Abstract
Background:
An efficient registration system with accurate and timely information on cancer incidence and mortality is key to development of policies to prevent and control cancer. A traditional registration system usually needs 3-4 years to collect data and publish a cancer report. However, researchers, policymakers and healthcare professionals need to know the latest cancer registration data quickly.
Methods:
A computer system has been operating with cases reported online by hospitals and followed up in communities at the Cancer Registry of Guangzhou (CRG) since 2008. The comparability, completeness, accuracy and timeliness of collected data were here evaluated.
Results:
From 2010 to 2014, 181,194 cancer cases from 1,916,253 medical records of cancer were reported to the CRG online. 53,473 cases were deleted as duplicates (47,906), wrong diagnoses (410) or residents of other places (5,157) during the follow up. Successful final follow-up rates were over 90% for both newly and previously diagnosed cases by general practitioners in community clinics. The CRG coding and classification system follows international standards. The annual trends for all sites by sex were stable from 2010 to 2014. All age-specific incidence rates for childhood cancers were within the limits of the respective international references. The overall M: I ratio for all sites but C44 was 56.7%., ratios for most sites in Guangzhou being between Hong Kong and Shanghai. A total of 75.7% of the cancer cases reported in 2010–2012 were morphologically verified. Ninety five percent of new cases completed registration within 29.0 months in 2010, reducing to 8.0 months in 2014.
Conclusion:
The online report system with community follow up at the CRG yields reasonably accurate and close-to-complete data. It takes less time to confirm diagnosis and other information so that reporting annual incidence one year after the close of registration becomes possible.
Keywords: Cancer, registration, quality, timeliness
Introduction
Cancer is a major burden of disease in the world and the first cause of deaths in many countries. An efficient registration system with accurate and timely information on cancer incidence and mortality is the key for policies to prevent and control cancer. Almost every country has its registry (Parkin, 2006). In 2015, China has 308 registries covering 300 million people (about 20% of total population) with a variety of models to collect cancer data from hospitals (Chen et al., 2016). The national central registry gathers data from local registries, then checks and analyses the data with software CanReg4 which is based on a standalone computer (Zhang, 2002). Local registries develop their systems respectively. In China, the first computer network registration system started in Shanghai in 2002 (Lu et al., 2002). Beijing and Dalian also developed their computer network data collection system afterwards (Wang et al., 2010; Zhao et al., 2008). Guangzhou has been one of the largest cities with the fastest economic development in the past decades in China. Guangzhou began cancer registration in 1998. The reporting of neoplasms to the Cancer Registry of Guangzhou (CRG) under Guangzhou Center for Disease Control and Prevention (GZCDC) has been compulsory following the Case Report and Follow-up Provisions of Cancer from the Guangzhou Health Bureau. All hospitals, laboratories and community doctors in Guangzhou are requires to report cancer cases from multiple sources and the trace-back routines have been built up at the registry since 1998. The data from 2000 to 2002 were accepted by Cancer Incidence in Five Continents, Volume IX. In 2007, a computer network system was established to collect data from hospitals. After 3 years’ trial, it has been expanded with follow-up function which is conducted by general practitioners (GP) in the community clinics in 2010 and formally named as Guangzhou Cancer Case Report and Follow-up System (GZCCRFS). All case reports, data check and information exchange for cancer registration are done online. The data quality and efficiency are improved by implementing the network system and follow-up in community. Several studies at the regional and site-specific level have been reported (Li et al., 2014; Zhou et al., 2015), but there was no comprehensive evaluation of the system. In this paper, we report our evaluation of the comparability, validity, completeness and timeliness of the registration. With the growth of online cancer registration worldwide, our experience in Guangzhou could be useful for the global networks of cancer registries.
Materials and Methods
Case reports
After a cancer patient is discharged, the person who is in charge of managing the medical records in the hospital, often the clinical statistician, picks up the cancer case after a preliminary review and checks if this is the first time the patient comes to see the doctors and has been diagnosed as cancer. If “yes”, a cancer report card with structured templates based on the form recommended by International Association for Cancer Registration (IACR) is set up for extracting information from the medical records. The information in the card after coding is entered into the computer. The computer system has set up the International Codes for Disease (ICD) classification and fuzzy query entry form with most items made in the report variable drop-down menu or options for convenient entry and reducing mistakes. Some hospitals with an advanced information system can extract automatically cancer report card information and input data by the special digital interface of GZCCRFS. The process of cancer registration has changed totally from manual to a computer network direct report system from 2007. Individual information was obtained from hospitals capable of diagnosing cancer in Guangzhou. For each incident case, information including medical identification number, China Identity Card Number (unique for each resident), ICD-10 code, name, sex, birth date, occupation, ethnicity, residential address, phone number, cancer site, basis for diagnosis, treatment, prognosis and pathological report if available (date of diagnosis, hospital and name of doctor who made the diagnosis) are all registered. All cases are distributed to community clinics (known as Community Health Service Centers) for follow up by the GP.
The CRG has also been collecting electric record data with discharge diagnosis from 1998 every year on all patients treated for malignant and central nervous system tumors in every hospital and outpatient clinic in Guangzhou. The discharge diagnoses are used as reference for checking missed and duplicated cases. Pathology notifications are from copies of the laboratory workers’ original reports, which are sent to the registry half yearly. The pathological notifications provide histological, hematological, cytological and/or autopsy information. Morphology codes have been developed for further subdivision of ICD-O-3 on the basis of additional information on morphology, cytochemistry and immunophenotype, especially within the haematological malignancies. Guangzhou Statistics Bureau provides information of sex and age-specific population data of Guangzhou citizens yearly.
Duplicated cases deletion
When the data of newly diagnosed cases of cancer enter the GZCCRFS, the machine automatically compare them with existing records by name and ID number. If the system finds a same name or a same ID number, it will remind CDC staffs to inspect and check. Two staff members in GZCD and at least a staff member in every of its 12 affiliated district CDCs, the preventive doctors who have been trained and mastered the GZCCRFS operation and ICD-03 coding, are put in charge for this job as examiners of the cards. Three kinds of cards will be deleted:
(i) The patient is a resident of other places (the registered permanent residence is not Guangzhou).
(ii) The patient previously diagnosed as cancer but the diagnosis is revised and confirmed to be non-cancer by follow up, and
(iii) The duplicated cards.
The cards for (i) and (ii) will be deleted directly. For (iii), the new report may contain some additional useful information such as better method of diagnosis or address change of patient. If so, the card will be deleted only after merging the useful items. At the same time, careful attention is paid to check whether the card shows a multi-primary cancer, and if so, it will be kept as a new card with a special mark to avoid deletion for duplication by mistake.
Community follow-up
All new cancer cases, after deleting duplication and merging items, are sent to community clinics for the GP to follow up. The GP is demanded to finish the follow up within 1 month after receiving the case. This is a very important process to confirm the diagnosis and registered residence, and collect survival data of the patients. Since 2009, cancer, diabetes and hypertension have been ranked as the topic public health project by the government of Guangzhou, so it is the community clinic’s duty to take care of the patients with cancer in its administrative region, which includes setting up health record, regular visit and rehabilitation. Generally, the GP in community clinics calls the patient, family member or relative who is the designated contact person in the medical record by telephone to confirm the basic information. When the patient has died or moved out of the city, the GP marks it as a terminated card. Otherwise, the GP will arrange a home visit to see the patient. During the home visit, the GP completes a questionnaire and health check for the patient, reexamines the diagnosis and other items of the cancer report card with the patient. A special cancer medical file is established for the patient. The patient situation is graded by the Karnofsky Performance Status (KPS) to determine when the next visit should be done (Schag et al., 1984). When KPS score is equal to 40 or bellow, the next visit should be scheduled in 3 months (4 times every year); KPS of 50 to less than 80, next visit in 6 months (2 times every year); KPS equal to 80 or higher, next visit in 1 year (once a year). Every month, all alive cases are checked in Guangzhou Vital Registration Data which is a population-based and online system. If the patient is found died, the card marks as terminated and follow up stops. All GPs involved in follow-up observe the privacy protection and informed consent of the patient by signing a Letter of Commitment drafted by CRG. If the patient or his/her guardian disagree the follow-up from clinic, the next visit will not be scheduled and the follow up stops.
Data in this study
Data for the period 2004–2014 were extracted from the CRG. The whole time span was used for the evaluation of comparability whereas the years of 2010- 2014 were selected to evaluate the validity, completeness and timeliness after the GZCCRFS was established. Analyses are presented at the two-digit ICD-10 level. Age-standardized rates were estimated by the direct method in accordance with the World Standard Population, and expressed as per 100, 000 individuals.
Evaluation of the system
We followed the evaluation method of Parkin and Bray (Parkin and Bray, 2009; Bray and Parkin, 2009). Three semi-quantitative methods were applied to evaluate the completeness, including stability of data over time (2004–2014), age-specific incidence rates of childhood cancer with the corresponding reference intervals based on deciles for childhood cancer published in CI5 Volume VIII and the mortality: incidence ratio (M:I). Validity was evaluated by the proportions with morphological verification (MV %) and proportions obtained through death certificate sources only (DCO %) by site. Timeliness was evaluated in terms of the time from diagnosis to report, and the time from report to confirming the diagnosis and the registered residence of the patient by telephone call or by visit.
Results
Quantity of reported cases in 5 years
From 2010 to 2014, 181,194 cancer cases were reported to the GZCCRFS online. After deleting and merging 53,473 duplicated cases, 127,721 newly diagnosed cases were kept for follow-up and comparison with cases diagnosed before 2010. In 2010, 24949 cases were reported from 110 hospitals online and 4715 cases were collected by traditional hand work from medical records in 59 hospitals to supplement the system. Then the number of cases and hospitals increased every year, reaching 46,636 cases and 196 hospitals in 2014. But still not all hospitals reported online for lack equipment or qualified personnel. The manual hospitals remained 8 and they provided 32 cases (Figure 2). More duplicated cases were reported with more hospitals reporting online. Table 1 shows, in 2010, the first year of online reporting, 24,949 new cases were identified and reported to GZCCRFS by hospitals, accounting for 8.3% of 29,8875 medical records of cancer in that year. Only 2454 (9.9% of 24949) were deleted for duplication by district or city examiners, leaving 22,495 (90.2%) as newly diagnosed cases. From 2011, more reported cases were deleted because of duplication, from 21.6% in 2011 to 37.9% in 2014. Five years of figures show that the system is practical.
Figure 1.
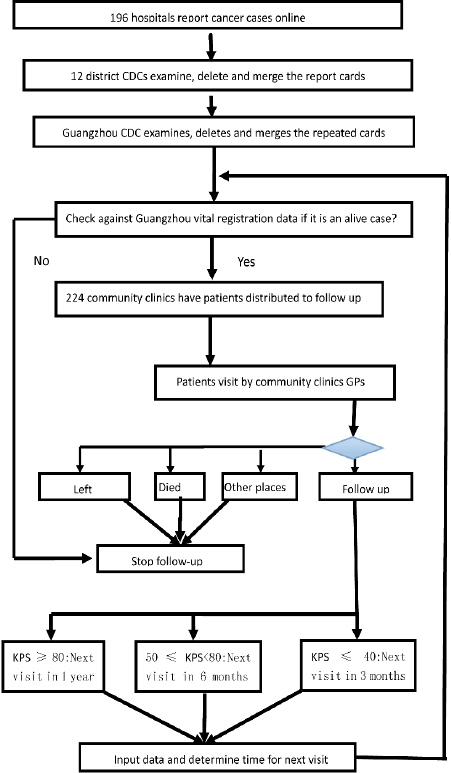
the Procedures of Cancer Registration and Follow-Up in Guangzhou
Figure 2.
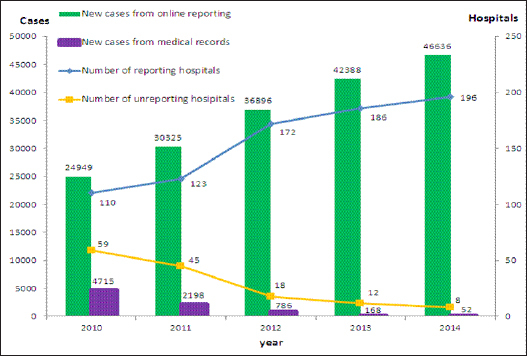
Cases and Hospitals in Guangzhou Cancer Reporting and Follow-Up System 2010-2014
Table 1.
Cases from Medical Records, Deleted and New Cases in Guangzhou 2010-2014
| Year | Cancer cases from records (a) | Reported cases | Deleted cases | New cases |
|---|---|---|---|---|
| (b)(b/a%) | (c)(c/b%) | (d)(d/b%) | ||
| 2010 | 298,875 | 24,949(8.3) | 2,454(9.9) | 22,495 (90.2) |
| 2011 | 344,569 | 30,325(8.8) | 6,563 (21.6) | 23,762 (78.4) |
| 2012 | 390,996 | 36,896(9.4) | 12,321 (33.4) | 24,575 (66.6) |
| 2013 | 428,315 | 42,388(9.9) | 14,452 (34.1) | 27,936 (65.9) |
| 2014 | 453,498 | 46,636 (10.3) | 17,683 (37.9) | 28,953 (62.1) |
| Total | 1,916,253 | 181,194 (9.5) | 53,473 (29.5) | 127,721 (70.5) |
a, Inpatients records for cancer collected from hospitals that had wards and treated inpatients with cancer, including the hospitals that had not taken part in reporting online yet; b, Cases reported by hospitals online before district and city level deletion; c, Cases deleted by district and city examiners; d, Cases kept as new diagnosis in the respective year
Item merging and duplicate deletion
Inpatient data were tremendous with 1,916,253 records of cancer during 5 years of 2010-2014 (Table 1). About 90% cases were selected out and 181,194 (9.5%) were reported by hospitals. About 30% reported cards (53,473) were deleted during follow-up. Table 2 shows that, in 2010, duplication accounted for 54.7% of deleted cards. This proportion increased with increasing number of reporting hospitals and cases, reaching 95.0% in 2014. Wrong diagnosis (previous diagnosis is cancer but denied later by further detection) and decreased remarkably from 3.4% to 0.4%. The proportion of cases from other places (i.e. not Guangzhou residents) decreased greater, from 41.9% to 4.6%. By examiners of different level, 66.7% of all deleted cases were done in the district CDCs in the 5 years.
Table 2.
Cases Deleted by Different Reasons and Examiners at Different Levels in Guangzhou 2010-2014
| Year | Total deleted cases | By reasons | By examiners | ||||
|---|---|---|---|---|---|---|---|
| Duplicated cases (%)* | Wrong diagnosis (%) # | Residents of other places (%) | District CDC (%) | City CDC (%) | |||
| 2010 | 2,454 | 1,342 (54.7) | 83 (3.4) | 1,029 (41.9) | 528 (21.5) | 839 (34.2) | |
| 2011 | 6,563 | 5,262 (80.2) | 84 (1.3) | 1,217 (18.5) | 3,309 (50.4) | 1,253 (19.1) | |
| 2012 | 12,321 | 11,086 (90.0) | 97 (0.8) | 1,138 (9.2) | 8,680 (70.5) | 1,915 (15.5) | |
| 2013 | 14,452 | 13,415 (92.8) | 74 (0.5) | 963 (6.7) | 9,547 (66.1) | 2,792 (19.3) | |
| 2014 | 17,683 | 16,801 (95.0) | 72 (0.4) | 810 (4.6) | 13,618 (77.0) | 1,842 (10.4) | |
| Total | 53,473 | 47,906 (89.6) | 410 (0.8) | 5,157 (9.6) | 35,682 (66.7) | 8,641 (16.2) | |
, Cases deleted after merging useful items as appropriate;
, Including wrong diagnoses found by GPs during follow-up and from original hospital reports
Follow up completion rates
After duplicate deletion, the reported cases were sent to community clinics online to follow up. Table 3 shows that about 93.9% of newly diagnosed cases had the first visit in 5 years. The timely rate of first visit increased from 64.2% in 2010 to 89.0% in 2014. For the old cases, the overall completion rate of the 5 years was 90.4%, and the timely completion rate was 88.8%.
Table 3.
Follow Up by Community Clinic GPs in Guangzhou 2010 to 2014
| Year | Newly diagnosed cases | Previously diagnosed cases# | |||||
|---|---|---|---|---|---|---|---|
| Num | First visit(%) | Timely first visit (%)$ | Survival | Requiring visit | Completed visit(%) | Timely completion (%)@ | |
| 2010 | 22,495 | 20,914 (93.0) | 14,450 (64.2) | 0 | 4,783 | 3,945(82.5) | 2,645(67.1) |
| 2011 | 23,762 | 22,310 (93.9) | 17,434 (73.4) | 17,174 | 30,503 | 25,795(84.6) | 19,948(77.3) |
| 2012 | 24,575 | 23,014 (93.7) | 20,338 (82.8) | 38,104 | 53,093 | 46,457(87.5) | 38,894(83,7) |
| 2013 | 27,936 | 26,237 (93.9) | 24,128 (86.4) | 55,387 | 72,256 | 66,784(92.4) | 60,751(91.0) |
| 2014 | 28,953 | 27,424 (94.7) | 25,757 (89.0) | 72,232 | 87,578 | 81,586(93.2) | 76,861(94.2) |
| Total | 127,721 | 119,900(93.9) | 102,108 (85.2) | 182,897 | 248,213 | 224,267(90.4) | 199,099(88.8) |
, Cancer cases diagnosed from 2010; some cases with Karnofsky Performance Status (KPS) score less than 80 needed follow up in respective year;
, Visit completed within 1 month from receipt of the online report;
, Visit completed within the time period according to KPS standar
Comparability
The National Central Cancer Registry of China (NCCR) developed the Guidelines for Chinese Cancer Registration (NCCR, 2004) according to the data-quality criteria of International Agency for Research on Cancer/International Association of Cancer Registries (IARC/IACR) (Ferley, et al., 2005). CRG follows these protocols. Incident cases of cancer in Guangzhou comprise all malignancies with the 5th-digit behavior code 3 according to ICD-O-3 for hematological malignancies and the 5th-digit behavior code 1 for tumors of the central nervous system. The recognition of two or more primary cancers does not depend on time, and the groups of topography codes considered as single sites (from ICD-O-2 and ICD-O-3) are followed, with systemic and multicentric cancers counted only once.
Completeness
Figure 3 shows the incidence trends, by sex, for some selected cancers. Using the log scales, the annual trends for all sites, lung, liver and colorectum did not fluctuate in any systematic pattern. Male nasopharynx cancer slightly decreased while female thyroid cancer increased more markedly. Table 3 shows all age-specific incidence rates for childhood cancer were within the limits of the respective references. The Guangzhou average M: I ratio of cancer in 2010-2012 for all sites but C44 was 56.7%. Figure 4 shows a comparison of the M: I ratios by cancer sites in 2010–2012 in Guangzhou, Hong Kong and Shanghai. The M: I ratios of Guangzhou were between Hong Kong and Shanghai except mesothelioma and cancer of soft tissue, hypopharynx, bladder, colon, brain, central nervous system, bone, liver, and pancreas. The M: I ratios of lung, liver, breast and colorectal cancers, which are leading cancers in China, were quite similar in the three areas. The M: I ratios of eye, lip, nose and tonsil were obviously different but they are rare cancers, with an annual average of 20, 7, 31 and 2 cases respectively in Guangzhou.
Figure 3.
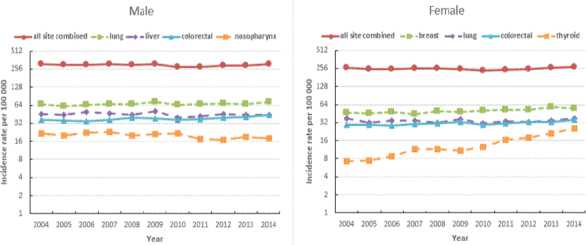
Annual Trends in Age-Standardized (World) Incidence Rates for All Sites Combined, and for Selected Sites, 2004-2013, Guangzhou
Figure 4.
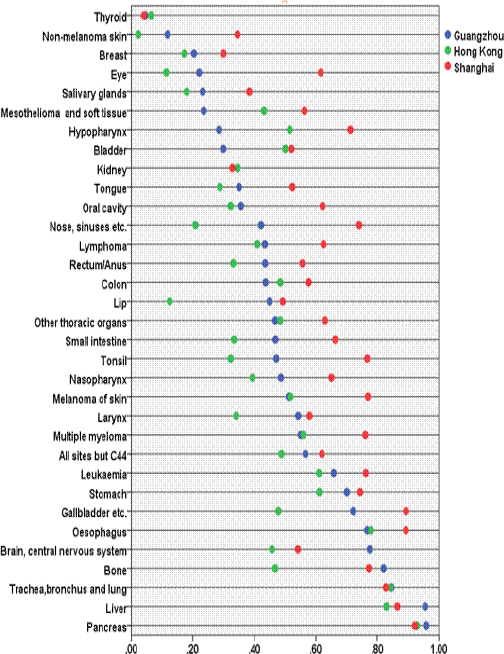
Mortality: Incidence Ratios by Cancer Sites Among Guangzhou, Shanghai and Hong Kong 2010-2012.
# Hong Kong and Shanghai did not publish the M: I of every site, here only including the items both of them have.
Validity (accuracy)
A total of 75.7% of the cancer cases reported in 2010–2012 were morphologically verified (Table 5). Liver cancer (6345 cases), the second commonest cancer, had the lowest of MV% (30.9%). Lung and trachea cancer, the commonest cancer (11966 cases), had a MV of only 61.8%. The proportion of DCO cases was 1.0% for all causes. Thirty eight among 49 of the cancer sites listed had a DCO of less than 1%. Mediastinum, pleura (3.2%) and soft tissue cancer (2.8%) had the highest DCO.
Table 4.
Age-Specific Incidence Rates Per 100,000 for Childhood Cancer by Gender, Guangzhou, 2010-2012
| Age | Boys | Reference | Girls | Reference |
|---|---|---|---|---|
| 0-4 | 22.2 | (12.3-24.7) | 15.1 | (9.7-21.4) |
| 5-9 | 13.4 | (8.5-15.6) | 9 | (6.9-12.0) |
| 10-14 | 11.9 | (8.5-15.0) | 11 | (6.8-13.6) |
Table 5.
Number of Cases and Percentage Morphologically Verified (MV%) and Percentage Obtained from Death Certification Only (DCO%), 2010-2012, Guangzhou
| ICD-10 | Site | Cases | MV% | DCO% |
|---|---|---|---|---|
| C00-95 | All sites | 62,192 | 75.7 | 1.0 |
| C00 | Lip | 20 | 100.0 | 0.0 |
| C01-02 | Tongue | 367 | 92.9 | 0.5 |
| C03-6 | Mouth, other | 294 | 92.9 | 0.0 |
| C07-8 | Salivary glands | 198 | 92.4 | 1.0 |
| C09-14 | Pharynx | 3,445 | 81.9 | 1.4 |
| C15 | Esophagus | 1,456 | 81.7 | 1.0 |
| C16 | Stomach | 2,714 | 85.6 | 0.5 |
| C17 | Small intestine | 325 | 81.8 | 0.6 |
| C18 | Colon | 5,118 | 86.5 | 0.6 |
| C19-21 | Rectum, anus | 3,129 | 89.8 | 0.7 |
| C22 | Liver | 6,345 | 30.9 | 2.6 |
| C23-24 | Gallbladder, bile ducts | 693 | 51.8 | 2.2 |
| C25 | Pancreas | 973 | 40.4 | 1.5 |
| C26 | Other digestive organs | 101 | 46.5 | 1.0 |
| C30-31 | Nose, sinuses | 108 | 87.0 | 0.0 |
| C32 | Larynx, epiglottis | 607 | 86.0 | 0.3 |
| C33-34 | Lung, trachea | 119,66 | 61.8 | 1.4 |
| C38 | Mediastinum, pleura | 222 | 73.9 | 3.2 |
| C40-41 | Bone | 240 | 67.9 | 2.1 |
| C43 | Melanoma of skin | 134 | 98.5 | 0.0 |
| C44 | Skin, non-melanoma | 777 | 95.4 | 0.1 |
| C45 | Mesothelioma | 53 | 100.0 | 0.0 |
| C46 | Kaposi’s sarcoma | 2 | 100.0 | 0.0 |
| C47 | Autonomic nervous system | 35 | 94.2 | 0.0 |
| C48-49 | Soft tissues | 522 | 74.3 | 2.8 |
| C50 | Breast | 6,134 | 94.5 | 0.3 |
| C53 | Cervix uteri | 1,682 | 92.6 | 0.4 |
| C54 | Corpus uteri | 1,423 | 95.7 | 0.3 |
| C55 | Uterus other | 112 | 78.6 | 1.8 |
| C56 | Ovary | 1,020 | 88.8 | 0.3 |
| C51-52,57 | Other female genital | 135 | 90.4 | 0.0 |
| C58 | Placenta | 17 | 76.5 | 0.0 |
| C61 | Prostate | 1,684 | 77.3 | 0.8 |
| C62 | Testis | 108 | 93.5 | 0.0 |
| C60,63 | Other male genital | 108 | 88.9 | 0.0 |
| C64 | Kidney excluding renal pelvis | 748 | 79.3 | 0.8 |
| c65 | Renal pelvis | 93 | 77.4 | 2.2 |
| C66-68 | Bladder, ureter, urethra | 1,420 | 82.6 | 0.2 |
| C69 | Eye | 60 | 76.7 | 0.0 |
| C70-72 | Central nervous system | 770 | 74.3 | 1.0 |
| C73 | Thyroid gland | 2,432 | 96.8 | 0.1 |
| C37, c74-75 | Other endocrine glands | 102 | 72.5 | 1.0 |
| C39, c76, c80 | Other or unspecified | 944 | 48.5 | 2.0 |
| C81 | Hodgkin lymphoma | 93 | 100.0 | 0.0 |
| C82-85, c96 | Non-Hodgkin lymphoma | 1,480 | 95.1 | 0.2 |
| C88 | Malignant immune proliferative disease | 9 | 100.0 | 0.0 |
| C90 | Multiple myeloma | 375 | 93.6 | 0.5 |
| C91-95 | Leukemia | 1,390 | 97.3 | 0.1 |
Timeliness
Figure 5 shows that the timeliness improved markedly from 2010 to 2014 with more and more cases reported and most cases (71.4%) confirmed with 3 months in 2014. The median time from the date of diagnosis to a report and confirmation of a new case reduced from over 9.7 months in 2010 to 2.1 months in 2014 (Table 6). Ninety five percent of news cases completed report and confirmation in 29.0 months in 2010, reducing to 8.0 months in 2014.
Figure 5.
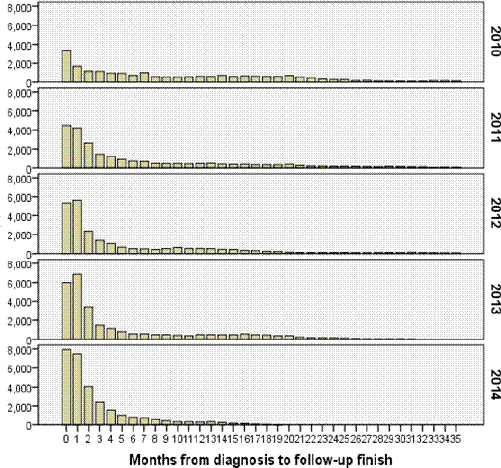
Time from Diagnoses to Completion of Report and Confirmation for New Cases (Months)
Table 6.
Time From Diagnosis to Completion of Report and Confirmation for New Cases (Months)
| Year | No. of cases | Mean | Standard deviation | Median | 95% Count |
|---|---|---|---|---|---|
| 2010 | 22,495 | 9.7 | 10.61 | 5 | 29 |
| 2011 | 23,762 | 5.7 | 8.00 | 2 | 20 |
| 2012 | 24,575 | 4.5 | 6.42 | 1 | 17 |
| 2013 | 27,936 | 3.5 | 4.96 | 1 | 15 |
| 2014 | 28,953 | 2.1 | 2.73 | 1 | 8 |
| Total | 127,721 | 5.9 | 7.58 | 2 | 22 |
Discussion
The prerequisites for population-based high quality cancer incidence data by online report are favorable in Guangzhou with mandatory reporting, unique personal identification number and computer equipment and software. The present evaluation of the quality of data shows that the registry has a high degree of comparability, completeness, accuracy and timeliness.
Some heterogeneity was, however, observed for some quality indicators for specific cancer sites. Cancers of the breast, cervical, colorectal and prostate cancer were found to have very favorable values for all aspects assessed, whereas cancers of the liver, lung and pancreas did not meet the highest standards of MV% recommended by IACR. This situation can also be seen in other high income European registries (Larsen et al., 2009). That is because biopsy with histology of breast, cervical, colorectal and prostate cancer is easier whereas biopsy of the liver, lung and pancreas is more difficult. Imaging technique combining with clinical experience can determine the diagnosis of most cases of the latter cancers.
The overall completeness estimates from the present study indicates that online reporting during 2010-2014 from Guangzhou have stably improved the data compared to the data before 2010 when traditional manual reporting was employed. The changes of incidence for nasopharyngeal cancer in males and thyroid cancer in females were obvious but reasonable for these trends are also witnessed in most countries or areas in the world. Incidence rates of nasopharyngeal cancer decreased significantly in southern and eastern Asia, North America and Nordic countries. Decreasing trends in nasopharyngeal cancer incidence are probably due to tobacco control, changes in diets and economic development (Tang et al., 2016). In many countries, a steady increase in the incidence of thyroid cancer (mainly papillary carcinomas) was observed in both sexes. The increases in the incidence are likely due to the increase in the detection of this neoplasm (La Vecchia et al., 2015). The childhood cancer incidence rates were stable between the lower and upper limit of the reference intervals, indicating that under-reporting is not likely. Shanghai and Hong Kong have the earliest registries established in China with over 50 years of experience (Jiang et al., 2003; Li et al., 1999). Their cancer data are accepted by Cancer in Five Continents (CI5) of IARC since Volume V (IARC 2015; IARC 2016), which means the quality reaches very high standards. Guangzhou had most M:I ratios between Shanghai and Hong Kong, and the M:I ratios of leading cancers (lung, liver, colorectal and breast) were similar. The differences in rare cancers were due to small numbers (lip and nose) or variations in screening and diagnostic practices (prostate and bladder cancer, respectively). The high degree of completeness must be supported by all sources and different channels as previously described (Parkin and Bray, 2009). This semi-quantitative assessments of completeness further supported the concept of close-to-complete incidence data of the GZCCRFS, but the quantitative methods, flow method and capture–recapture, are necessary for further completeness estimate of the system (Bray and Parkin, 2009). Some of our reporting hospitals automatically defined cancer cases by the automated registration system and reported cases to our system online. A study in Venetian shows these reported cases, especially based only on a single cytology record, are unreliable (Tognazzo, 2005). We collected hospital discharge source and pathology records to decrease the missed and inaccurate cases every year. During recent years, many cancer registries have extended beyond collecting information at the time of diagnosis, to include more clinical data on treatment and follow-up of patients. Registered incident cases were linked to database of hospital discharge and matched with those in the all-cause mortality database from vital statistics division to get the therapeutic schedule, health status and the date of death and underlying cause of death if the patient had died (Znaor et al., 2012; Van de Poll-Franse et al., 2012). In China, follow-up of cancer patients is very important for the registry to confirm diagnosis and residence in densely-populated cities with many migrants. Guangzhou is also the largest city with most advanced cancer diagnosis and treatment choices in southern China. Many patients elsewhere come to Guangzhou for doctor consultation and could be mistakenly reported as local residents. The identification and deletion of these cases depends on follow- up. From 2010 to 2014, the first visit rates and the timely completion rates increased and the deleted cases of non-Guangzhou residents decreased. The traditional registration system usually needs 3-4 years to collect data and publish a cancer report (Parkin and Bray, 2009). But researchers, policy makers and healthcare professionals want to know the latest cancer registration data quickly. It is recommended the registry should contain at least 95% of the expected cases of reportable cancer occurring in residents within 23 months of the close of a diagnosis year (Havener, 2016). In Icelandic Registry, a nationwide registry with over 60 years of history, the median time from diagnosis to availability for research is 8 months (238 days) and the cancer data (94.8%) is collected, processed and reported relatively reliable and complete within 15 months (Sigurdardottir et al., 2012). Bulgarian registry needs 18 months to collect acceptably complete data of new cases (Dimitrova, 2015). Timeliness has been greatly improved due to the introduction of computerized online system and follow-up by GP in CRG. At present, 95% of news cases in residents completed report and confirmation within 8.0 months. So the publication of cancer statistics in one year becomes available.
References
- Bray F, Parkin DM. Evaluation of data quality in the cancer registry: Principles and methods. Part I. Comparability, Validity and Timeliness. Eur J Cancer. 2009;45:747–55. doi: 10.1016/j.ejca.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Dimitrova N, Parkin DM. Quality at the Bulgarian national cancer registry: An overview of comparability, completeness, validity and timeliness. Cancer Epidemiol. 2015;39:405–13. doi: 10.1016/j.canep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Burkhard C, Whelan S, Parkin DM. IARC Technical Report. Vol. 42. Lyon, France: IARC Press; 2005. Check and conversion programs for cancer registries (IARC/IACR Tools for Cancer Registries) pp. 1–46. [Google Scholar]
- Havener LA. Standards for cancer registries volume III: Standards forcompleteness, quality, analysis, management, confidentiality and security of data. Spring field (IL): North American association of central cancer registries 2008; 2008. [Accessed 20 Feb 2016]. http://www.naaccr.org/ [Google Scholar]
- IARC (International Association of Cancer Registry) CI5-Concer in Five Continents. [Accessed 21 Oct 2015]. http://www.iacr.com.fr/
- IARC (International Agency for Research on Cancer) CI5 I-X, Cancer Incidence in Five Continents Volumes I to X. [Accessed 21 Mar 2017]. http://ci5.iarc.fr/CI5I-X/Pages/database.aspx .
- Jiang F, Li XJ, Zheng Y. The past and future challenge of cancer registry in Shanghai. Sh J Prev Med. 2003;15:154–55. [Google Scholar]
- La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 1015;136:2187–95. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45:1218–31. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Li K, Lin GZ, Shen JC, et al. Time trends of nasopharyngeal carcinoma in urban Guangzhou over a 12-year period (2000-2011): declines in both incidence and mortality. Asian Pac J Cancer Prev. 2014;15:9899–903. doi: 10.7314/apjcp.2014.15.22.9899. [DOI] [PubMed] [Google Scholar]
- Li CK, Mang OWK, Foo W. Epidemiology of pediatric cancer in Hong Kong, 1982 to 1991 Hong Kong cancer registry. Hong Kong Med J. 1999;5:128–34. [PubMed] [Google Scholar]
- Lu W, Zheng Y, He YF, et al. Shanghai cancer registry computer information system. China Cancer. 2002;6:311–3. [Google Scholar]
- NCCR (National Center for Cancer Registration) Guideline for Chinese Cancer Registration. Beijing, China: Peking Union Medical College Publishing House; 2004. pp. 1–289. [Google Scholar]
- Parkin DM, Bray F. Evaluation of data quality in the cancer registry: Principles and methods. Part II. Completeness. Eur J Cancer. 2009;45:756–64. doi: 10.1016/j.ejca.2008.11.033. [DOI] [PubMed] [Google Scholar]
- Parkin DM. The evolution of the population-based cancer registry. Nat Rev Cancer. 2006;6:603–12. doi: 10.1038/nrc1948. [DOI] [PubMed] [Google Scholar]
- Schag CC, Heinrich RL, Ganz PA. Karnofsky Performance status revised: reliability, validity and guidelines. J Clin Oncol. 1984;2:187–93. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir LG, Jonasson JG, Stefansdottir S, et al. Data quality at the Icelandic cancer registry: comparability, validity, timeliness and completeness. Acta Oncol. 2012;51:880–9. doi: 10.3109/0284186X.2012.698751. [DOI] [PubMed] [Google Scholar]
- Tang LL, Chen WQ, Xue WQ, et al. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016;374:22–30. doi: 10.1016/j.canlet.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Tognazzo S, Andolfo A, Bovo E, et al. Quality control of automatically defined cancer cases by the automated registration system of the Venetian Tumour Registry. Eur J Public Health. 2005;15:657–64. doi: 10.1093/eurpub/cki035. [DOI] [PubMed] [Google Scholar]
- Van de Poll-Franse LV, Haak HR, Coebergh JW, et al. Disease-specific mortality among stage I-III colorectal cancer patients with diabetes: a large population- based analysis. Diabetologia. 2012;55:2163–72. doi: 10.1007/s00125-012-2555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Zhu WX, Xing XM. Construction and improvement of information systems of cancer registry in Beijing. China Cancer. 2010;3:150–4. [Google Scholar]
- Zhang SW. Brief introduction of CanReg4 computer program for cancer registration. China Cancer. 2002;5:15–6. [Google Scholar]
- Zhao L, Zhang LM, Wang SP, et al. Management and application of cancer registration network information system in Dalian. Chin J of Public Health. 2008;2:219–20. [Google Scholar]
- Zhou Q, Li K, Lin GZ, et al. Incidence trends and age distribution of colorectal cancer by subsite in Guangzhou 2000-2011. Chin J Cancer. 2015;34:358–64. doi: 10.1186/s40880-015-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znaor A, Brenner H, Holleczek B, et al. Has there been progress in cancer care in Croatia?Assessing outcomes in a partially complete mortality follow-up setting. Eur J Cancer. 2012;48:921–8. doi: 10.1016/j.ejca.2011.05.027. [DOI] [PubMed] [Google Scholar]


