Abstract
Cancer is one of the most important issues in modern medicine and the most common cause of death after cardiovascular diseases in many countries. Brain cancer is one of the most common causes of cancer death among men and women, ranking third. Chemotherapeutic drugs that aim to prevent uncontrolled proliferation of cells in tissues of the body and induce apoptosis of tumor cells are prominent candidates for development. Since cisplatin has an apoptosis-inducing role, it is widely used as an anticancer agent. In this research, toxicity of cisplatin was studied with the C6 rat glioma cell lined using the MTT method. In addition, nanoparticles underwent SEM microscopic imaging. Particle average size, size distribution, polydispersity index (PDI) and zeta potential of poly butyl cyanoacrylate nanoparticles were found to be 222 nm, 0.470 ± 0.04 and 5.1 ± 0.2 mV, respectively. The results showed that nanoconjugates of cisplatin have more cytotoxic effects on C6 cells than the free drug (P<0.05), pointing to an enhanced potential of the synthesized nano-particles as a new nanocarrier for chemotherapy.
Keywords: Brain cancer cells, cisplatin, cell cytotoxicity
Introduction
Glioma is the most common and deadliest of malignant primary brain and CNS tumors in adults and consists 80% of malignant cerebral tumors. Using different options such as surgery, radiotherapy, and chemotherapy, average survival rates of patients with Glioblastoma is 14.6 months. The blood–brain barrier (BBB) is the main hurdle for cerebral delivery of therapeutic materials (Izadi et al., 2016). In recent years biodegradable polymeric nanoparticles have attracted attention as drug carriers. These materials have shown great potential in the controlled delivery of drugs to target tissues and organs, in acting as DNA carriers in gene therapy and oral transfer of proteins, peptides and genes (Langer, 2000, Lanza et al., 2011). Based on the process used for their production, two types of nanoparticles can be obtained: nanospheres and nanocapsules, which are shown in Figure 1 (Mathiowitz, 1999).
Figure 1.
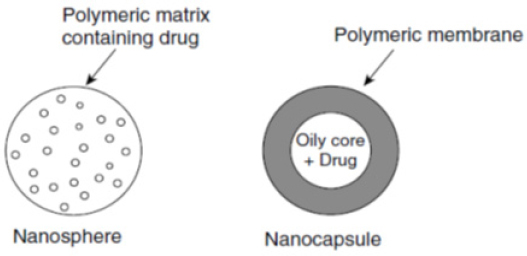
Schematic Representation of Nanospheres and Nanocapsules
Apoptosis or programmed cell death is a regulated process of the typical suicide of cells that enables an organism to control the number of cells in its body and eliminate the unwanted cells that threaten its survival. The right balance between apoptosis and its inhibition has a crucial role in tissues homeostasis and organs morphogenesis (Kemnitzer et al., 2004, O’Driscoll et al., 2003). During apoptosis, specific biochemical and cytological changes occur in a cell that includes compaction of nucleoplasm and cytoplasm, DNA fragmentation, and formation of apoptotic bodies attached to the membrane which is recognized and removed by adjacent cells (Reed and Tomaselli, 2000, Kemnitzer et al., 2005). Any faults in this process result in pathologic states. Deviation from the course of apoptosis can lead to spreading the tumor and metastasis, and apoptosis induction could cause nerve atrophy diseases like Alzheimer (Reed, 1999, Robertson et al., 2000).
In this study, we analyzed the toxicity effect of nano-cisplatin at Pasteur Institute of Tehran, Iran. Up to today, several carriers have been used to deliver cisplatin. efforts to produce a suitable nanoparticle formulation of cisplatin were not very fruitful which can be attributed to its low hydrophilic properties and lipophilicity which result in low drug loading on the nanocarrier. In this project, we plan to load cisplatin on poly butyl cyanoacrylate nanoparticles and compare its impact with a standard drug on rat glioma cell line C6, C6 and examine its properties in vitro.
Materials and Methods
This experimenttal study was performed at Nano-biotechnology Pilot Laboratory of Pasteur Institute of Iran, Tehran.
A: Cell lines and cell culture
C6 cell line was purchased from National Cell Bank of Iran (NCBI). The cells were cultured in RPMI with 10% fetal bovine serum (FBS), 100 µg/ml streptomycin (to prevent the growth of Gram-negative bacteria) and 100 U/ml penicillin (to prevent the growth of Gram-positive bacteria). A solution of trypsin-EDTA was used to separate the cells from the flask. Cell count was performed with a hemocytometer. In all tests, the viability was determined by trypan blue and was always above 90%.
B: Nanoparticles production
The nanoparticles were obtained by anionic polymerization method in pH<3. 1% N-butyl cyanoacrylate monomer (Evobond® Tong Shen Enterprise Co., Ltd., Taiwan) was added to 1% dextran 70000 solution (Floka) in 0.1 normal HCl (Merck, Germany) with 1.3 mg of cisplatin (Sigma-Aldrich Co., UK) in a polymerization medium containing distilled water (pH 2.5) under stirring by a magnetic stirrer and stirred for 3 hours. Afterward, the suspension of nanoparticles was neutralized with 0.1 N NaOH and then nanoparticles were deposited by centrifugation for 30 minutes at 15,000 rpm. Next, deposited particles were lyophilized. Nanoparticles were stored in 2 ml vials at 4°C until use. The particle size, zeta potential, and polydispersity were calculated from size distribution measured by Zetasizer (Nano ZS3600, Malvern Instruments, UK) and by optical correlation spectroscopy. Also, scanning electron microscopy (SEM) was used to characterize nanoparticles.
MTT test
MTT assay was used to investigate the cytotoxicity effects of nano-cisplatin formulation, cisplatin and control nanoparticles. C6 cells were cultured in DMEM at a concentration of 1x104 per well in 96 well plates. The medium was supplemented with 10% FBS and 1% penicillin/streptomycin and incubated under 10% carbon dioxide and 37°C. The supernatant was removed 24 hours after cell culture. Cells were treated with equal concentrations of 0, 12, 24, 48, 96, 300 and 600 µM of nano-cisplatin, free cisplatin, and nanoparticle formulations. After 24 and 48 hours of incubation, the medium was removed, and 100 µl MTT (0.5 mg/ml PBS, pH 4.7) was added to each well and incubated for three hours at 37°C. MTT solution is then removed, and 200 µl 100% isopropanol was added to each well to dissolve formazan crystals formed in wells. Absorbance was read at 570, and 540 nm using ELISA Reader (BioTek Instruments, VT, USA) was read. Tests were done in triplicates and repeated three times. The cytotoxicity and viability were calculated based on absorption of cells treated with the formulation of drug/adsorption of control cells ratio from formula 1 and 2.
(1) % Cytotoxicity= 1 - (Average absorption of cells treated with toxin)/(Average absorption of control) × 100
(2) % Viability = 100 – cytotoxicity (%)
IC50 (concentration of any derivatives that reduces cell growth by half compared to controls) was obtained from the MTT test and calculated with Pharm-PCS statistical package (Springer-Verlag. New York).
Statistical analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data were statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and statistical significance was set at p < 0.05.
Results
Suitable nanoparticles produced (Figure 2) had size, a polydispersity of size distribution and zeta potential of 222 nm (Figure 3), 0.470 ± 0.04 and 5.1 ± 0.2 mV, respectively.
Figure 2.

Poly-Butyl Cyanoacrylate Nanoparticles at 1% Dextran Concentration
Figure 3.
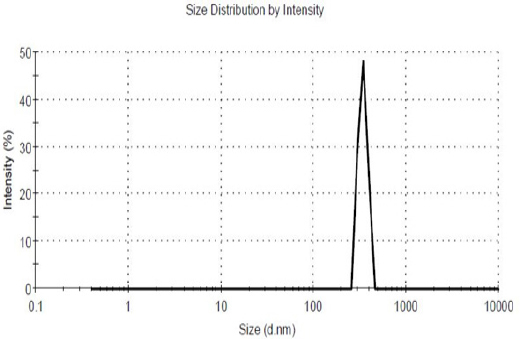
Size of Poly Butyl Cyanoacrylate Nanoparticles
Cytotoxicity
The results of the cytotoxicity tests of nano-cisplatin and free drug are summarized in Table 1. Control nanoparticles were devoid of toxicity, even at high concentrations. IC50 is reported in micromolar. Results show that nano-conjugated cisplatin is more cytotoxic than cisplatin. In other words, nano-conjugated cisplatin’s IC50 is less than cisplatin.
Table 1.
IC50 Cytotoxicity of Nano-Conjugated Cisplatin, Free Cisplatin, and Control Group at front Side Category of Cell Cancer the Brain in Vitro (Rat Glioma Cell Line C6)
| C6 (Nano Drug) IC50 (µM) | C6 (Free Drug) IC50 (µM) | C6 (Control Drug) IC50 (µM) |
|---|---|---|
| 67.7±4.7 | 99.5±8.8 | 164.1±16.0 |
IC50 (in terms of µM) shows the average result from three experiments. (Results were expressed as mean ± 5% values).
Discussion
Cancer is a disease where cells start to divide and multiply abnormally and spread to healthy tissues. Most anti-cancer therapeutic agents exert their effects in the form of apoptosis induction. Apoptosis is a critical step in the regulation of cell metabolism and growth. If apoptosis is stopped, the metabolism is disturbed, and tumors start to form and grow. Induction of apoptosis is one of the most important methods of destroying cancer cells without side effects (Kerr et al., 1994). In this research, Cytotoxic effects of cisplatin induction effects of nano-conjugated cisplatin was evaluated. Based on other studies we know that alkylating agents such as cisplatin add an alkyl group (CnH2n + 1) to DNA molecules, so DNA replication is prevented in this way which provokes apoptosis induction in tumor cells (Knox et al., 1986). The MTT test is one of the most common methods of measuring cell viability so was chosen to check cell survival and toxicity assessment of nano- cisplatin. Induce. Nanoparticles produced in this study had favorable size, size distribution, Zeta potential, and form. Nano-conjugated cisplatin also has IC50 smaller than free cisplatin which shows that nano-conjugated cisplatin is more effective in killing C6 brain cancer cells. However, more studies are recommended to increase the effectiveness of this new drug further so that it became a better candidate for the treatment of brain cancer or other types of cancer. Therefore, using folate ligands, dextran or monoclonal antibody is recommended. Formulation robustly intensified the cytotoxicity effects of drug increased with compared to the standard drug. In the present study, Poly Butyl Cyanoacrylate nanoparticles improve the anticancer activity of cisplatin against rat glioma cell line C6. Cytotoxicity effects were directly associated with drug concentrations. Nanodrug with the IC50 of 67 µM showed the superior cytotoxicity compared to the standard cisplatin with IC50 of 99 µM. As far as we know, this is the first study that have evaluated the efficacy of cisplatin loaded Poly Butyl Cyanoacrylate nanoparticles on the rat glioma cell line C6. In conclusion, Poly Butyl Cyanoacrylatenanoparticles are proper carrier for cisplatin delivery to rat glioma cell line C6.
Taking collectively, our research confirms that cisplatin on nanoparticles has more cytotoxic effects than free cisplatin on brian cell line (rat glioma cell line C6). Thus, this formulation may be an alternative chemotherapeutic candidate for brian cancer in the future.
Co-author contributions
Meysam Ebrahimi Far and Nejad Mohamadi contributed to develop and characterize the nanoparticles. Maryam Kazemi and Azim Akbarzadeh designed the study. Hasan Ebrahimi Shahemabadi and Mahshid Mohammadi anconducted the MTT assay. Attabak Toofani Milani performed the statistical analysis. Younes Moradi and Maryam Yasemi performed theSEM image.
Conflict of interest
The authors declare that there is noconflict of interest.
Acknowledgments
This work was supported financially by Pilot Nanobiotechnology Department, Pasteur Institute of Iran.
References
- Izadi M, Shahemabadi HE, Kanaani L, et al. Investigation the characteristics of carboplatin loaded onto pegylated liposomal nanoparticles on the rat Glioma cell line C6. Adv Biores. 2016;7:113–18. [Google Scholar]
- Kemnitzer W, Drewe J, Jiang S, et al. Discovery of 4-Aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay 1. structure- activity relationships of the 4-Aryl group. J Med Chem. 2004;47:6299–310. doi: 10.1021/jm049640t. [DOI] [PubMed] [Google Scholar]
- Kemnitzer W, Kasibhatla S, Jiang S, et al. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay 2 Structure–activity relationships of the 7-and 5-, 6-, 8-positions. Bioorg Med Chem Lett. 2005;15:4745–51. doi: 10.1016/j.bmcl.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Friedlos F, Lydall DA, Roberts JJ. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum (II) and cis-diammine-(1, 1 cyclobutanedicarboxylato) platinum (II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986;46:1972–79. [PubMed] [Google Scholar]
- Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- Lanza R, Langer R, Vacanti JP. Principles of tissue engineering. second ed. New York: Academic press; 2002. p. 995. [Google Scholar]
- Mathiowitz E. Encyclopedia of controlled drug delivery. New York: Wiley; 1999. p. 45. [Google Scholar]
- O’ Driscoll L, Linehan R, Clynes M. Survivin: role in normal cells and in pathological conditions. Curr Cancer Drug Targets. 2003;3:131–52. doi: 10.2174/1568009033482038. [DOI] [PubMed] [Google Scholar]
- Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–53. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- Reed JC, Tomaselli KJ. Drug discovery opportunities from apoptosis research. Curr Opin Biotechnol. 2000;11:586–92. doi: 10.1016/s0958-1669(00)00148-8. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–92. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


