Abstract
We investigated the anti-cholangiocarcinoma effect of α-mangostin from Garcinia mangostana pericarp extract (GM) in a human cholangiocarcinoma (CCA) cell line and a hamster CCA allograft model. In vitro, human CCA cells were treated with GM at various concentrations and for different time periods; then cell-cycle arrest and apoptosis were evaluated using flow cytometry, and metastatic potential with wound healing assays. In vivo, hamster allografts were treated with GM, gemcitabine (positive control) and a placebo (negative control) for 1 month; tumor weight and volume were then determined. Histopathological features and immunostaining (CK19 and PCNA) characteristics were examined by microscopy. The present study found that α-mangostin could: inhibit CCA cell proliferation by inducing apoptosis through the mitochondrial pathway; induce G1 cell-cycle arrest; and inhibit metastasis. Moreover, α-mangostin could inhibit CCA growth, i.e. reduce tumor mass (weight and size) and alter CCA pathology, as evidenced by reduced positive staining for CK19 and PCNA. The present study thus suggested that α-mangostin is a promising anti-CCA compound whose ready availability in tropical countries might indicate use for prevention and treatment of CCA.
Keywords: Inhibitory effect, cholangiocarcinoma, α-mangostin
Introduction
Cholangiocarcinoma (CCA), caused by chronic liver fluke infection in combination with other factors, remains a major health problem in the northeastern part of Thailand (IARC, 2011). Currently, the most effective treatment of CCA is dissection of the tumor, which leads to a good prognosis in the early stage but not in the late stage (Chamadol et al., 2014). Several studies have shown the effectiveness of anti-cholangiocarcinoma drugs in in vitro cell culture (Nakajima et al., 2012; Ramírez-Merino et al., 2013; Kamada et al., 2014), but so far these have not been effective in vivo against human CCA (Marumoto et al., 2014). Therefore, prevention of CCA seems to be the best choice. However, there have been several recent reports on the use of traditional medicine for relief of suffering from several diseases, including CCA (Janeklang et al., 2014; Lee et al., 2014).
To date, there have also been numerous reports on the anti-cancer activity of medicinal plants against CCA. Garcinia mangostana Linn. or G. mangostana pericarp extract (GM), the hull of the mangosteen fruit, is a traditional folk medicine that has many medicinal properties: antioxidant (Aukkanimart et al., 2015), anti-inflammatory (Lee et al., 2014), antimicrobial (Pothitirat et al., 2009), anti-adipogenesis (Liu et al., 2015), treatment of systemic lupus erythematosus (Li et al., 2015), hepatoprotective (Wang et al., 2015), antimalaria (Upegui et al., 2015), Wound healing (Charernsriwilaiwat et al., 2013), anti-parasite (Aukkanimart et al., 2015), as well as anti-colon cancer (Aisha et al., 2012), and anti-breast cancer (Lee et al., 2010). We therefore investigated the anti-cholangiocarcinoma effect of GM extract in an in vitro model (cell-cycle arrest, wound healing assay, and apoptotic gene expression) and in a hamster CCA model.
Materials and methods
Preparation of G. mangostana Linn. pericarp extract
Fresh GM was bought from a market in Khon Kaen, Thailand from the same areas as previously reported (Aukkanimart et al., 2015). Authentication was achieved by a taxonomist, Asst. Prof. Dr. Prathan Luecha, Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand. The voucher specimen (Collecting no. KKU-TB001) of the plant material has been deposited in Laboratory of Assoc. Prof. Dr. Thidarut Boonmars, Department of Parasitology, Faculty of Medicine, Khon Kaen University. GM pericarps were cleaned with distilled water several times until no dust and dirt remained on the fruit pericarp. The white flesh was then removed and the pericarp washed again. Pericarp were chopped and dried at 60°C in an incubator for 24 h and then ground into powder. The GM powder was weighed, dissolved with distilled water and then filtrated with filter paper, to be immediately used for oral treatment of the assigned hamster groups (Figure 1).
Figure 1.

Crude Extracts of Garcinia mangostana Hull
Preparation of cancer drug and compound
α- mangostin (C6H26O6, Lot No. 97072) was purchased from the Indofine Chemical Co. (Hillsborough Township, NJ, USA). The α-mangostin solution was immediately diluted with 0.05% dimethylsulfoxide (DMSO, Amresco®), and various concentrations were prepared based on the assigned group. Gemcitabine (Gemita®) was purchased from Fresenius Kabi Oncology (Haryana, India). Before use, gemcitabine was diluted with 0.85% normal saline for injection at the assigned dose.
Thin-layer chromatography for determination of α-mangostin
Before starting the experiment, thin-layer chromatography was used for determination of α-mangostin: stationary phase, silica (60 F254) aluminum plate; mobile phase, dichloromethane: ethyl acetate: methanol (16:2:1);detector, 2% w/v vanillin and 10% v/v sulfuric acid heated at 60 °C for 5 min. The Rf of crude GM extract was 0.79, which is similar to that of standard α-mangostin (Figure. 2).
Figure 2.

Thin-Layer Chromatogram of G. mangostana Pericarp. Standard α-mangostin (lane 1), G. mangostana pericarp extracts with methanol (lane 2) and crude G. mangostana pericarp extracts (lane 3). Stationary phase: siliga aluminium plate. Mobile phase: dichloromethane: ethyl acetate: methanol; (16:2:1). Detector: 2% w/v vanilline and 10% v/v sulphuric acids heated at 60°C for 5 minute.
Cell lines
The human intrahepatic CCA cell line (KKU-M214) was established in the Liver Fluke and Cholangiocarcinoma Reasearch Center, Khon Kaen University, Thailand. The hamster CCA cell line (Ham-1) was derived from the Department of Biochemistry, Faculty of Medicine, Khon Kaen University, Thailand. KKU-M214 cells and Ham-1 cells were cultured in RPMI-1640 medium and DMEM (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum, 100 units/ml of penicillin and 100 mg/ml streptomycin (Gibco BRL) at 37 ºC in 5% CO2 humidified incubator.
Cytotoxic effects on normal white blood cells
The effect of α-Mangostin on cell viability of normal white blood cells at difference concentration (1.5, 4, 30 and 60 µg/ml) and for difference time periods (24 and 48 h) was determined. After that, cell viability was determined by SRB assay.
Inhibitory assay
The M214 CCA cell line (JCR1556) was used for analysis of the inhibitory effect of α-mangostin; 5×105 cells were incubated with various concentrations of α-mangostin (0.5, 1, 1.5, 2 and 2.5µg/ml) and incubated at 37 °C with 5% CO2 for 24, 48 and 72 h were performed 3 times in triplicate. Cells in all treatment groups were harvested at each time point for evaluation of the IC50 and for protein extraction.
Cell-cycle arrest and apoptosis assays
M214 CCA cells treated with α-mangostin or gemcitabine (an anticancer drug used as the reference drug) at concentrations of 0.5, 1 and 1.5 µg/ml were used for cell arrest assay, and concentrations of 1, 1.5, 2, 4 and 8 µg/ml were used for apoptosis assay were performed 3 times in triplicate. Cells were harvested after 48 h and fixed with 70% ethanol, then stained with propidium iodide for 1 h. Pelleted cells were suspended in PBS and then stained with annexin V fluorescein isothiocyanate (FITC)/ PI for 10-15 min at room temperature in the dark. The cell cycle distribution and apoptosis induction were analyzed by flow cytometry (BD FACSCantoTM II; BD Biosciences, San Jose, CA, USA).
Wound healing assay
M214 CCA cells were cultured at 37 °C and 5% CO2 in an incubator for 48 h until cells covered the plate. All wells were then cut with a sterilized tip and this cut space was measured a distance in µm width and then plate of each group was continue cultured using untreated diluents and treated with α-mangostin at concentrations of 0.5, 1 and 1.5 µg/ml were performed 3 times in triplicate. The width distance was measured at 24 h post-treatment under a microscope.
Protein extraction and apoptotic protein expression
M214 cell line pellets in each group (control, diluents and treated groups) were harvested at each time point, 24 and 48 h. Each group was homogenized with a sonicator and the concentration measured at OD280. Protein from each group was run in 12% acrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (Bio-RAD, Lot no. BR 9203034, CA, USA). The transferred nitrocellulose was probed with an anti-housekeeping protein (β-actin) and apoptotic proteins (caspase-3, p53, BAX and Bcl-2), then color-developed using chemiluminescence (ECL™Prime, Lot no. 9659205; GE Healthcare, Uppsala, Sweden), and then photographed. The relationship of each apoptotic protein and β-actin was calculated and plotted in a graph.
Hamster CCA allograft model
Six-week-old male Syrian hamsters, approximately 90 g each, were injected with hamster CCA (Ham-1) cells and treated with GM extract and gemcitabine; after that, tumor development was observed. In brief, hamsters were intradermally injected with Ham-1 (2.5× 105) cells into the dorsal region (2sites/hamster). Three days after injection, hamsters received GM extract 100mg/kg orally (at 9.00 AM, daily) and gemcitabine 20 mg/kg intraperitoneally (at 9.00 AM for 3times/week) for 21 days. Tumor development in treated and untreated control groups was determined by measuring the size of the tumor every week. Tumor volume was calculated as follows: ½ (W2×L), where W and L were the shortest and longest diameters, respectively. At the end of each time period, hamsters were sacrificed and tumor mass was collected and weighed. Tissues were fixed in 10% neutral-buffered formalin for histological examination and immunohistochemical staining. Blood was collected for determination of the effect of GM pericarp extract on liver and kidney function. All protocols were approved by the Animal Ethics Committee, Khon Kaen University (AEKKU 62/2556).
Histopathological study
Paraffinized sections of the CCA mass from each group were cut into 4 µm slices, deparaffinized with xylene, washed with phosphate buffered saline (PBS), stained with hematoxylin and eosin, and then photographed under a microscope (Siraj et al., 2013).
Immunohistochemistry for CK19 and PCNA
Deparaffinized sections were washed with phosphate buffered saline and blocked with 5%skim milk in PBS incubated with primary antibodies, anti-cytokeratin 19 (CK19; Abcam, Cambridge, MA, USA) or anti-proliferating cell nuclear antigen (PCNA; Novocastra Laboratories, Newcastle-upon-Tyne, UK), then incubated with secondary antibody and developed for color using 3-amino-9-ethylcarbazole (AEC;Sigma-Aldrich,). The tissue slides were photographed under a microscope (Olympus CX31, Tokyo, Japan) and then graded based on positive staining (Juasook et al., 2013).
Liver and kidney function test
To determine the cytotoxic effect of GM crude extract, serum from hamsters in each group was used to analyze alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN) and creatinine (Cr) levels using Infinity™ Liquid Stable Reagents (Thermo Fisher Scientific, Waltham, MA, USA).
Results
Cytotoxic effects on normal white blood cells
α-mangostin at concentrations of 1.5, 4, 30 and 60 µg/ml had no cytotoxic effect on white blood cells (Figure 3).
Figure 3.
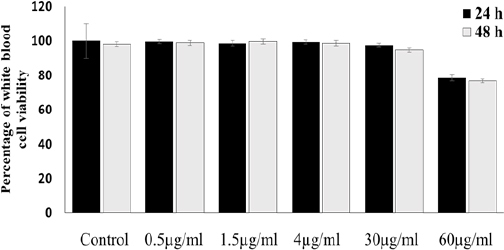
The Percentage of White Blood Cells Viability in Control and Treatment Groups (α-Mangostin 0.5, 1.5, 4, 30, 60 µg/mi) are no Significantly Different Compared with Control Group (p>0.05)
Cytotoxic effects of α-mangostin on the M214 CCA cell line
Figure 4 shows the cytotoxic effects of α-mangostin at concentrations of 0.5, 1, 1.5, 2 and 2.5 µg/ml on the human M214 CCA cell line (Figure 4 A) at each time point (Figure 4 B).
Figure 4.
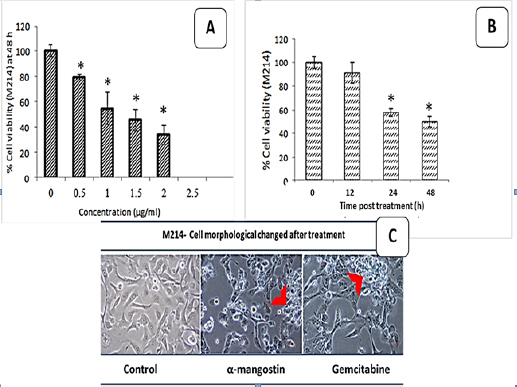
The Cytotoxic Effects of α-Mangostin (0.5, 1, 1.5, 2, 2.5 µg/ml) on M214- Human CCA Cell Lines (A) and cytotoxic effects of α-mangostin on M214- human CCA cell lines of each time points (B) resulted in a dose and time dependent. Morphological change post treatment (C). *p<0.05 compared to control, red arrow; indicated apoptotic cell
α-mangostin at a concentration of 1.36 µg/ml could reduce or inhibit CCA cell line growth and development by 50%, as shown in Figure 4A and 4B. Morphological changes after treatment with α-mangostin and gemcitabine included membrane blebbing, cell shrinkage, nuclear condensation, and fragmentation (Figure 4 C). Viability of cells was reduced in a dose-and time-dependent manner.
Wound healing assay
At 0 h, the distance between the left and right side of wounds in the normal control, untreated treatment and treated groups (0.5, 1, 1.5 µg/ml) were 44.2±1.1, 44.4±0.8, 44.34±0.9, 44.4±0.3 and 44.4±0.8 µm, respectively. At 18 and 24 h, a very short distance between the left and right side of the normal control and untreated treatment groups were observed. Normal cell development and proliferation were evidenced by space between the left and right side compared with the time point of 0 h. For treated groups at 24h, a space between the left and right side of the wound was observed; the distance between the left and right side in treated groups (0.5, 1, 1.5 µg/ml) was 11.3±0.13, 14.9±0.16 and 26.9±0.6 µm, respectively (Figure 5).
Figure 5.

Wound Healing Assay of the M214- Human CCA Cell Lines Showed the Inhibition of Cell Migration in Response to Treatment 0.5, 1, 1.5 µg/ml of α-Mangostin at 18 and 24 h. The Results are Expressed as mean±SD are Significantly Different Compared with Control Group (at 24 h *p< 0.05, **p<0.001)
Cell-cycle arrest and apoptosis
α-mangostin (1, 1.5 µg/ml) could induce cell arrest at the G1 phase, as evidenced by the percentage of cells at the G1 phase increasing from 74.2% to 85.2% after treatment for 48 h (Figure 6A and B). Clearly, α-mangostin (1, 1.5, 2, 4, 8 µg/ml) treatment could induce apoptosis in a human CCA cell line; the apoptosis-inductive effect appeared to be concentration-dependent, i.e. 22.6%, 54.7%, 49.96%, 55%, and 75.4%, respectively. Interestingly, the apoptosis effect of 8 µg/ml of α-mangostin was similar to that of gemcitabine, 75.4% and 75.6%, respectively. Figure 7A and 7B, show the percentage of necrotic cells, early apoptotic cells, viable cells and late apoptotic cells at 48 h.
Figure 6.

Analysis of Cell Cycle Phases, DNA Content Histogram Showing Distribution of M214 Cells at Various Phases of Cell Cycle after Treatment with α-Mangostin (A, B). The results are expressed as mean±SD are significantly different compared with control group (*p<0.05)
Figure 7.

Apoptosis Induction was Increased in KKU-M214 Cells Compare with Control. Flow Cytometry of Phosphatidylserine Exposure for M214-CCA Cells. CCA cell were exposed DMSO (Control), α-mangostin (1, 1.5, 2, 4, 8 µg/ml) and gemcitabine 10 µM for 48 h (A). The experiment was performed in three independent experiments and results are expressed as mean±SD. The percentage of apoptotic cells at 48 h are indicated (B). KKU-M214 cells express caspase3, P53, Bax and Bcl-2 after treated with various concentration of α-mangostin at 24 and 48 h (C). density of the band was normalized with internal actins (D), *,**p<0.05 compared to control.
Apoptotic protein expression
Expression of caspase-3, p53, Bax and Bcl-2, increased in intensity, as shown in Figure 7C and 7D. The α-mangostin-treated group demonstrated the highest expression of caspase-3, p53 and Bax, in a time- and dose-dependent manner (*p< 0.05), but this was not observed for Bcl-2.
Hamster CCA allograft model
The body weight of hamsters in each group increased in a time-dependent manner, but there was no significant difference among the untreated control, gemcitabine-treated and G. mangostana-treated groups. The effect of gemcitabine and G. mangostana extract on hamster tumor size was significantly decreased compared with the control group (Figure 8A; p< 0.001). The tumor volume in the untreated control, gemcitabine-treated and GM-treated groups was 1.4±0.9, 0.2±0.1 and 0.3±0.1, respectively, as shown in Figure 8B, and the tumor weight was 1.23±0.3, 0.7±0.2 and 0.8±0.2 mg, respectively (Figure 8 C).
Figure 8.

The Gross Anatomy of Tumor Tissue the Groups of Control, Administered with Gemcitabine 20 mg/kg Intraperitoneal Injection and Administered with G. Mangostana 100 mg/kg per Orally (A). Tumor volume (B) and tumor weight (C) were significantly decreased compare to control group (*; p<0.001)
Histological examination of tumors treated with gemcitabine and GM extract
The pathology of hamster tumor allografts was also examined (Figure 9); the histological observations are shown in Figure 9 (A-I). In the untreated control group, increased bile duct proliferation was observed (Figure 9A), as well as high expression of PCNA in the tumor mass (Figure 9 D). For treated groups (gemcitabine and GM extract), there was decreased bile duct proliferation (Figure 9 B and C) and low expression of PCNA in the cell nucleus (Figure 9 E and F). The gemcitabine-treated and GM-treated groups showed a statistically significantly lower expression of PCNA in the nuclei of tumor tissue when compared with the control group (p< 0.05) (Table 1).
Figure 9.

In Vivo Anti-Tumor Effect on Tumor Allograft Model for 21 Days of Treatment. Garcinia mangostana extracts 100 mg/kg/day oral daily (Figure 9 C, F and I) and gemcitabine 20 mg/kg per intraperitoneal (Figure 9 B, E and H) for 3 times/ week. drinking water was given to control group as the same volume (Figure 9 A, D and G). Tumor tissues were stained by hematoxylin and eosin (Figure 9 A-C), PCNA (Figure 9 D-F) and CK19 (Figure 9 G-I)
Table 1.
The Expression of Antigen in Tumor Tissue by the immunohistochemical Observations
| Antigen expression | Score | Experimental group | ||
|---|---|---|---|---|
| Control (n=8) | Gemcitabine (20 mg/kg) (n=8) | GM (100mg/kg) (n=8) | ||
| % (n) | %(n) | % (n) | ||
| Cytokeratin 19 antigen (CK 19) | 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 0 (0.0) | 4 (50.0) | 3 (37.5) | |
| 2 | 6 (75) | 2 (25.0) | 2 (25) | |
| 3 | 1 (12.5) | 1 (12.5) | 2 (25) | |
| 4 | 1 (12.5) | 1 (12.5) | 1 (12.5) | |
| Proliferating cell nuclear antigen (PCNA) | 0 | 0 (0.0) | 5 (62.5)* | 4 (50)* |
| 1 | 0 (0.0) | 3 (37.5) | 3 (37.5) | |
| 2 | 1 (12.5) | 0 (0.0) | 1 (12.5) | |
| 3 | 4 (12.5) | 0 (0.0) | 0 (0.0) | |
| 4 | 3 (37.5) | 0 (0.0) | 0 (0.0) | |
Score: 0, no or a few positive area (< 10%); 1, positive area (< 25%); 2, no or a few positive area (< 50%); 3, no or a few positive area (< 75%); 4, no or a few positive area (> 75%); Significantly different compared with untreated control at the same time point
(p< 0.05)
Immunohistochemical staining for anti-CK19 and anti-PCNA
The untreated control allograft CCA showed a lot of CCA development in each area, while CCA was decreased in the treated area and most of the tumor mass was mixed with fibrosis (Figure 9). PCNA was very strongly stained in the untreated control group, but there was only very light staining (a few nuclei) in the treated groups. CK19 was stained in all groups at the CCA area, but only light staining was observed in groups treated with gemcitabine and GM.
Liver and kidney function tests
Table 2 shows that the serum levels of ALT, ALP, BUN and creatinine in hamsters administered gemcitabine or GM were below normal levels when compared with the normal control; this confirmed that there was no hepatotoxicity and nephrotoxicity from either gemcitabine or GM.
Table 2.
Serum Level of Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Kidney Function Tests; Blood Urea Nitrogen (BUN), Creatinine (Cr) Levels in the Group of Untreated Control, Administered with Gemcitabine, Administered with GM and Normal Hamster
| Group | ALT (U/L) | ALP (U/L) | BUN (mg/dL) | Cr (g/dL) |
|---|---|---|---|---|
| Mean± SD | Mean± SD | Mean± SD | Mean± SD | |
| Untreated | 33.2±14.85 | 93.4±11.89 | 19.8±2.28 | 0.3±0.0 |
| Gemcitabine | 36.2±4.43 | 81.6±13.16 | 21±0.7 | 0.3±0.0 |
| GM | 30.4±6.6 | 77.2±14.04 | 21.4±1.52 | 0.3±0.0 |
| Normal control | 39.75±6.85 | 86±2.70 | 22.73±0.21 | 0.4±0.0 |
No statistical significant; p> 0.05
Discussion
In our previous studies we found that α-mangostin from GM could improve the hepatobiliary tract in hamster opisthorchiasis via its anti-inflammatory and antioxidant properties. In the present study, we found that α-mangostin has anti-CCA properties, as evidenced by the induction of apoptotic cell death through the mitochondrial pathway by inducing caspase-3, p53, Bax and Bcl-2 protein expression, as well as by reducing cell proliferation through cell-cycle arrest at the G1 phase, inhibiting metastasis (as shown in a wound healing assay), and retarding tumor mass (as demonstrated in a hamster allograft model).
The results show that α-mangostin could inhibit the M214 CCA cell line in a time- and dose-dependent manner, with an indicated IC50 value of 1.36 µg/ml. Previous reports have shown cytotoxic, anti-cancer and anti-proliferative effects of α-mangostin at concentrations of 1–20 µg/ml against various human or animal cell lines, and a strong inhibitory effect against various cancer cell lines (Krajarng et al., 2011; Hafeez et al., 2014). In addition, the present data reveal that α-mangostin suppressed M214 CCA cell line migration, as shown in Figure 5, which is supported by Lee et al., (2010). Treatment with α-mangostin induced G1-phase cell-cycle arrest (Figure 6); this was due to inhibition of entry into the S phase and G2/M phase of cell-cycle arrest (Matsumoto et al., 2005), which was completed by 48 h. α-mangostin has a powerful antiproliferative effect by inducing apoptosis. Annexin V is a phospholipid-binding protein with a strong affinity for phosphatidylserine, which appears on the cell surface as a general indicator of apoptosis. As shown in Figure 7, the percentage of annexin V-positive cells (right quadrants) increased to 22.6%, 54.7%, 50%, 55.1% and 75.4% after treatment with 1, 1.5, 2, 4 and 8 µg/ml of α-mangostin, respectively, compared with only 11.13% of the control cells; this is in accordance with previous results showing that α-mangostin induces apoptosis in SK-Hep-1 cells, i.e. canine osteosarcoma (Krajarng et al., 2012; Hsieh et al., 2013). Interestingly, the results of treatment with 8 µg/ml (0.0195 µM) of α-mangostin and gemcitabine (10 µM) were similar, inducing apoptosis in 75.6% and 75.4% of cells, respectively. The present results show that α-mangostin induced apoptosis in M214 CCA cells via increased caspase-3 activation, a pathway similar to sinomenine which was shown to induce apoptosis in a murine osteoclast cell line (He et al., 2014). Caspases (cysteinyl aspartate–specific proteases), which are part of the cysteine–aspartic acid protease family, are regulators of apoptosis. Caspases are common to both the extrinsic and intrinsic mitochondrial pathways. Caspase-3 activation is regulated by members of the Bcl-2 family (Wang et al., 2001). An imbalance of anti-apoptotic proteins such as Bcl-2 and Bcl-xL and pro-apoptotic proteins such as Bax and Bak usually occurs in the apoptotic process (Tsujimoto et al., 2002; Gomez-Lazaro et al., 2007). The expression of PCNA and CK19 staining was correlated with enhanced CCA development (Juasook et al., 2013; Sriraj et al., 2013). Our study demonstrated that α-mangostin can significantly decrease proliferating cell nuclear antigen (PCNA), similar to the another Thai herbal medicine, Annona muricata which demonstrated down-regulation of PCNA and up-regulation of Bax (Zorofchian Moghadamtousi et al., 2015). In a future study, an in vivo model could be used to examine and clarify the effectiveness of α-mangostin as an anti-cancer agent and for prevention of cholangiocarcinoma invasion and migration, thereby further our understanding of its mechanism of action on CCA cells.
Funding
This research was supported by: the National Research University Initiative and Research Promotion in Higher Education Project, Office of the Higher Education Commission, Thailand, through the HealthCluster (SHeP-GMS), Faculty of Medicine, Khon Kaen University (Golden Srinagarind grant number GR57201); and the Thailand Research Fund (grant number RTA 5880001)
Conflict of interests
No competing financial interests exist.
Acknowledgments
We would like to thanks the Department of Parasitology and Research Affair, Faculty of Medicine, Khon Kaen University for providing lab facility.
References
- Aukkanimart R, Boonmars T, Sriraj P, et al. Anthelmintic, anti-inflammatory and antioxidant effects of Garcinia mangostana extract in hamster opisthorchiasis. Exp Parasitol. 2015;30:5–13. doi: 10.1016/j.exppara.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Aisha AF, Abu-Salah KM, Ismail Z, Majid AM. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement Altern Med. 2012;20:104–108. doi: 10.1186/1472-6882-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chamadol N, Pairojkul C, Khuntikeo N, et al. Histological confirmation of periductal fibrosis from ultrasound diagnosis in cholangiocarcinoma patients. J Hepatobiliary Pancreat Sci. 2014;21:316–22. doi: 10.1002/jhbp.64. [DOI] [PubMed] [Google Scholar]
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Sukma M, Opanasopit P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int J Pharm. 2013;16:333–43. doi: 10.1016/j.ijpharm.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Gomez-Lazaro M, Galindo MF, Melero-Fernandez de Mera RM, et al. Reactive oxygen species and p38 mitogen-activated protein kinase activate Bax to induce mitochondrial cytochrome c release and apoptosis in response to malonate. Mol Pharmaco. 2007;71:736–43. doi: 10.1124/mol.106.030718. [DOI] [PubMed] [Google Scholar]
- Hafeez BB, Mustafa A, Fischer JW, et al. α-Mangostin: a dietary antioxidant derived from the pericarp of Garcinia mangostana L. inhibits pancreatic tumor growth in xenograft mouse model. Antioxid Redox Signal. 2014;10:682–99. doi: 10.1089/ars.2013.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LG, Li XL, Zeng XZ, et al. Sinomenine induces apoptosis in RAW 264.7 cell-derived osteoclasts in vitro via caspase-3 activation. Acta Pharmacol Sin. 2014;35:203–210. doi: 10.1038/aps.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SC, Huang MH, Cheng CW, et al. α- Mangostin induces mitochondrial dependent apoptosis in human hepatoma SK-Hep-1 cells through inhibition of p38 MAPK pathway. Apoptosis. 2013;18:1548–60. doi: 10.1007/s10495-013-0888-5. [DOI] [PubMed] [Google Scholar]
- IARC. Opisthorchis viverrini and Clonorchis sinensis. IARC Monogr Eval Carcinog Risks Hum. 2011;100:347–76. [PMC free article] [PubMed] [Google Scholar]
- Janeklang S, Nakaew A, Vaeteewoottacharn K, et al. In vitro and In vivo antitumor activity of Tiliacorinine in human cholangiocarcinoma. Asian Pac J Cancer Prev. 2014;15:7473–8. doi: 10.7314/apjcp.2014.15.17.7473. [DOI] [PubMed] [Google Scholar]
- Juasook A, Aukkanimart R, Boonmars T, et al. Tumor-related gene changes in immunosuppressive Syrian hamster cholangiocarcinoma. Pathol Oncol Res. 2013;19:785–94. doi: 10.1007/s12253-013-9645-x. [DOI] [PubMed] [Google Scholar]
- Kamada M, Akiyoshi K, Akiyama N, et al. Cholangiocarcinoma cell line TK may be useful for the pharmacokinetic study of the chemotherapeutic agent gemcitabine. Oncol Rep. 2014;32:829–34. doi: 10.3892/or.2014.3227. [DOI] [PubMed] [Google Scholar]
- Krajarng A, Nilwarankoon S, Suksamrarn S, et al. Antiproliferative effect of α-mangostin on canine osteosarcoma cells. Res Vet Sci. 2012;93:788–94. doi: 10.1016/j.rvsc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Krajarng A, Nakamura Y, Suksamrarn S, Watanapokasin R. Alpha-mangostin induces apoptosis in human chondrosarcoma cells through downregulation of ERK/JNK and AKT signaling pathway. J Agric Food Chem. 2011;59:5746–54. doi: 10.1021/jf200620n. [DOI] [PubMed] [Google Scholar]
- Lee GR, Jang SH, Kim CJ, et al. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK-NF-κB signaling pathway. Clin Exp Metastasis. 2014;31:897–907. doi: 10.1007/s10585-014-9678-x. [DOI] [PubMed] [Google Scholar]
- Lee YB, Ko KC, Shi MD, et al. alpha- Mangostin, a novel dietary xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions through the ERK signaling pathway in MCF-7 human breast adenocarcinoma cells. J Food Sci. 2010;75:13–23. doi: 10.1111/j.1750-3841.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- Li W, Li H, Zhang M, et al. Isogarcinol extracted from Garcinia mangostana L. Ameliorates systemic lupus erythematosus-like disease in a Murine model. J Agric Food Chem. 2015;30:8452–9. doi: 10.1021/acs.jafc.5b03425. [DOI] [PubMed] [Google Scholar]
- Liu QY, Wang YT, Lin LG. New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct. 2015;6:383–93. doi: 10.1039/c4fo00758a. [DOI] [PubMed] [Google Scholar]
- Marumoto M, Yamasaki T, Marumoto Y, et al. Systemic gemcitabine combined with hepatic arterial infusion chemotherapy with cisplatin, 5-fluorouracil, and isovorin for the treatment of advanced intrahepatic cholangiocarcinoma: a pilot study. Hepatogastroenterology. 2014;61:162–7. [PubMed] [Google Scholar]
- Matsumoto K, Akao Y, Kobayashi E, et al. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2005;66:1124–7. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Takagi H, Kakizaki S, et al. Gefitinib and gemcitabine coordinately inhibited the proliferation of cholangiocarcinoma cells. Anticancer Res. 2012;32:5251–62. [PubMed] [Google Scholar]
- Pothitirat W, Chomnawang MT, Supabphol R, Gritsanapan W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia. 2009;80:442–7. doi: 10.1016/j.fitote.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol. 2013;15:171–6. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriraj P, Aukkanimart R, Boonmars T, et al. Does a combination of opisthorchiasis and ethyl alcohol consumption enhance early cholangiofibrosis, the risk of cholangiocarcinoma? Parasitol Res. 2013;112:2971–81. doi: 10.1007/s00436-013-3469-1. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Bcl-2 family of proteins: life-or-death switch in mitochondria. Biosci Rep. 2002;22:47–58. doi: 10.1023/a:1016061006256. [DOI] [PubMed] [Google Scholar]
- Upegui Y, Robledo SM, Gil Romero JF, et al. In vivo antimalarial activity of α- Mangostin and the new Xanthone δ- Mangostin. Phytother Res. 2015;29:1195–201. doi: 10.1002/ptr.5362. [DOI] [PubMed] [Google Scholar]
- Wang A, Liu Q, Ye Y, Wang Y, Lin L. Identification of hepatoprotective xanthones from the pericarps of Garcinia mangostana guided with tert- butylhydroperoxide induced oxidative injury in HL-7702 cells. Food Funct. 2015;2:3013–21. doi: 10.1039/c5fo00573f. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- Zorofchian Moghadamtousi S, Rouhollahi E, Karimian H, et al. The Chemopotential effect of Annona muricata leaves against Azoxymethane-Induced colonic Aberrant Crypt Foci in rats and the Apoptotic effect of Acetogenin Annomuricin E in HT-29 Cells: A bioassay-guided approach. PLoS One. 2015;10:e0122288. doi: 10.1371/journal.pone.0122288. [DOI] [PMC free article] [PubMed] [Google Scholar]


