educational, and social settings may be needed. New therapies that have less long-term side effects should be developed, and future studies should evaluate whether modifications of primary therapy intended to reduce risk for late effects have been effective.
Medulloblastoma and primitive neuroectodermal tumor (PNET) of the CNS are embryonal tumors arising in the infratentorial and supratentorial brain, respectively. This classification was maintained until 2016, when the World Health Organization retained the term “medulloblastoma” for posterior fossa tumors but replaced the term “PNET” with “embryonal tumors” for other small-cell, hyperchromatic primary CNS tumors.1 CNS PNET and medulloblastoma comprise approximately 10%–15% of pediatric brain tumors.2,3 As early as the 1970s, the use of adjuvant craniospinal irradiation (CSI) after surgery resulted in improved survival in patients with medulloblastoma/PNET.4 The typical radiation doses delivered were 35–36 Gy to the neuraxis (CSI) with an additional boost to the tumor bed. This postoperative “standard dose” CSI approach alone resulted in 5-year event-free survival rates of approximately 60%.5 More recently, the combination of surgical resection, radiation therapy, and chemotherapy has improved survival to 75%–80% for patients with standard risk medulloblastoma.6–8 However, these survivors, even at a young age, are at high risk of neurologic late effects,9–18 which may contribute to poor quality of life. While previous data from the Childhood Cancer Survivor Study (CCSS) have been published in aggregate for all CNS tumor survivors, focused assessment of this important population of medulloblastoma/PNET survivors highlights their specific long-term complications.
Aging survivors of childhood medulloblastoma and CNS PNET now face common adult milestones that include self-management of chronic health conditions, living independently, engaging in intimate relationships, and preparing for the future. It is important to evaluate the impact of late effects on these common adult functions given the standard use of CSI and increasing use of chemotherapy often associated with long-term health risks.19 The purpose of this study was to estimate risk for neurologic late effects after diagnosis and treatment for medulloblastoma/PNET within the CCSS cohort and to evaluate the personal outcomes and psychosocial function in adulthood.
Methods
Participants
Participants were drawn from the CCSS, a retrospective cohort with prospective follow-up of children diagnosed and treated for cancer, including CNS tumors, from 1970 to 1986 at 26 collaborating institutions in the United States and Canada. The cohort methodology and study design have been previously described in detail.20,21 Eligibility for participation in the CCSS included a diagnosis of cancer before age 21 and survival for at least 5 years from diagnosis. A randomly selected subset of survivors was asked to identify their siblings. A comparison group of the sibling closest in age to the survivor was recruited to participate. The current analysis compares 380 five-year survivors of medulloblastoma/PNET (Supplementary Fig. 1) from this CCSS cohort with the sibling comparison sample (n = 4031). Institutional review boards of all participating centers reviewed and approved the CCSS protocol.
Demographic and health-related outcomes were collected via a self-administered baseline questionnaire. Parents completed the baseline questionnaire if participants were younger than 18 years or if participants older than 18 years were unable to complete the questionnaire themselves.
Updated questionnaires and interviews were subsequently administered (see Supplementary Fig. 1). Questions about specific neurologic conditions and sensory impairments were phrased as, “Have you ever been told by a doctor or other health care professional that you have or have had [condition]?” If the respondent indicated “Yes,” he or she was asked to report the age at first occurrence of the condition. The baseline and 2003 and 2007 follow-up questionnaires also included the Brief Symptom Inventory–18 (BSI-18). The BSI-18 is a set of 18 items designed to assess symptoms of depression, anxiety, and somatic complaints. Participants answer the questions with regard to the past 7 days to describe the extent that they have been distressed by each symptom using a Likert scale ranging from 1 (not at all) to 5 (extremely). In addition to the 3 subscales, responses to all 18 items are summed to provide a global score (Global Severity Index).22 BSI scores greater than or equal to the 90th percentile on standardized norms were classified as “impaired.” Ratings of general health, cancer-related anxiety, and concerns about future health were reported on 5-point Likert scales, and self-reported memory problems were identified as being present or not.
Subsequent malignant neoplasms (SMNs) included new malignancies collected from the cohort, not including recurrence of the primary childhood malignancy, and were initially ascertained through self- or proxy-report questionnaires and/or death certificates. Cases were subsequently confirmed by pathology report or, when not available, by death certificate or other medical records reviewed by study investigators. Only subsequent malignant neoplasms occurring 5 or more years following the childhood cancer diagnosis were evaluated.
Cancer diagnosis and treatment data including chemotherapy and radiotherapy exposures were abstracted from medical records at treating institutions utilizing standardized CCSS protocols, for survivors who provided authorization.
Statistical Methods
Demographic data and cancer treatment factors were summarized with descriptive statistics. Neurologic, SMN, and sensory outcomes were analyzed as time-to-event data. Cumulative incidence curves with 95% CIs were used to summarize the pattern of onset for time-to-event outcomes from study entry at 5 years post–cancer diagnosis forward. Death was treated as a competing risk in the cumulative incidence estimations.23 Cox regression models with age as the time scale were used to estimate cause-specific hazard ratios (HRs) and 95% CIs relative to the sibling comparison population, adjusted for sex. Within the survivor cohort, Cox models evaluated associations between cancer treatment exposures and neurologic outcomes. The treatment exposures considered were: cranial radiation (maximum dose to region), chemotherapy (yes or no), extracranial ventricular shunt, and, for auditory outcomes, platinum agents (yes or no). Sex, age at primary cancer diagnosis, occurrence of an SMN, and recurrence of medulloblastoma/PNET were considered as time-varying covariates. For each outcome, factors that were not statistically significant in the multivariable context were omitted from the final model unless doing so caused a 10% or greater change in the HR estimate for another factor in the model. In cases in which a study participant reported a condition but did not report the age at first occurrence, multiple imputation for the missing event time was performed using a modified version of the technique of Taylor and colleagues.24 Ten imputed values for each missing age were generated. The models for generating imputed age values included sex, age, and, for cancer survivors, age at cancer diagnosis and type of therapy. Parameter estimates and 95% CIs were obtained using standard formulas for combining analyses across the imputed datasets.25
Memory complications and psychosocial outcomes, including BSI, educational status, marital status, independent living, employment status, and income were assessed in cross-sectional analyses. Outcome measures were dichotomized, and generalized linear models were used to estimate relative risks (RRs). A log-link model, rather than a logistic model, was chosen because outcomes were not rare; hence, the odds ratio from a logistic model would not provide a good approximation of RR. The generalized linear model utilized a Poisson error distribution with robust variance estimation.26 RR estimates were adjusted for age at last follow-up, sex, and race.
Results
Among 380 five-year survivors of medulloblastoma/PNET, 58% were male, and the median age at last follow-up was 30 years (interquartile range 24–36; Table 1). Seventy-three percent of survivors were younger than 10 years of age at diagnosis. Among survivors, 94% received CSI, with the majority (74%) receiving greater than 30 Gy. The median dose of CSI was 35 Gy (interquartile range 30–36), and the mean total dose to the tumor bed was 52 Gy (interquartile range 50–54). In this era, few survivors received chemotherapies now accepted as standard therapy for medulloblastoma/PNET, including: cisplatinum (24%), CCNU (34%), and cyclophosphamide (21%). See Table 1 for further characteristics of survivors and siblings.
Table 1.
Demographic and treatment characteristics of survivors of medulloblastoma/PNET and sibling comparison population
| Survivors (N = 380) | Siblings (N = 4031) | |||
|---|---|---|---|---|
| Characteristics | N | % a | N | % a |
| Sex | ||||
| Female | 158 | 42% | 2088 | 52% |
| Male | 222 | 58% | 1943 | 48% |
| Race/ethnicity | ||||
| White, Non-Hispanic | 308 | 86% | 3509 | 90% |
| Black, Non-Hispanic | 24 | 7% | 112 | 3% |
| Hispanic | 18 | 5% | 149 | 4% |
| Other | 10 | 3% | 111 | 3% |
| Unknown | 20 | -- | 150 | -- |
| Age at diagnosis, y | ||||
| 0–4 | 127 | 33% | -- | |
| 5–9 | 150 | 40% | -- | |
| 10–14 | 76 | 20% | -- | |
| >14 | 27 | 7% | -- | |
| Radiation therapy (RT) | ||||
| None | 8 | 2% | -- | |
| Cranial | 11 | 3% | -- | |
| Craniospinalb | 312 | 94% | -- | |
| Unknownc | 49 | -- | -- | |
| Maximum cranial radiation dose | ||||
| ≥30 Gy | 319 | 96% | -- | |
| >1 to <30 Gy | 5 | 2% | -- | |
| No RT | 8 | 2% | -- | |
| Unknown | 48 | -- | -- | |
| Maximum spinal radiation dose | ||||
| ≥30 Gy | 245 | 74% | -- | |
| >4 to <30 Gy | 67 | 20% | -- | |
| Scatter exposure from cranial RT only | 11 | 3% | -- | |
| No RT | 8 | 2% | ||
| Unknown | 49 | -- | -- | |
| Chemotherapy | ||||
| Yes | 202 | 59% | -- | |
| No | 140 | 41% | -- | |
| Unknown | 38 | -- | -- | |
| Cisplatinum | ||||
| Yes | 81 | 24% | -- | |
| No | 261 | 76% | -- | |
| Unknown | 38 | -- | -- | |
| CCNU | ||||
| Yes | 116 | 34% | -- | |
| No | 226 | 66% | -- | |
| Unknown | 38 | -- | -- | |
| Cyclophosphamide | ||||
| Yes | 71 | 21% | -- | |
| No | 271 | 79% | -- | |
| Unknown | 38 | -- | -- | |
| Extracranial ventricular shunt | ||||
| Yes | 136 | 40% | -- | |
| No | 207 | 60% | -- | |
| Unknown | 37 | -- | -- | |
aPercentages based on survivors for whom data are available.
bMedian cranial dose = 52 Gy; median spinal dose = 35 Gy.
cForty-eight survivors were missing both the cranial and spinal RT dosimetry data; one survivor had known cranial RT dose, but unknown spinal RT status.
Chronic Neurosensory and Neurologic Health Conditions
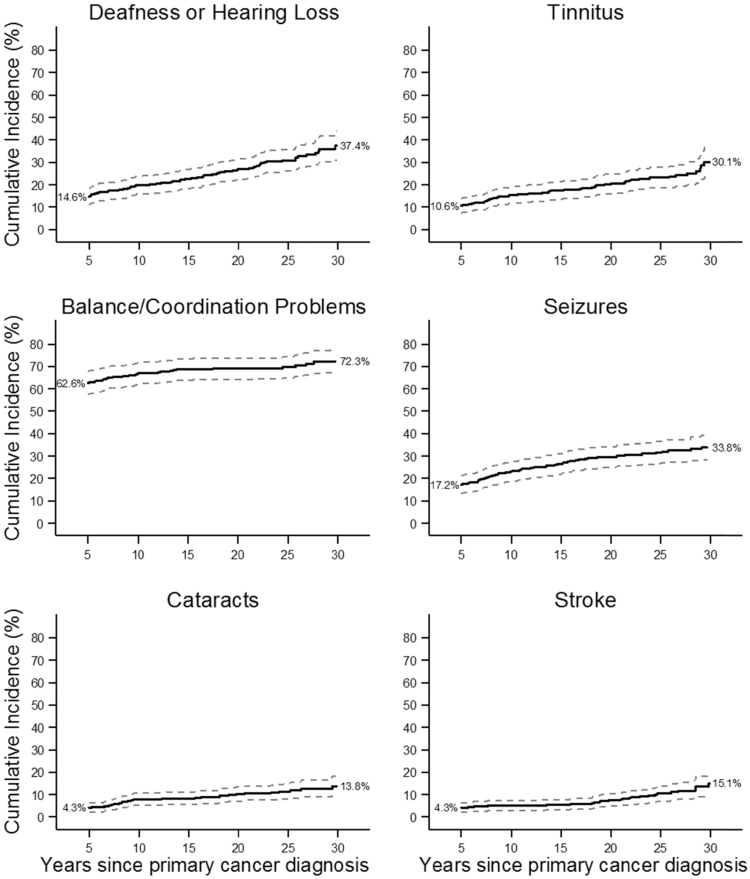
For most neurologic outcomes, the majority of events occurred in the first 5 years postdiagnosis, but substantial numbers of survivors developed new onset of neurologic conditions between 5 and 30 years postdiagnosis (Table 2). At 30 years after diagnosis, the cumulative incidence of hearing loss was 37.4%; tinnitus, 30.1%; and seizures, 33.8% (Fig. 1). Problems with balance, coordination, and/or tremors were very common at CCSS cohort entry, and the cumulative incidence rose further to 72.3% by 30 years post–cancer diagnosis (Fig. 1).
Table 2.
Chronic neurologic health conditions among 5-year survivors of medulloblastoma/PNET
| Timing of Onset of Chronic Health Condition | ||||
|---|---|---|---|---|
| Prior to Diagnosis | Diagnosis to 5-Y Postdiagnosis | ≥5-Y Postdiagnosis | ||
| Condition | Hazard Ratio (95% CI)a | |||
| Deafness or hearing loss requiring hearing aid | 6 | 48 | 61 | 36.0 (23.6–54.9)** |
| Tinnitus | 5 | 25 | 36 | 4.8 (3.5–6.8)** |
| Vertigo | 7 | 35 | 17 | 2.7 (1.7–4.4) ** |
| Problems with balance, coordination, and/ or tremors | 18 | 192 | 22 | 10.4 (6.7–15.9)** |
| Seizures | 3 | 57 | 49 | 12.8 (9.0–18.1)** |
| Problems with stuttering or stammering | 6 | 14 | 5 | 2.7 (1.1–6.7)* |
| Cataracts | 1 | 14 | 25 | 31.8 (16.7–60.5)** |
| Stroke | 1 | 15 | 25 | 33.9 (17.8–64.7)** |
aCox regression models compare survivor versus sibling risks adjusted for sex and accounting for age in the time scale.
* P < .05.
** P < .0001.
Fig. 1.
Cumulative incidence of chronic health conditions among long-term survivors of medulloblastoma and PNET.
Chronic sensory impairments were more commonly reported among survivors than siblings. A total of 61 survivors reported significant hearing loss (deafness or hearing loss that requires a hearing aid) with onset occurring more than 5 years post–cancer diagnosis (see Table 2). The age- and sex-adjusted HR for survivors compared with siblings was 36.0 (95% CI: 23.6–54.9). Seizures, tinnitus, and vertigo were more common in survivors than siblings (see Table 2). Twenty-five survivors reported cataracts more than 5 years after cancer diagnosis (HR: 31.8, 95% CI: 16.7–60.5). Forty (10.5%) survivors reported a history of stroke; 25 suffered the stroke more than 5 years after their original diagnosis (HR: 33.9; 95% CI: 17.8–64.7). Problems with balance, coordination, and/or tremors were also common (HR: 10.4; 95% CI: 6.7–15.9). Temporal/frontal lobe radiotherapy of 50 Gy or more increased risk for hearing loss (HR: 1.9; 95% CI: 1.1–1.3; see Table 3), seizure (HR: 2.1; 95% CI: 1.1–3.9), stroke (HR: 3.5; 95% CI: 1.3–9.1), and tinnitus (HR: 2.0; 95% CI: 1.0–3.9).
Table 3.
Treatment-related risk factors for specific health conditions among survivors of medulloblastoma/PNET
| Health Conditiona | Risk Factor | Category | Hazard Ratio (95% CI) | P |
|---|---|---|---|---|
| Deafness or hearing loss requiring hearing aid | Age at primary cancer diagnosis | <5 y | 1.0 (referent) | -- |
| 5–9 y | 1.8 (0.7–4.2) | .20 | ||
| 10–14 y | 1.8 (0.7–4.8) | .23 | ||
| ≥15 y | 1.5 (0.4–5.6) | .52 | ||
| Temporal and/or frontal lobe radiation dose | <50 Gy | 1.0 (referent) | -- | |
| ≥50 Gy | 1.9 (1.1–3.3) | .02 | ||
| Platinum | No | 1.0 (referent) | -- | |
| Yes | 1.8 (0.9–3.6) | .12 | ||
| Recurrence of primary cancer | No | 1.0 (referent) | -- | |
| Yes | 2.2 (0.9–5.2) | .08 | ||
| Tinnitus | Age at primary cancer diagnosis | <5 y | 1.0 (referent) | -- |
| 5–9 y | 0.9 (0.4–2.4) | .90 | ||
| 10–14 y | 1.6 (0.6–4.2) | .37 | ||
| ≥15 y | 1.5 (0.4–6.2) | .56 | ||
| Temporal and/or frontal lobe radiation dose | <50 Gy | 1.0 (referent) | -- | |
| ≥50 Gy | 2.0 (1.0–3.9) | .04 | ||
| Platinum | No | 1.0 (referent) | -- | |
| Yes | 2.6 (1.2–5.5) | .02 | ||
| Subsequent malignant neoplasms | No | 1.0 (referent) | -- | |
| Yes | 3.4 (0.9–12.8) | .06 | ||
| Problems with balance, coordination, and/or tremors | Sex | Female | 1.0 (referent) | -- |
| Male | 1.5 (0.6–3.8) | .39 | ||
| Any chemotherapy | No | 1.0 (referent) | -- | |
| Yes | 3.1 (1.0–9.6) | .05 | ||
| Recurrence of primary cancer | No | 1.0 (referent) | -- | |
| Yes | 2.5 (0.8–7.3) | .10 | ||
| SMN | No | 1.0 (referent) | -- | |
| Yes | 11.7 (1.0–136.0) | .05 | ||
| Seizures | Temporal and/or frontal lobe radiation dose | <50 Gy | 1.0 (referent) | -- |
| ≥50 Gy | 2.1 (1.1–3.9) | .02 | ||
| Any chemotherapy | No | 1.0 (referent) | -- | |
| Yes | 2.4 (1.2–4.9) | .01 | ||
| Recurrence of primary cancer | No | 1.0 (referent) | -- | |
| Yes | 2.9 (1.4–6.3) | .005 | ||
| Stroke | Temporal and/or frontal lobe radiation dose | <50 Gy | 1.0 (referent) | -- |
| ≥50 Gy | 3.5 (1.3–9.1) | .01 | ||
| Any chemotherapy | No | 1.0 (referent) | -- | |
| Yes | 2.7 (1.1–6.6) | .04 | ||
| Recurrence of primary cancer | No | 1.0 (referent) | -- | |
| Yes | 3.9 (1.1–14.3) | .04 |
aTreatment-related risk factors were evaluated for all health conditions listed in Table 3 For vertigo, stuttering/stammering and cataracts, no statistically significant associations with treatment factors were found.
Abbreviations: SMN, Subsequent malignant neoplasm.
At 30 years postdiagnosis, 70.6% of the survivors were alive (Supplementary Fig. 2). The cumulative incidence of primary tumor recurrence was 18% (95% CI: 14–22) by 30 years after cancer diagnosis. The 30-year cumulative incidence of a subsequent malignancy was 8% (95% CI: 5–12). Almost half (48%) of subsequent malignancies were thyroid cancers (Supplementary Table), followed by malignant brain tumors (16%) and sarcomas (16%). Tumor recurrence independently increased risk for seizure (HR: 2.9; 1.4–6.3) and stroke (HR: 3.9; 1.1–14.3).
Educational Attainment and Social Independence
Among medulloblastoma/PNET survivors, 25% earned a bachelor’s degree or higher, compared with 55% of the sibling comparison group (RR: 0.49; 95% CI: 0.39–0.60; Table 4). Fewer than half of the survivors reported working more than 30 hours per week, and almost 90% had personal income less than $40 000. Compared with siblings, survivors were less likely to report having ever married or lived as being married (RR: 0.35; 95% CI: 0.29–0.42).
Table 4.
Sociodemographic outcomes and psychosocial function of survivors of medulloblastoma/PNET
| Survivor vs Sibling | ||||
|---|---|---|---|---|
| N | % | RR b | 95% CI | |
| Sociodemographic Outcomes | ||||
| College degreea | ||||
| Survivors | 66 | 25.4% | 0.49 | (0.39–0.60) |
| Siblings | 1797 | 55.3% | 1.0 | |
| Marital status—ever married or lived as married | ||||
| Survivors | 78 | 22.2% | 0.35 | (0.29–0.42) |
| Siblings | 2897 | 73.4% | 1.0 | |
| Independent living* | ||||
| Survivors | 123 | 53.0% | 0.58 | (0.52–0.66) |
| Siblings | 2627 | 93.6% | 1.0 | |
| Employed ≥30 h/wk | ||||
| Survivors | 84 | 43.8% | 0.59 | (0.50–0.69) |
| Siblings | 1635 | 73.5% | 1.0 | |
| Personal income ≥$40 000/y | ||||
| Survivors | 18 | 10.1% | 0.19 | (0.12–0.29) |
| Siblings | 1159 | 54.1% | 1.0 | |
| Psychological Status | ||||
| Current learning or memory problems | ||||
| Survivors | 120 | 60.6% | 19.8 | (15.1–25.9) |
| Siblings | 71 | 3.0% | 1.0 | |
| Impaired on global BSI scalec | ||||
| Survivors | 9 | 6.8% | 2.00 | (1.03–3.89) |
| Siblings | 78 | 3.4% | 1.0 | |
| Impaired on BSI depression subscalec | ||||
| Survivors | 14 | 10.5% | 2.62 | (1.55–4.45) |
| Siblings | 93 | 4.1% | 1.0 | |
| Impaired on BSI anxiety subscalec | ||||
| Survivors | 5 | 3.8% | 1.18 | (0.48–2.88) |
| Siblings | 71 | 3.1% | 1.0 | |
| Impaired on BSI somatization subscalec | ||||
| Survivors | 8 | 6.0% | 1.71 | (0.84–3.51) |
| Siblings | 90 | 4.0% | 1.0 | |
| Good/very good/excellent general health status | ||||
| Survivors | 158 | 77.8% | 0.82 | (0.76–0.88) |
| Siblings | 2212 | 94.2% | ||
| Concerned about future healthc | ||||
| Survivors | 84 | 62.2% | 1.05 | (0.91–1.21) |
| Siblings | 1364 | 60.2% | ||
| Survivor level of anxiety/fear as a result of cancer/cancer treatmentc | ||||
| None | 92 | 71.3% | ||
| Small amount | 32 | 24.8% | ||
| Medium amount | 4 | 3.1% | ||
| A lot | 1 | 0.8% | ||
| Extreme | 0 | 0% | ||
aEducational attainment and independent living restricted to observations obtained at age ≥25 years of age.
bCross-sectional analyses adjusted for age at last follow-up, sex, and race.
cAnalyses of the measures of distress, anxiety, and concerns about the future are restricted to observations self-reported by the subject. Questionnaires that were completed by a parent or other surrogate reporter on behalf of a study subject were not used in these analyses.
Memory Impairment and General Psychological Distress
Over 60% of survivors reported learning or memory problems, as opposed to only 3% of the sibling comparison group (RR: 19.8; 95% CI: 15.1–25.9; see Table 4). Among those who reported learning or memory problems, 30% of the survivors and 9% of the siblings reported these problems as disabling. On the BSI-18, survivors were twice as likely to have impaired global function (RR: 2.00; 95% CI: 1.03–3.89) and almost 3 times more likely to report depression (RR: 2.62; 95% CI: 1.55–4.45). There were no significant differences between survivors and siblings on the anxiety and somatization subscales (see Table 4).
Discussion
Five-year survival for children with medulloblastoma or PNET has significantly improved over the last 4 decades. However, the use of craniospinal radiotherapy and the more recent addition of chemotherapy to achieve survival still place them at significant risk for long-term, chronic neurologic conditions and impaired psychosocial functioning. The current study estimates risks for these late effects in one of the largest cohorts to date of aging adult survivors of medulloblastoma/PNET.
Based on these results, it is clear that the cumulative incidence of chronic neurologic late effects continues to increase as this population ages. Almost 40% of survivors had significant hearing loss by 30 years posttreatment. Interventions such as amplification devices and cochlear implants are available and may improve hearing,27 yet many survivors may not continue evaluation for hearing loss after they leave their primary oncology care clinic and establish community-based care during adulthood. Despite the well-established relationships among hearing loss, platinum-based therapies, and radiation,28,29 hearing loss is often known as the “invisible disability,” being frequently ignored.30 Survivors are also at risk for visual impairments due to cataracts, which were 30 times more likely in the survivor cohort compared with siblings; thus, regular ophthalmologic follow-up is imperative. Both the survivor and providers need to be aware of the survivor’s treatment history to prioritize health screening and open the gateway to interventions.
The risk of stroke was much higher in medulloblastoma/PNET survivors than in siblings. Recent CCSS publications have enumerated first and recurrent stroke risk among all survivors with a history of cranial radiation.31–33 Additionally, in single-center retrospective studies, approximately 5% of survivors with a history of cranial or cervical radiation had a stroke by 10 years postdiagnosis.34,35 Among survivors of medulloblastoma or PNET, we found an even higher proportion (11.3%) with a history of stroke, with 59.5% of the strokes occurring more than 5 years postdiagnosis. Previous analysis of data from the Surveillance, Epidemiology, and End Results program for all children treated for CNS malignancies demonstrated an almost 8-fold increased risk of death from cardiovascular or cerebrovascular disease.36 Thus, increased awareness of this high risk of stroke and considerations for prevention are essential for improved survivorship health.
A substantial number of survivors experienced learning or memory problems in the current study. Multiple investigators have documented the cognitive challenges that brain tumor survivors face.37–39 The data are also consistent with smaller studies that documented unemployment among survivors of medulloblastoma.40 Cognitive ability is a determinant of educational attainment, earning potential, and health outcomes.41 Based on the findings of the current study, survivors are greatly at risk for poorer after-effects across these outcomes. These findings highlight the challenges of long-term planning for families of these survivors to ensure a support system.
The majority of survivors in this cohort were treated with CSI with a median focal dose of 52 Gy (interquartile range 50–54) and a median CSI dose of 35 Gy (interquartile range 30–36). While this dose remains the standard of care for patients with high-risk medulloblastoma and embryonal tumors, in the past decade, patients with standard-risk medulloblastoma usually have received 23.4 Gy. Further research will be needed to evaluate long-term effects in patients receiving lower-dose CSI. Additionally, while the total dose to the posterior fossa remains 54 to 55.8 Gy, newer and more conformal techniques (eg, intensity modulated radiation therapy, proton beam radiation therapy) now allow for adequate target coverage with a lower dose of radiation to the cochlea and surrounding supratentorial brain.42,43 Additionally, current chemotherapy dosing includes more dose modifications in response to certain morbidities such as high-frequency hearing loss. The overall impact of these temporal changes in therapy upon the high rates of morbidity we have identified in this manuscript among the first generation of medulloblastoma/PNET survivors will need to be demonstrated.44
There are limitations to the current study that should be considered. Although this is one of the largest cohorts of medulloblastoma/PNET cases reported, the total number of participants represent 25%–30% of the children diagnosed with these tumors in the United States from the period 1970–1986.45 Therefore, there may be limitations in the generalizability of the results. While all of the treatment-related variables were collected via medical record abstraction and SMNs were validated, chronic neurologic conditions and psychosocial functioning were self-reported. However, with regard to the impact on daily living, the perspective of the survivor may be the most valuable. Another limitation is inability to separate patients diagnosed with medulloblastoma from those with PNET. During the 1970s through the mid-1980s, medulloblastomas and PNETs were treated in the same manner, although the “boost” site of radiation varied depending on tumor location. It is possible that patients with PNET experience different long-term toxicity, given the supratentorial location of the tumor and increased radiation dose to the cerebrum due to the boost location. However, we were not able to accurately segregate the survivors to complete reliable subgroup analyses because primary tumor location was not collected on the cohort at initial data capture. Lastly, we chose to focus primarily on neurologic side effects, including cognitive and functional outcomes. Future studies are needed to comprehensively assess and intervene on psychosocial outcomes of these survivors.
Among adult survivors of childhood medulloblastoma/PNET, significant neurologic morbidity and lower levels of educational attainment and social independence exist relative to siblings. Given the high survival rates for these children, interventions to reduce these sequelae and support the survivors should be a high priority.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the National Cancer Institute (CA55727, G. T. Armstrong, Principal Investigator). Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748) for authors CAS and KCO. Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Conflict of interest statement. The authors had no conflicts of interest in writing this manuscript. Investigators interested in potential uses of this resource are encouraged to visit: http://ccss.stjude.org.
Supplementary Material
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–1544. [DOI] [PubMed] [Google Scholar]
- 3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. 2012; http://seer.cancer.gov/csr/1975_2010/. Accessed August 2, 2013. [Google Scholar]
- 4. Hirsch JF, Renier D, Czernichow P, et al. Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien). 1979;48(1–2):1–15. [DOI] [PubMed] [Google Scholar]
- 5. Jenkin D, Goddard K, Armstrong D, et al. Posterior fossa medulloblastoma in childhood: treatment results and a proposal for a new staging system. Int J Radiat Oncol Biol Phys. 1990;19(2):265–274. [DOI] [PubMed] [Google Scholar]
- 6. Packer RJ, Macdonald T, Vezina G, et al. Chapter 35 - Medulloblastoma and primitive neuroectodermal tumors. In: Wolfgang G, Riccardo S, eds. Handbook of Clinical Neurology. Vol 105: Elsevier;2012:529–548. [DOI] [PubMed] [Google Scholar]
- 7. Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 8. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 9. Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23(6):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perkins SM, Fei W, Mitra N, et al. Late causes of death in children treated for CNS malignancies. J Neurooncol. 2013;115(1):79–85. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong GT, Pan Z, Ness KK, et al. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010;28(7):1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oeffinger KC, Mertens AC, Sklar CA, et al. ; Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 15. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21(17):3255–3261. [DOI] [PubMed] [Google Scholar]
- 16. Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88(10):4731–4739. [DOI] [PubMed] [Google Scholar]
- 17. Speechley KN, Barrera M, Shaw AK, et al. Health-related quality of life among child and adolescent survivors of childhood cancer. J Clin Oncol. 2006;24(16):2536–2543. [DOI] [PubMed] [Google Scholar]
- 18. Hudson MM, Mertens AC, Yasui Y, et al. ; Childhood Cancer Survivor Study Investigators. Health status of adult long-term survivors of childhood cancer: a report from the childhood cancer survivor study. JAMA. 2003;290(12):1583–1592. [DOI] [PubMed] [Google Scholar]
- 19. Tonning Olsson I, Perrin S, Lundgren J, et al. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51(4):515–521. [DOI] [PubMed] [Google Scholar]
- 20. Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. [DOI] [PubMed] [Google Scholar]
- 21. Robison LL, Armstrong GT, Boice JD, et al. The childhood cancer survivor study: a national cancer institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derogatis LR. Brief Symptom Inventory–18: Administration, Scoring, and Procedures Manual. Minneapolis: Pearson; 2001. [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 24. Taylor JM, Muñoz A, Bass SM, et al. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Multicentre AIDS cohort study. Stat Med. 1990;9(5):505–514. [DOI] [PubMed] [Google Scholar]
- 25. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 26. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 27. Kuthubutheen J, Hedne CN, Krishnaswamy J, et al. A case series of paediatric hearing preservation cochlear implantation: a new treatment modality for children with drug-induced or congenital partial deafness. Audiol Neurootol. 2012;17(5):321–330. [DOI] [PubMed] [Google Scholar]
- 28. Nitz A, Kontopantelis E, Bielack S, et al. Prospective evaluation of cisplatin- and carboplatin-mediated ototoxicity in paediatric and adult soft tissue and osteosarcoma patients. Oncol Lett. 2013;5(1):311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology boston ototoxicity scale. J Clin Oncol. 2012;30(19):2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shohet JA, Bent T. Hearing loss: the invisible disability. Postgrad Med. 1998;104(3):81–83, 87–90. [DOI] [PubMed] [Google Scholar]
- 31. Fullerton HJ, Stratton K, Mueller S, et al. Recurrent stroke in childhood cancer survivors. Neurology. 2015;85(12):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–5282. [DOI] [PubMed] [Google Scholar]
- 33. Mueller S, Fullerton HJ, Stratton K, et al. Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: a report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2013;86(4):649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(4):643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43(11):3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perkins SM, Fei W, Mitra N, et al. Late causes of death in children treated for CNS malignancies. J Neurooncol. 2013;115(1):79–85. [DOI] [PubMed] [Google Scholar]
- 37. Edelstein K, Spiegler BJ, Fung S, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13(5):536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boman KK, Hovén E, Anclair M, et al. Health and persistent functional late effects in adult survivors of childhood CNS tumours: a population-based cohort study. Eur J Cancer. 2009;45(14):2552–2561. [DOI] [PubMed] [Google Scholar]
- 39. Maddrey AM, Bergeron JA, Lombardo ER, et al. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–253. [DOI] [PubMed] [Google Scholar]
- 40. Frange P, Alapetite C, Gaboriaud G, et al. From childhood to adulthood: long-term outcome of medulloblastoma patients. The Institut Curie experience (1980–2000). J Neurooncol. 2009;95(2):271–279. [DOI] [PubMed] [Google Scholar]
- 41. Heckman JJ. The economics, technology, and neuroscience of human capability formation. Proc Natl Acad Sci U S A. 2007;104(33): 13250–13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merchant TE, Gould CJ, Xiong X, et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58(4):1194–1207. [DOI] [PubMed] [Google Scholar]
- 43. Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9): 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McNeil DE, Coté TR, Clegg L, et al. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39(3):190–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.