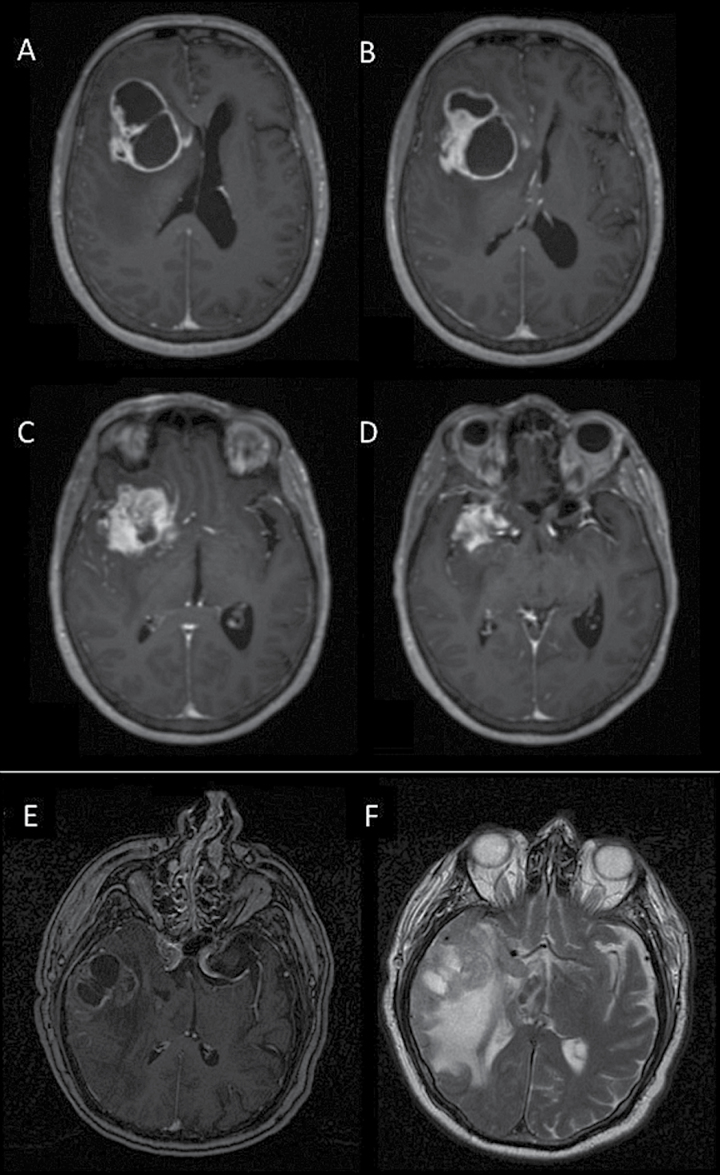
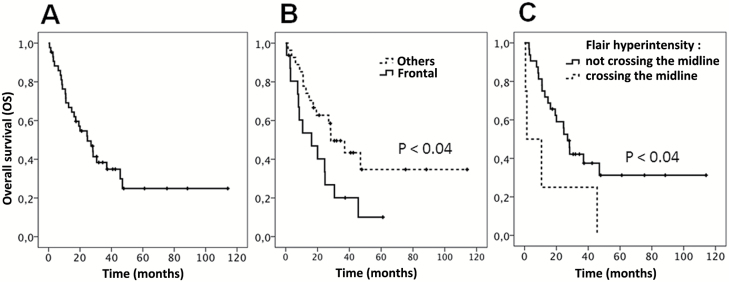
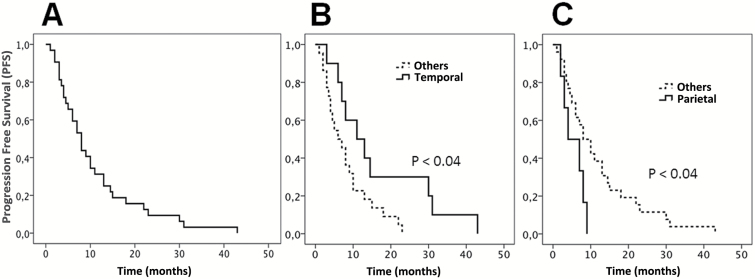
Louis-Marie Terrier
Louis-Marie Terrier
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Luc Bauchet
Luc Bauchet
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Valérie Rigau
Valérie Rigau
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Aymeric Amelot
Aymeric Amelot
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Sonia Zouaoui
Sonia Zouaoui
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Isabelle Filipiak
Isabelle Filipiak
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Agnès Caille
Agnès Caille
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Fabien Almairac
Fabien Almairac
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Marie-Hélène Aubriot-Lorton
Marie-Hélène Aubriot-Lorton
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Anne-Marie Bergemer-Fouquet
Anne-Marie Bergemer-Fouquet
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Eric Bord
Eric Bord
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Philippe Cornu
Philippe Cornu
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Alain Czorny
Alain Czorny
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Phong Dam Hieu
Phong Dam Hieu
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Bertrand Debono
Bertrand Debono
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Marie-Bernadette Delisle
Marie-Bernadette Delisle
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Evelyne Emery
Evelyne Emery
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Walid Farah
Walid Farah
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Guillaume Gauchotte
Guillaume Gauchotte
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Catherine Godfraind
Catherine Godfraind
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Jacques Guyotat
Jacques Guyotat
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Bernard Irthum
Bernard Irthum
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Kevin Janot
Kevin Janot
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Pierre-Jean Le Reste
Pierre-Jean Le Reste
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Dominique Liguoro
Dominique Liguoro
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Hugues Loiseau
Hugues Loiseau
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Guillaume Lot
Guillaume Lot
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Vincent Lubrano
Vincent Lubrano
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Emmanuel Mandonnet
Emmanuel Mandonnet
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Philippe Menei
Philippe Menei
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Philippe Metellus
Philippe Metellus
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Serge Milin
Serge Milin
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Bertrand Muckenstrum
Bertrand Muckenstrum
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Pierre-Hugues Roche
Pierre-Hugues Roche
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Audrey Rousseau
Audrey Rousseau
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Emmanuelle Uro-Coste
Emmanuelle Uro-Coste
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Anne Vital
Anne Vital
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Jimmy Voirin
Jimmy Voirin
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Michel Wager
Michel Wager
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Marc Zanello
Marc Zanello
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Patrick François
Patrick François
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Stéphane Velut
Stéphane Velut
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Pascale Varlet
Pascale Varlet
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Dominique Figarella-Branger
Dominique Figarella-Branger
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Johan Pallud
Johan Pallud
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,
Ilyess Zemmoura
Ilyess Zemmoura
1CHRU de Tours, Service de Neurochirurgie, Tours, France (L.-M.T., P.F., S.V., I.Z.); Université François-Rabelais de Tours, Inserm, Imagerie et Cerveau UMR U930, Tours, France (L.-M.T., S.V., I.Z.); Department of Neurosurgery and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (L.B., S.Z.), French Brain Tumor DataBase, ICM, Montpellier, France (L.B., S.Z., V.R.); Department of Neuropathology and INSERM U1051, Hôpital Saint Eloi - Gui de Chauliac, Montpellier, France (V.R.); Department of Neurosurgery, Hôpital La Pitié Salpétrière, APHP, Paris, France (A.A., P.C.); Plateforme CIRE, UMR-PRC, 37380 Nouzilly, Centre INRA Val de Loire (I.F.); Université François-Rabelais de Tours, Tours, France (A.C.); Inserm, CIC 1415, CHRU de Tours, Tours, France (A.C.); Department of Neurosurgery, Hôpital Pasteur, University Hospital Center, 06000, Nice, France (F.A.); Department of Pathology, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (M.-H.A.-L.); Service d’Anatomie et Cytologie Patholgique, CHRU de Tours, Tours, France (A.-M.B.-F.); Department of Neurosurgery and Neurotraumatology, Nantes University Hospital, Nantes, France (E.B.); Service de Neurochirurgie, CHU Jean-Minjoz, 3 boulevard Alexander-Fleming, Besançon cedex, France (A.C.); Department of Neurosurgery, CHU de la Cavale Blanche, Brest, France (P.D.H.); Department of Neurosurgery, Cèdres Hospital, Toulouse, France (B.D.); Laboratoire Universitaire d’Anatomie Patholgique, Neuropathologie humaine et expérimentale, CHU Rangueil, Toulouse, France (M.-B.D.); Department of Neurosurgery, University Hospital of Caen, Caen, France (E.E.); Service de Neurochirurgie, Hôpital François Mitterand, CHU de Dijon, 14 rue Paul Gaffarel, 21000 Dijon, France (W.F.); Department of Pathology, CHU Nancy and INSERM U954, Faculty of Medicine, Université de Lorraine, France (G.G.); Department of Pathology, CHU de Clermont-Ferrand, Clermont-Ferrand, France (C.G.); Department of Neurosurgery, Neurological Hospital, Lyon, France (J.G.); Service de neurochirurgie, hôpital Gabriel-Montpied, CHU de Clermont-Ferrand, 58, rue Montalembert, 63003 Clermont-Ferrand, France (B.I.); Service de Neuroradiologie, CHRU de Tours, Tours, France (K.J.); Department of Neurosurgery, University Hospital Pontchaillou, 2, Rue Henri Le Guilloux, 35000, Rennes, France (P.-J.L.R.); Service de neurochirurgie A, place Amélie-Raba-Léon, 33076 Bordeaux cedex, France (D.L.); Université de Bordeaux - Service de Neurochirurgie B, hôpital Pellegrin Tripode, Bordeaux, France (H.L.); Department of Neurosurgery, Fondation Ophtalmologique Rothschild, Paris, France (G.L.); Service de neurochirurgie, hôpital de Rangueil, CHU de Toulouse, 1, avenue du Professeur-Jean-Poulhès, TSA 50032, 31059 Toulouse, France (V.L.); Department of Neurosurgery, Lariboisière Hospital, 75010 Paris, France (E.M.); Département de neurochirurgie, CHU d’Angers, 4, rue Larrey, 49940 Angers cedex 9, France (P.M.); Département de neurochirurgie, Aix-Marseille université, CHU Timone, Assistance publique-Hôpitaux de Marseille, 264, rue Saint-Pierre, 13385 Marseille cedex 05, France (P.M.); Department of Pathology, CHU de Poitiers, Hôpital la Milétrie, Poitiers, France (S.M.); Department of Neurosurgery, Orléans Hospital, France (B.M.); Service de Neurochirurgie, Hôpital Nord, APHM, University Hospital of Marseille Aix-Marseille Univ, Marseille, France (P.-H.R.); Département de Pathologie Cellulaire et Tissulaire, Centre Hospitalo-universitaire d’Angers, 4 rue Larrey, 49933, Angers Cedex 09, France (A.R.); CHU Toulouse, Hôpital de Rangueil, Service d’Anatomie et Cytologie Pathologique, 31400 Toulouse, France (E.U.-C.); Bordeaux Institute of Neuroscience, CNRS UMR 5227, F-33076, Bordeaux, France (A.V.); Department of Neurosurgery, Strasbourg-Colmar Hospital, France (J.V.); Department of Neurosurgery, Imaging Laboratory, University Hospital Poitiers, 2 Rue de La Miletrie, Poitiers Cedex, France (M.W.); Paris Descartes University, Sorbonne Paris Cité, Paris, France (M.Z., P.V., J.P.); Department of Neurosurgery, Sainte-Anne Hospital, Paris, France (M.Z., J.P.); Department of Neuropathology, Sainte-Anne Hospital, Paris, France (P.V.); Department of Pathology and Neuropathology, Assistance Publique des Hôpitaux de Marseille (APHM), CHU Timone, Marseille, France (D.F.-B.)
1,✉;
Club de Neuro-Oncologie of the Société Française de Neurochirurgie