Abstract
Background.
Complete prevalence proportions illustrate the burden of disease in a population. This study estimates the 2010 complete prevalence of malignant primary brain tumors overall and by Central Brain Tumor Registry of the United States (CBTRUS) histology groups, and compares the brain tumor prevalence estimates to the complete prevalence of other common cancers as determined by the Surveillance, Epidemiology, and End Results Program (SEER) by age at prevalence (2010): children (0–14 y), adolescent and young adult (AYA) (15–39 y), and adult (40+ y).
Methods.
Complete prevalence proportions were estimated using a novel regression method extended from the Completeness Index Method, which combines survival and incidence data from multiple sources. In this study, two datasets, CBTRUS and SEER, were used to calculate complete prevalence estimates of interest.
Results.
Complete prevalence for malignant primary brain tumors was 47.59/100000 population (22.31, 48.49, and 57.75/100000 for child, AYA, and adult populations). The most prevalent cancers by age were childhood leukemia (36.65/100000), AYA melanoma of the skin (66.21/100000), and adult female breast (1949.00/100000). The most prevalent CBTRUS histologies in children and AYA were pilocytic astrocytoma (6.82/100000, 5.92/100000), and glioblastoma (12.76/100000) in adults.
Conclusions.
The relative impact of malignant primary brain tumors is higher among children than any other age group; it emerges as the second most prevalent cancer among children. Complete prevalence estimates for primary malignant brain tumors fills a gap in overall cancer knowledge, which provides critical information toward public health and health care planning, including treatment, decision making, funding, and advocacy programs.
Keywords: brain tumors, epidemiology, glioblastoma, glioma, prevalence
Importance of the study
Prevalence of brain and other CNS tumors compared with other common cancers provides a perspective which helps us understand the relative impact of this cancer within the US population. By using a novel method to combine cancer registry databases to estimate prevalence proportions, we were able to provide a more accurate estimate of the complex relationships among incidence, survival, and population demographics. The increasing accuracy in prevalence estimates can strengthen the focus of research on this rare cancer.
Malignant primary brain and CNS tumors are rare cancers that cause significant morbidity and mortality across all ages. However, they are one of the major cancer sites whose statistics are not regularly reported by the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI)1 in its regular reporting of SEER Cancer Statistics Review for common cancer sites. In this paper, brain tumors refer to malignant primary brain and other CNS tumors.
The average annual age-adjusted incidence rate of brain tumors in the United States between 2007 and 2011 was 7.25 per 100000 population.2 An incidence rate is defined as the number of at-risk cases per population in a specific time period. While median survival after diagnosis of brain tumor is often short, approximately 34% of patients will live 5 years after diagnosis, and approximately 29% will live 10 years.3
Incidence and survival are the most commonly reported statistics in cancer surveillance, but these may fail to fully describe the burden of cancer within a population. Complete prevalence proportions estimate the total number of people living with a disease and include both new diagnoses within the year of interest and patients surviving diagnoses during previous years. We target a point prevalence at January 1, 2010 and any references to 2010 refer to the first day of the year. Complete prevalence reflects the complex relationships among incidence, survival, and population demographics and provides important information about the total burden of disease in a population. Estimates of complete prevalence provide critical information toward public health and health care planning, including treatment, decision making, funding, and advocacy programs.
Though SEER annually publishes complete prevalence estimates for a number of major cancer types, it does not currently publish these statistics by specific brain tumor histologies. Brain tumors are a heterogeneous group of cancer types with varying patterns of incidence, prognosis, and survival. Although there are over 100 histologies as defined by the World Health Organization (WHO),4 this study focuses on the top 10 major malignant histologies that can be estimated given the available data: pilocytic astrocytoma, diffuse astrocytoma, anaplastic astrocytoma, glioblastoma, oligodendroglioma, anaplastic oligodendroglioma, oligoastrocytic tumors, ependymal tumors, embryonal tumors, and malignant meningioma (WHO grade III). Historically, pilocytic astrocytoma has been coded as malignant in cancer registries based on a decision made when cancer collection was guided by a presidential proclamation. Pilocytic astrocytoma is a tumor classified by WHO as grade I and coded by the International Coding for Disease of Oncology, third edition (ICD-O-3), with an uncertain behavior code of /1. However, tumor registrars are instructed to disregard the /1 behavior code and include pilocytic astrocytoma in the reporting of tumors with a malignant behavior /3, as these tumors have historically been included in malignant cancer surveillance.5 Since data limitations have consistently been an obstacle to accurately estimate statistics for brain tumors, new methods are required to estimate statistics that utilize both older and newer data. This paper brings an extension to the Completeness Index Method (CIM), where the novelty in the method alters the CIM to utilize data from multiple registries.6
In addition, we estimate the 2010 complete prevalence of brain tumors in the US and compare these proportions to the complete prevalence of other common cancers reported by SEER Cancer Statistics Review 1975–2010 to illustrate the importance of including brain tumors in cancer surveillance reports.7 Having complete brain and CNS tumor prevalence estimates for 2010 is critical, since estimates use data from the decennial census. Complete prevalence estimates by Central Brain Tumor Registry of the United States (CBTRUS) histology groups and by age at prevalence are also included.3 Therefore, the primary aim of this study is to estimate the overall 2010 complete prevalence of malignant brain tumors overall, as well as by histology group, and compare these prevalence estimates for those of the most common malignant cancers in the US by standard surveillance age groups. This study utilized the most up-to-date and complete data on brain tumor incidence and survival to landmark the decennial influence, and contextualizes these statistics with the most common cancers by age group to illustrate the broader national impact of brain tumors.
Methods
Data Collection
This work was conducted under approval from the University Hospitals Case Medical Center institutional review board. The data for prevalence calculations were obtained from 2 cancer registries: CBTRUS and SEER. CBTRUS is the largest aggregation of population-based incidence data on brain tumors in the US, with incidence data from 51 registries, representing over 99% of the US population between 2008 and 2012.3 The incidence data were obtained from CBTRUS for 1995–2010 and from SEER 9 for 1975–1994, which includes data from 9 central cancer registries representing approximately 15% of the US population as of the 2010 census.8,9 The survival statistics used in this study were obtained from SEER 18 for the years 1975–2010, which includes malignant data from 18 central cancer registries that represent approximately 28% of the US population as of the 2010 census.8,10 The common cancers used for comparison were obtained from SEER Cancer Statistics Review 1975–2010 for 21 major cancer sites.7 SEER*Stat 8.2.1 statistical software11 was used to generate age-specific incidence rates and overall survival rates for the SEER databases for first malignant primary only criteria. Though nonmalignant brain tumors contribute significantly to the overall burden of morbidity due to brain and CNS tumors in the United States, estimates could not be made for these histologies due to the long survival periods after diagnosis and short lengths of survival data available for these tumors (2004–2010). As a result, this study focuses on malignant tumors only. Population data used for these prevalence estimates were derived from the 2010 US Census.
Statistical Analysis
Complete prevalence can be estimated by the CIM, which sums the observed cases with the unobserved cases from a cancer registry.6 Observed cases are prevalent cases computed from observed incident cases that registries have captured. Unobserved cases are prevalent cases defined by statistically calculated incident cases. The models used to estimate prevalence estimates for cancer generally assume irreversibility of a diagnosis. Thus, once a diagnosis of cancer has been made for a person, that person is assumed to have cancer for life, regardless of whether the cancer progresses after initial treatment.
The CIM states that complete prevalence is a sum of the observed cases diagnosed between the interval [x-L,x], and the unobserved cases diagnosed at previous ages and still living at x in the interval [0,x-L].6 In our case, x denotes the prevalence date (x = 2010), and L denotes the length registry data (L = 15), since we have 15 years of CBTRUS data.
where
N(x) is the complete prevalence at date x. Our study estimates N(2010).
is the prevalence estimated with observed incident data at date 2010 for a registry length of L = 15 for the 15 years of available CBTRUS data.
is the prevalence estimated with unobserved incident data at date 2010.
is the incidence function at age x for a single birth cohort.
is the hypothesis made on disease irreversibility; in our case , which assumes irreversibility of a disease.
is the cumulative relative survival function.
The estimates generated by this model are based on the assumption of disease irreversibility, which is a common assumption used in estimating and measuring cancer prevalence. Diagnosis prevalence assumes that once a case is diagnosed, it is always a cancer case, even though this may not necessarily be true for all individuals.5
The measure of closeness of the observed prevalence to the complete prevalence is given by the completeness index, R:
R is computed from the characteristics of a specific registry and epidemiological characteristics of the disease when exact values of or are unavailable.6 Complete prevalence estimates for common cancer sites in SEER Cancer Statistics Review 1975–2010 are based on 2010 cancer prevalence proportions from the SEER 9 registries, and the CIM was used with the ComPrev software for cases that were diagnosed prior to the initiation of data collection by SEER (>35 y ago).6,7,12
The CIM was not used specifically to compute R for brain tumor registry data, since we wanted to use the most complete data for both incidence and survival from 2 different cancer databases. SEER’s software that computes the CIM is loaded with a subset of the total SEER data and does not include the appropriate data to generate estimates for malignant brain and CNS tumors. To perform the most accurate calculations, using data from a registry that has been around for a long time (SEER) and data from the most current registry (CBTRUS) can make the estimates more accurate. We adopted the main concepts from the CIM, where complete prevalence is a sum of N0(x,L) and NU(x,L), but used regression techniques to predict CBTRUS’ incomplete data from SEER 9’s biased downward data to estimate the unobserved incident cases. Utilizing regression techniques controls for period effects, whereas the CIM’s original method for estimating the unobserved incident cases does not. To align with the standard categorization of age groups for cancer in surveillance by the NCI, brain tumor estimates are separated by age at prevalence (2010): children (0–14 y), adolescent and young adult (AYA) (15–39 y), and adult (40+ y). These separations represent 3 distinct groups with different clinical needs that are relevant for reporting brain tumor statistics.
Histology Complete Prevalence Estimate
Since CBTRUS data represent approximately 99% of the US population, the CBTRUS incidence data were assumed to be nearly complete and used as the observed cases. SEER 18 has the most complete and accurate population-based survival data for malignant brain tumors and was used to generate overall survival estimates for each histology. It was assumed that survival drops to zero after 15 years for each histology, and the CBTRUS data provided 15 years of complete incidence. As a result, the histology analysis only required computation for using direct methods,13 and the equation for estimating complete prevalence simplified to
Overall Brain Tumors Complete Prevalence Estimate
Similar methods utilizing the CBTRUS and SEER 18 datasets were also used to estimate the incidence and survival functions for overall brain tumors. For calculations of malignant brain tumor prevalence overall, we did not assume that survival dropped to zero after 15 years. Therefore, CBTRUS data account for only the observed portion of incident cases. Incidence and survival data for the SEER 9 registries represent the source of longest period of population-based data collection for malignant brain tumors (1975–2010). Incidence data from SEER 9 between 2000 and 2010 were used to perform a linear regression, to allow for 5 years of stabilization in the CBTRUS data between 1995 and 2000. This linear model was created to describe the relationship between the databases to predict what CBTRUS’ incidence data may have been during the years when only SEER 9 data (1975–1994) were available. This estimate represents the unobserved incident cases. The unobserved incident cases were combined with SEER 18’s survival estimates to determine the prevalence of malignant brain tumors in 2010 from those cases diagnosed between 1975 and 1994. This analysis assumed that survival dropped to zero for cases diagnosed prior to 1975. The equation for estimating complete prevalence for brain tumors is:
To illustrate the importance of stratifying cancers by age, the relative proportions of prevalence counts by age at prevalence were calculated.
Cases diagnosed by autopsy alone or from death certificates were excluded from this analysis. Since cancer incidence and survival vary significantly by age, all calculations were stratified by age at prevalence into 5-year age groups using the counting approach for prevalence estimation. Based on this method, we broke time groupings down to 5-year intervals. Computations are done by these time groupings which define incidence and survival for that particular grouping. These 5-year intervals were combined to create the respective age and histology groupings for prevalence estimates of brain tumors. This method is used in the prevalence session of the SEER*Stat software. All prevalence calculations were performed using R 3.2.2 and the ggplot2 graphics package.14,15
Results
Approximately 608804 incident cases of brain tumors were diagnosed between 1975 and 2010 (294666 observed cases; 314138 unobserved cases). The numbers of incident cases for the most prevalent histologies stratified by age were: 4311 childhood and 6852 AYA pilocytic astrocytoma, and 130627 adult glioblastoma.
Brain tumors overall had low 15-year survival rates: in children, the lowest 15-year survival rates by age for each age group were 43% for children at age 0 years, 41% for AYA at age 24 years, and 0% for adults older than 85 years. Survival rates were highest in younger age groups across all common cancers compared with older persons.
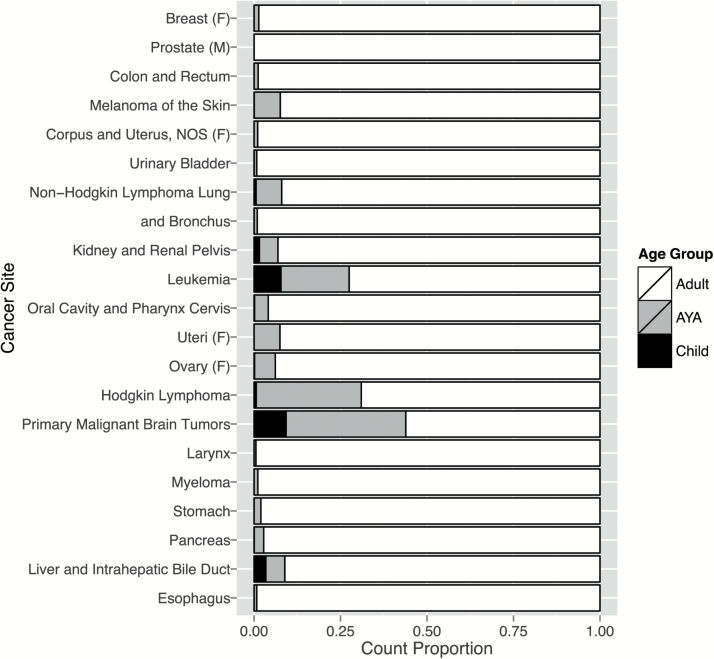
When comparing our estimates of complete prevalence proportions for brain tumors using the altered CIM versus the complete prevalence of cancer sites reported by SEER using its standard CIM software, the overall top 3 cancers remained the same: breast (female only) (914.57 per 100000), prostate (male only) (846.24 per 100000), and colon/rectum (373.22 per 100000) (Table 1). Cancer in adults was more prevalent than in any other age group. The most prevalent childhood cancer was leukemia (36.65 per 100000); brain tumors were the second most prevalent among children, at 22.31 per 100000 population. The most prevalent cancer among AYA was melanoma of the skin (66.21 per 100000), and brain tumors were the fourth most prevalent cancer among AYA at 48.49 ± 0.69 per 100000. The most common cancer site for adults was female breast (1949.00 per 100000). Brain tumors were not among the top cancers for adults (57.75 ± 0.50 per 100000) (Table 1).
Table 1.
US complete prevalence counts, invasive cancers only, 2010 by age at prevalence
| Site | All Agesc | Children (0–14 y)c | AYA (15–39 y)c | Older Adult (40+ y)c | ||||
|---|---|---|---|---|---|---|---|---|
| Counts | Prevalencea | Counts | Prevalencea | Counts | Prevalencea | Counts | Prevalencea | |
| Breast (female only) | 2,829,041 | 914.57 | 0 | 0.00 | 39,084 | 37.23 | 2,789,956 | 1,949.00 |
| Prostate (male only) | 2,617,682 | 846.24 | 46 | 0.08 | 473 | 0.45 | 2,617,166 | 1,828.30 |
| Colon and rectum | 1,154,481 | 373.22 | 21 | 0.03 | 13,722 | 13.07 | 1,140,728 | 796.89 |
| Melanoma of the skin | 921,780 | 297.99 | 504 | 0.82 | 69,510 | 66.21 | 851,765 | 595.03 |
| Corpus and uterus, NOS (female only) | 600,346 | 194.08 | 0 | 0.00 | 6,244 | 5.95 | 594,103 | 415.03 |
| Urinary bladder | 563,640 | 182.21 | 131 | 0.21 | 4,330 | 4.12 | 559,180 | 390.63 |
| Non-Hodgkin lymphoma | 509,065 | 164.57 | 2,964 | 4.84 | 37,772 | 35.98 | 468,327 | 327.16 |
| Lung and bronchus | 399,431 | 129.13 | 104 | 0.17 | 3,592 | 3.42 | 395,735 | 276.45 |
| Kidney and renal pelvis | 341,505 | 110.40 | 5,275 | 8.62 | 18,293 | 17.43 | 317,937 | 222.10 |
| Leukemia | 287,963 | 93.09 | 22,429 | 36.65 | 56,761 | 54.07 | 209,773 | 146.54 |
| Oral cavity and pharynx | 275,193 | 88.96 | 423 | 0.69 | 10,857 | 10.34 | 263,911 | 184.36 |
| Cervix uteri (female only) | 249,496 | 80.66 | 22 | 0.04 | 18,668 | 17.78 | 230,806 | 161.24 |
| Ovary (female only) | 186,138 | 60.17 | 287 | 0.47 | 11,151 | 10.62 | 174,700 | 122.04 |
| Hodgkin lymphoma | 181,928 | 58.81 | 1,150 | 1.88 | 55,252 | 52.63 | 125,525 | 87.69 |
| Primary brain tumorsb | 147,220 | 47.59 ± 0.43 | 13,657 | 22.31 | 50,902 | 48.49 ± 0.69 | 82,661 | 57.75 ± 0.50 |
| Larynx | 89,029 | 28.78 | 0 | 0.00 | 480 | 0.46 | 88,550 | 61.86 |
| Myeloma | 77,617 | 25.09 | 0 | 0.00 | 852 | 0.81 | 76,764 | 53.63 |
| Stomach | 72,269 | 23.36 | 16 | 0.03 | 1,422 | 1.35 | 70,834 | 49.48 |
| Pancreas | 41,609 | 13.45 | 23 | 0.04 | 1,135 | 1.08 | 40,450 | 28.26 |
| Liver and intrahepatic bile duct | 41,404 | 13.39 | 1,418 | 2.32 | 2,272 | 2.16 | 37,713 | 26.35 |
| Esophagus | 33,839 | 10.94 | 0 | 0.00 | 270 | 0.26 | 33,569 | 23.45 |
a Rates are per 100000 and age-adjusted to the 2010 US standard population.
b US 2010 cancer prevalence counts are based on the 2010 cancer prevalence proportions from CBTRUS 1995–2011 and statistical analyses from the SEER 9 registries.
c Due to rounding, the sum of the age specific estimates may not equal the all ages estimate.
Listed cancer sites are ordered by 2010 prevalence estimates.
US 2010 cancer prevalence counts are based on 2010 cancer prevalence proportions from the SEER 9 registries (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta) on 1/1/2010.
US population estimates are based on the average of 2009 and 2010 populations.
Cases diagnosed more than 35 years ago were estimated using the completeness index method (Capocaccia et al, 1997; Merrill et al, 2000).
NOS, not otherwise specified.
There was significant variation in the distribution of age at prevalence by cancer site (Fig. 1). Brain tumors overall had a complete prevalence proportion of 47.59 ± 0.43 per 100000 population. Among brain tumors, adults represented the largest proportion of prevalent cases (56.1%), followed by AYA (34.6%), then children (9.3%). Although female breast cancer was the most prevalent cancer, there were no prevalent cases among children (0.0%) (Fig. 1). While brain tumors were not one of the most prevalent cancers overall, children represented a greater proportion of prevalent cases than any other common cancer site listed.
Fig. 1.
The relative prevalence count proportions of SEER Cancer Statistics Review 1975–2010 common cancer sites and malignant primary brain and CNS tumors were stratified by age at prevalence groups. NOS, not otherwise specified.
The adjustment for the unobserved incident cases for complete prevalence of overall malignant brain tumors contributed the largest increases of prevalent cases to the AYA age group. Due to the age group utilized for children, all incident cases for these prevalence calculations came from CBTRUS and had no effect when adjusting for complete prevalence. Among AYA, the complete prevalence proportion increased from 29.81 (observed cases only) to 48.49 (observed and unobserved cases) per 100000 population (63% increase). For adults, the complete prevalence increased from 33.80 (observed cases only) to 58.71 (observed and unobserved cases) per 100000 population (74% increase) (Table 2).
Table 2.
Pre-adjusted prevalence vs adjusted prevalence for primary malignant brain tumors in the US, 2010
| Age | Counts | Adjusted Counts | Prevalencea | Adjusted Prevalencea |
|---|---|---|---|---|
| Child (0–14 y) | 13,657 | 13,657 | 22.31 | 22.31 |
| 00-04 | 2,319 | 2,319 | 11.49 | 11.49 |
| 05-09 | 4,838 | 4,838 | 23.79 | 23.79 |
| 10–14 | 6,499 | 6,499 | 31.43 | 31.43 |
| AYA (15–39 y) | 31,299 | 50,902 | 29.81 | 48.49 |
| 15–19 | 6,971 | 7,759 | 31.72 | 35.30 |
| 20–24 | 6,185 | 9,410 | 29.31 | 51.98 |
| 25–29 | 5,930 | 10,890 | 28.05 | 51.50 |
| 30-24 | 5,983 | 11,173 | 29.81 | 55.67 |
| 35–39 | 6,229 | 11,671 | 31.03 | 58.13 |
| Adult (40+ y) | 58,678 | 82,661 | 33.80 | 58.71 |
| 40–44 | 7,065 | 12,272 | 40.99 | 57.75 |
| 45–49 | 8,423 | 13,241 | 37.21 | 58.50 |
| 50–54 | 8,686 | 12,710 | 38.86 | 56.87 |
| 55–59 | 8,407 | 11,761 | 42.47 | 59.42 |
| 60–64 | 7,869 | 10,493 | 46.32 | 61.76 |
| 65–69 | 6,134 | 7,777 | 48.99 | 62.11 |
| 70–74 | 4,694 | 5,626 | 50.28 | 60.26 |
| 75–79 | 3,599 | 4,181 | 49.18 | 57.12 |
| 80–84 | 2,573 | 3,007 | 44.68 | 52.22 |
| 85+ | 1,228 | 1,594 | 22.16 | 28.75 |
| All | 103,634 | 147,220 | 33.50 | 47.59 |
aProportions are per 100000 population.
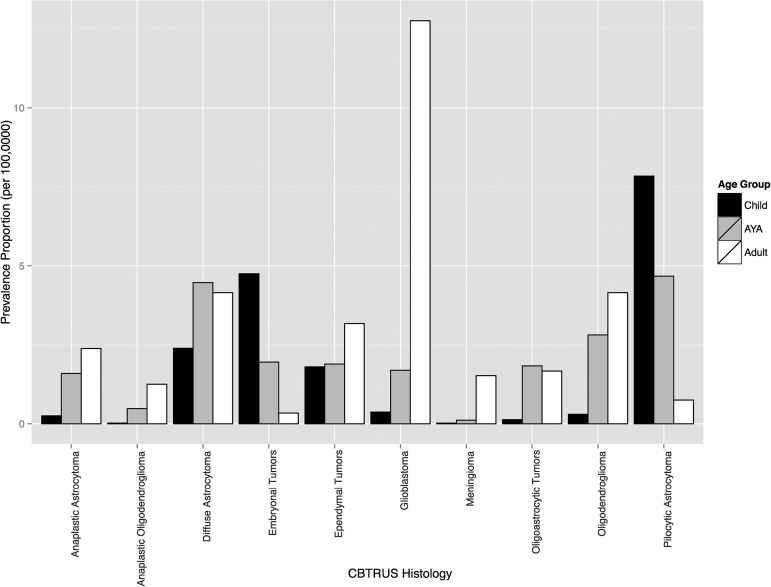
The most prevalent brain tumor histologies varied by age at prevalence (Table 3, Fig. 2). The most prevalent histologic types were glioblastoma (6.46 per 100000), diffuse astrocytoma (3.76 per 100000), and pilocytic astrocytoma (3.71 per 100000). The most prevalent tumor types by age were childhood pilocytic astrocytoma (30.6% of cases, prevalence 6.82 per 100000), AYA pilocytic astrocytoma (12.2% of cases, prevalence 5.92 per 100000), and adult glioblastoma (22.1% of cases, prevalence 12.76 per 100000) (Table 3, Fig. 2). Malignant meningioma (WHO grade III) had the lowest prevalence of the reported histologies. The reported brain tumor histologies were selected specifically to illustrate the most prevalent histologies.
Table 3.
Prevalence proportions by CBTRUS histology groupings for selected malignant brain tumors and by age groups in the United States, 2010
| Histologyc | Allb | Children (0–14 y)b | AYA (15–39 y)b | Older Adult (40+ y)b | ||||
|---|---|---|---|---|---|---|---|---|
| Count | Prevalence a | Count | Prevalence a | Count | Prevalence a | Count | Prevalence a | |
| Tumors of the Neuroepithelial Tissue | ||||||||
| Pilocytic astrocytoma | 11,464 | 3.71 | 4,177 | 6.82 | 6,215 | 5.92 | 1,072 | 0.75 |
| Diffuse astrocytoma | 11,644 | 3.76 | 1,260 | 2.06 | 4,437 | 4.23 | 5,947 | 4.15 |
| Anaplastic astrocytoma | 4,933 | 1.59 | 126 | 0.21 | 1,401 | 1.33 | 3,406 | 2.38 |
| Glioblastoma | 19,972 | 6.46 | 207 | 0.34 | 1,506 | 1.43 | 18,259 | 12.76 |
| Oligodendroglioma | 8,523 | 2.76 | 121 | 0.20 | 2,464 | 2.35 | 5,939 | 4.15 |
| Anaplastic oligodendroglioma | 2,201 | 0.71 | 6 | 0.01 | 405 | 0.39 | 1,790 | 1.25 |
| Oligoastrocytic tumors | 4,017 | 1.30 | 70 | 0.11 | 1,559 | 1.48 | 2,388 | 1.67 |
| Ependymal tumors | 7,605 | 2.46 | 1,053 | 1.72 | 2,011 | 1.92 | 4,541 | 3.17 |
| Embryonal tumors | 6,050 | 1.96 | 2,855 | 4.66 | 2,712 | 2.58 | 483 | 0.34 |
| Tumors of the Meninges | ||||||||
| Meningioma | - | - | - | - | 94 | 0.09 | 2,170 | 1.52 |
| All other | 68,547 | 22.16 | 3,781 | 6.18 | 28,099 | 26.77 | 36,667 | 25.61 |
| Age group TOTAL† | 147,219 | 47.59 ± 0.43 | 13,656 | 22.31 ± 0 | 50,902 | 48.49 ± 0.69 | 82,661 | 57.75 ± 0.50 |
a Proportions are per 100,000 and age-adjusted to the 2010 US standard population.
b Due to rounding, the sum of the age specific estimates may not equal the all ages estimate.
c See Ref. 2, Table 2 for specific ICD-O-3 histology codes.
Counts and proportions are not presented when fewer than 16 cases were reported for the specific histology category. The suppressed cases are included in the counts and proportions for totals.
All CBTRUS histology groups have malignant brain tumors included, although some groups may be known to have a large proportion of nonmalignant brain tumors; this study focused on malignants only.
NOS, not otherwise specified.
Fig. 2.
Prevalence proportion per 100000 population of selected major CBTRUS histologies by age at prevalence groups.
Discussion
Prevalence proportions are a critical component for estimating the impact of a disease on a population and are an important complement to the more commonly reported statistics for cancer incidence and survival. Prevalence estimates provide important population-based information to researchers, clinicians, public health officials, and disease interest groups and clinicians and influence the direction of future research. In rare cancers such as malignant brain tumors, these statistics may not be commonly calculated as part of omnibus cancer prevalence reporting. This analysis used the most up-to-date and complete data available at the time of this analysis and included specific brain tumor histology groups.16,17
Brain and other CNS tumor prevalence estimates vary widely by age and by histology groups. Incidence of malignant brain tumors peaks among young children and in the fifth to seventh decades of life.3 Though malignant brain tumors are not as commonly occurring among AYA, brain tumors (especially nonmalignant brain tumors) are a significant contributor to cancer incidence in this age group.18 The pre-adjusted prevalence proportions of this study aligned with this observation, but after including the unobserved incident cases using a variation of the CIM, we noticed that prevalence increased with age. AYA had a brain tumor prevalence proportion of 48.49 per 100000 compared with 22.31 in children and 57.75 in adults. However, the relative impact of the disease on the age group was inversely related to the age (Fig. 1). AYA had the largest adjustments on complete prevalence proportions (unobserved cases) of brain tumors with the linear model because childhood incidence had been completely accounted for by CBTRUS, and survival has been reported as the poorest among adults. Pilocytic astrocytoma was the most prevalent tumor type for both children and AYA (6.82 and 5.92, respectively, per 100000). Prevalence estimates for this analysis were calculated using age at prevalence, rather than age at diagnosis. As a result, it is likely that many of the prevalent AYA cases are childhood brain tumor survivors. This is reflected in the relatively high ratio of prevalence to incidence in pilocytic astrocytomas (prevalence proportion: 5.92 per 100000, incidence rate [2008–2012]: 0.28 per 100000) and diffuse astrocytoma (prevalence proportion: 4.23 per 100000, incidence rate [2008–2012]: 0.47 per 100000). These are common tumors in childhood with comparatively long survival, and it is likely that increasing the completeness of the prevalence estimates would more accurately describe the prevalence of histologies in this age group.
This is the first analysis utilizing these methods to estimate prevalence for specific brain tumor histologies, which uses data from the 2010 decennial census rather than predictions from the previous decade. Just as there are over 100 different types of brain tumor histologies as identified by WHO, the prevalence of specific histologies is different and varies significantly over the lifespan.4 Given that there is significant heterogeneity in patterns of incidence, prognosis, and survival between these different brain tumor histologic subtypes, presenting data at this level of detail is warranted.
Glioblastoma (WHO grade IV astrocytoma) was the most common malignant brain tumor histology in 2010 and represents 13.6% of all malignant brain tumors (Table 3, Fig. 2). These tumors are the most common malignant brain tumor in adults, and their incidence rate is 6.95 per 100000.3 Prognosis after diagnosis of glioblastoma is very poor, with a median survival of 12–14 months.19 Diffuse astrocytoma represents the second most prevalent brain tumor histology and has a prevalence proportion of 3.76 per 100000, which represents 7.9% of all brain tumors. The broad category of diffuse astrocytoma includes specific astrocytoma histologies that are generally assigned a WHO grade of II.2 Incidence of these tumors in 2010 was 0.57 per 100000, the second highest of all glioma subtypes. These tumors have significantly better prognoses than WHO grade III (eg, anaplastic astrocytoma) and WHO grade IV gliomas (eg, glioblastoma), and approximately 9.6% of all persons diagnosed with these tumors survive at least 15 years.
The calculated prevalence estimates for brain tumors in this analysis are higher than those previously calculated by Davis et al16 and Porter et al.17 There are several differences in data and methodology that may have caused these differences. The previous estimates were calculated with less complete incidence data; the incidence of the most recent prior report covered approximately 33% of the US population,17 while this analysis was based on incidence data from approximately 99% of the US population. Utilizing regression techniques with an existing database (SEER 9) to estimate the unobserved incident cases controls for period effects, whereas the CIM utilizes a projection of modeled incidence to calendar years prior to the observed period.13 This analysis also did not directly assume that survival drops to zero after 15 years. The survival data used for this project were more complete than data previously used, and the decision for suitable cutoffs for when survival dropped to zero were data driven. This is a necessary limitation of the model that may not reflect clinical reality, and may lead to underestimation of prevalence for some histologies with higher long-term survival. This analysis used survival data from the SEER 18, which includes 18 population-based registries that represent approximately 28% of the US population. The previous analyses used survival data from 2 collaborating cancer registries within the SEER 18 that were not necessarily representative of the broader US population. The maturation of databases resulted in differences from Davis’ and Porter’s algorithms and allowed for consideration of an observed and unobserved portion of the prevalence estimate. The unobserved portion of the data was based on SEER 9 incidence data to project CBTRUS data, since SEER 9 has been collected for the longest period of time. We assumed that the method of data collection for SEER 9 has aligned with standard protocol. In Porter’s analysis, the authors projected prevalence for 2010, which was 6 years beyond the available data at that time. Previous analyses used the 2000 US census data, whereas this analysis used the 2010 US census data. Hence, the statistics presented in the current paper represent the most up-to-date prevalence estimates overall of brain and other CNS tumors by histology groups. While there are differences between databases used and methods used, the 38-year limited duration estimates by SEER Cancer Statistics Review are similar to the prevalence estimates that were generated using this new method incorporating the patching of data from 2 different cancer registries.7
The prevalence of nonmalignant brain tumors has not been estimated because reporting of nonmalignant brain tumors to state cancer registries had not became mandatory until January 1, 2004 with the implementation of the Benign Brain Tumor Cancer Registries Amendment Act.20 Though several registries collected data on these nonmalignant tumors prior to the mandate, there were only 7 years of data on nonmalignant brain tumors (2004–2011) from all central (state) cancer registries in the US at the time of this analysis. Nonmalignant meningiomas (WHO grade I and II) have the highest survival rate, with 53% at 20 years.21 Thus, at least 20 years of survival is needed to perform reliable estimates of nonmalignant brain and other CNS tumor prevalence proportions given patients’ longer overall survival.
One limitation of all prevalence estimations is the inability to account completely for all living cases of any cancer.17 Hence, all diagnosed cases reported between 1975 and 2010 were used for this analysis. A limitation of this approach is that the survival data are based upon approximately only 28% of the US population and may inadequately reflect the number of prevalent cases. Additionally, the statistical assumption of irreversibility of a disease used in the generation of these estimates is not necessarily accurate. Patients can experience remission from their cancer and remain completely cancer free for the rest of their lives (also known as fatal cases prevalence).6 However, the fatal cases prevalence would be difficult to estimate, since the definition of “cured” is subjective between disease types. This analysis also assumed relatively stable incidence and survival rates over time, and if these assumptions are inaccurate, these rates may inaccurately contribute to prevalence. Nevertheless, incidence rates have stabilized in recent years for most cancers, including brain tumors.22 Cancer registries in the United States do not have the infrastructure to conduct central pathology reviews, and as a result this analysis used only the diagnosis assigned by the pathologist involved in each patient’s clinical care. During the time period covered by this analysis, there have been revisions to the pathologic criteria for several of the histologic categories (notably embryonal tumors, meningioma, and oligodendrogliomas) used in these estimates that may have some effect on incidence estimates. The 2016 WHO Classification of Tumours of the Central Nervous System23 contains significant revisions to diagnostic criteria for glioma to more accurately reflect prognosis. With this recent revision to the WHO criteria for CNS tumors,23IDH1/2 mutation and 1p/19q codeletion will become the primary factors by which gliomas are classified. Data on isocitrate dehydrogenase (IDH)1/2 mutation status are not currently collected in the US cancer registry system, and while 1p/19q deletion data are collected, these data vary significantly in completeness by histology.24 Though the coding changes contained within this revision are not currently adopted by the US cancer registry system, it is likely that these changes to diagnostic criteria may affect the incidence and survival estimates of these tumor types in future years. This revision does not affect this analysis, which focuses solely on estimating prevalence in the year 2010. For the vast majority of malignant brain tumors, there have not been significant advances in treatment modalities and improved survival for adults within the last decade. Survival estimates were generated for each cohort using the survival times observed for their period of diagnosis, which would take into account any survival benefit conferred by new treatments utilized during the time period covered by this analysis.
Conclusions
Although the presented prevalence proportions are higher than previous estimates for brain and other CNS tumors, the results of this study suggest that higher estimates resulted directly from the availability of more complete and accurate incidence and survival rates. These improved rates can in part be explained by the maturing of rubrics guiding the standardization of the resource databases. Even though brain tumors are not the most prevalent cancer in all age groups, these tumors are consistently prevalent throughout life and therefore merit documentation. The routine availability of prevalence estimates for brain and other CNS tumors provides a resource to support research proposals, public health projections, and health care decisions for cancer patients and contributes useful information to standard cancer reporting practices.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 200-2016-M-90304, The Sontag Foundation, Genentech, Novocure, Celldex, AbbVie, along with the Musella Foundation, Voices Against Cancer, Elekta, and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in kind donations.
Acknowledgments
This study was presented at the 20th Annual Society for Neuro-Oncology meeting in San Antonio, Texas, November 19–22, 2015 and in part at the North American Association of Central Cancer Registries 2016 Annual Conference in St. Louis, Missouri, June 11–16, 2016.
Conflict of interest statement. There is no conflict of interest.
References
- 1. Surveillance Epidemiology and End Results Program. Public Law 107–260 training.seer.cancer.gov/brain/non-malignant/history/public.html. Accessed November 14, 2016.
- 2. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(s4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Data Collection of Primary Central Nervous System Numbers National Program of Cancer Registries Training Materials. Atlanta, Georgia: Department of Health and Human Services, Centers for Disease Control and Prevention;2004. [Google Scholar]
- 6. Capocaccia R, De Angelis R. Estimating the completeness of prevalence based on cancer registry data. Stat Med. 1997;16(4):425–440. [DOI] [PubMed] [Google Scholar]
- 7. Howlader N, Noone AM, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975–2010, Based On November 2012 SEER Data Submission, Posted to the SEER Web Site, April 2013. Bethesda, MD: National Cancer Institute;2013. [Google Scholar]
- 8. National Cancer Institute. Overview of the SEER Program http://seer.cancer.gov/about/overview.html. Accessed November 14, 2016.
- 9. Surveillance Epidemiology and End (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012)—Linked To County Attributes—Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission . [Google Scholar]
- 10. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973–2013 varying)—Linked To County Attributes—Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission . [Google Scholar]
- 11. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.2.1; www.seer.cancer.gov/seerstat, 2015, Accessed November 14, 2016.
- 12. Surveillance Research Program. Complete Prevalence (ComPrev) Software version 2.0, https://surveillance.cancer.gov/comprev/, 2015. Accessed November 14, 2016. [Google Scholar]
- 13. Verdecchia A, De Angelis G, Capocaccia R. Estimation and projections of cancer prevalence from cancer registry data. Stat Med. 2002;21(22):3511–3526. [DOI] [PubMed] [Google Scholar]
- 14. Wickham H. ggplot2: elegant graphics for data analysis. Springer Science & Business Media, 2009.
- 15. R Core Team. R: A language and environment for statistical computing http://www.R-project.org/. Accessed November 14, 2016.
- 16. Davis FG, Kupelian V, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol. 2001;3(3):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porter KR, McCarthy BJ, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostrom QT, Gittleman H, de Blank PM, et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2016;18(Suppl 1):i1–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 20. Benign Brain Tumor Cancer Registries Amendment Act 2002; http://www.gpo.gov/fdsys/pkg/PLAW-107publ260/pdf/PLAW-107publ260.pdf. Accessed November 14, 2016.
- 21. van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14(5):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gittleman HR, Ostrom QT, Rouse CD, et al. Trends in central nervous system tumor incidence relative to other common cancers in adults, adolescents, and children in the United States, 2000 to 2010. Cancer. 2015;121(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Louis DN, Ohgaki H, Wiestler OD, et al. eds. WHO Classification of Tumours of the Central Nervous System.Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 24. Ostrom QT, Gittleman H, Kruchko C, et al. Completeness of required site-specific factors for brain and CNS tumors in the Surveillance, Epidemiology and End Results (SEER) 18 database (2004–2012, varying). J Neurooncol. 2016;130(1): 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]