Abstract
Fentanyl is an opioid analgesic that it is widely used in cancer patients. Since there have been reports of effects of analgesic medications on the recurrence and development of resistance to treatment, influences of of fentanyl on MCF-7 and HEK293 cells were evaluated. Cell viability and apoptosis were assessed by MTT assay and flow cytometry, respectively. Gene expression analysis was performed by quantitative real-time PCR assay for the Oct4, Sox2 and Nanog genes as stem cell markers and Bax, Bcl2, and p53 genes as apoptosis markers. MTT assay results showed that fentanyl significantly inhibited the growth of MCF-7 cells in a dose-and time-dependent manner while significantly increasing apoptosis. In contrast, decrease was noted in HEK-293 cells. In MCF-7 derived cancer stem cells, fentanyl treatment decreased the expression of Bax, Bcl2, Oct4, Sox2, Nanog genes when compared to untreated cells. In HEK-293 stem cells, decrease was noted for Sox2, Nanog and Bax, but increase for Oct4. Our study supports an antitumor role of fentanyl by inducing apoptosis and reducing numbers of cancer stem cells in the MCF-7 human breast adenocarcinoma line.
Keywords: Fentanyl, stem cell markers, Tumorigenesis, stem cell
Introduction
Opioids are important medications for the management of acute and chronic pain in cancer patients. They have been accepted as indispensable drugs for severe malignant and non-malignant pain (Mercadante 2013; Grapeet et al., 2010 ; Darwishet et al., 2008 ;Taylor, 2007). Opioid drugs can be classified into many categories based on onset of effect and duration of analgesic action. Within them, there are rapid-onset opioids, which are releasedimmediately to molecular structure and effect with a very rapid onset (3-15 minutes) and short duration (1-2 hours). One of the opioid drugs is fentanyl citrate, a highly lipophilic molecule that passes easily through thecell membrane and effectively provides the analgesic requirements of severepain, with a high analgesic effect (Davis, 2011; Greco et al., 2011).
Fentanyl isan effective and fast acted synthetic opioid analgesic. It has a strong agonistic effect on the μ-opioid receptors (Rauck et al., 2009; Rauck et al., 2010; Kress et al., 2009). Historically, it has been used to treat breakthrough pain and is commonly used in pre-procedures as a pain reliever as well as an anesthetic medication withacombinationof benzodiazepine (Taylor, 2007; Davis, 2011; Niosh, 2014; Taylor et al., 2010; Davies, 2011). Fentanyl is more effective than morphine and pharmaceutical heroin (100%pure) approximately 80 to 100 and 40 to 50 times respectively. Fentanyl was the most widely used synthetic opioid in cancer patients and it has new delivery methods including a sublingual spray and transdermal patches for cancer patients (Niosh, 2014; Taylor et al., 2010; Davies et al., 2011).
Chemotherapy is themaintreatment for cancers and patients with different cancers derive more survival benefits through chemotherapy. However drug resistance occurs in many cancers and this may a cause of relapse and metastasis (Chabner et al., 2005; AIHW, 2015). Several reasons have been discussed related to resistance. A cause of failure of conventional treatment is considered to be dormant or resistant cancer cells (Greaves and Maley, 2012). Recent studies have shown that these cells are exhibiting stem cell characteristics and called as cancer stem cells (CSC). An increasing number of studies have shown that one of thereasonsforrelapse and metastasis is cancer stem cells and these cells have anunlimitedcapacity for self-renewal anddifferentiation (Weiberg, 2011).
The CSC model suggests that the main mass of a tumor is composed of differentiated cells, just a small amount of cells are CSC (Reya et al., 2011; Ginestier et al., 2007) and CSCs are responsible for metastasis, recurrence and drug resistance (Zhou et al., 2009; Sakariassen et al., 2007). Drug response of differentiated cells and CSCs are different. It looks like currently accepted chemotherapeutics aremoreeffective against to the differentiated tumor cells, that representing the main mass of the tumor, than CSCs. In fact, recent studies have shownthatthemost of the current chemotherapeutic agents and radiation therapy fails to kill CSCs (Hirsch et al., 2009; Shafee et al., 2008; Bao et al., 2006). Interestingly, these conventional therapies are indicated to increase the CSCs population (Tanei et al., 2009; Guptaet al., 2009).
With the often usage of fentanyl inthetreatmentof cancer patients, reports about the effectsof analgesic medications on recurrence and development of resistance to treatment and the important role of cancer stem cells on cancer recurrence and drug resistance, we aimed to study the effect of fentanyl on cancer stem cells.
Materials and Methods
Cells and reagents
MCF-7 cells (human breast adenocarcinoma cell line) and HEK293 cells (embryonic kidney) were purchased from American Type Culture Collection (ATCC, Rockville, Maryland 20852, USA), and grown in RPMI 1640 supplemented with 10% fetal bovine serum and 1% antibiotic-penicillin solution at 37 °C in a humidified atmosphere of 5% CO2. After the cell culture reached 80% confluency, cells were trypsinized with 0.25% trypsin and then harvested. Both MCF-7 and HEK293 cells divided into two groups; study group and control group. Study groups were treated with various concentrations of fentanyl (10, 15, and 20μM) for 24, 48 and 72 h. Control groups cultured in same conditions with study groups and subjected to same analysis. Results compared between treated and untreated groups. HEK293 embryonic kidney cells were used for determining the effect of fentanyl on normal stem cells.
MTT assay to assess cell viability and to determine the IC50
For assay of cell viability, cells were seeded into 96 well-plate (10,000 cells per well) and cultured for 48 hours. Then, Fentanyl treatment was performed in predetermined doses to the cell lines with 24, 48, 72 hours. Fentanyl-treated and untreated cell linesCells were subjected to MTT analysis in accordance with the manufacturer’s instructions using the cell profiling kit (MTT, Sigma-Alrich, St. Louis, MO). Purple formation of formazan in wells was quantified by absorbance value (OD) at 570 nm.
Apoptosis analysis by Annexin-V Binding Assay and Flow Cytometry
The effect of fentanyl on apoptosis was determined using Annexin V-FITC Apoptosis Detection Kit (APOAF; Sigma-Aldrich, St. Louis, USA). The analysiswas performed in accordance with the instructions of the manufacturer. Briefly, cells were treated with Fentanyl (IC50) for 24 hours, washed twice with PBS and 1x106 cells resuspended in annexin-V binding buffer. Then, 5 μl of FITC conjugated annexin-V and 10 μl of propidium iodide solution (1 mg/ml) was added to the cell suspensions and incubated for 1 hour at room temperature. Cells were washed twice, resuspended in 500 μl of annexin-binding buffer and about 10,000 events were acquired in FACS machine (BD FACSAriaTM III cell sorter). Data was analyzed by using BD FACSAria TM IIII software (BD Biosciences, CA, USA).
Isolation of stem cells in the MCF-7 and HEK293 cell lines
MCF-7 and HEK293 cells were cultured in RPMI 1,640 medium (containing 10% FBS and penicillin/ streptomycin) in 25 ml culture flasks at 37 °C in a 5% CO2 incubator. The culture medium was changed daily, and cell morphology was observed. Cultured cells were removed by trypsinization and centrifuged. The cell was dissolved in 1-2 ml of HBSS according to the density of the precipitate and the cells were counted by hemocytometer. Each FACS tube was labeled according to the antibody to be used. Counted cells were transferred to labeled FACS test tubes according to the desired amount. After the cells in the FACS tube were centrifuged, the precipitates were suspended in 100µl of 2% FBS / HBSS. Each conjugate anticordin (CD44-APC, Sigma-Aldrich Corporation, Germany; CD24-FITC, Sigma-Aldrich Corporation, Germany) was taken to be 20 μl for 106 cells and added to the appropriate test tube in the dark. DAPI was added to all the tubes at the latest stage. The tubes were kept in the dark (covered with aluminum foil) for one hour with shaking at 4 ° C for 10 minutes. Cells in each tube were washed with 3 mL of 2% FBS / HBSS to remove unbound antibody. After an extra wash with HBSS, the cell suspension was diluted with 400 μl of DAPI (0.4 ng / ml) (Sigma-Aldrich Corporation, Germany) and cells were separated with a FACS instrument (FACS Aria III - BD Pharmingen, USA).
Soft agar colony assays
Soft agar colony assaywas performed to confirm the stem cells obtained from HEH293 and MCF cell lines by flow cytometry. Basal agar was prepared as 0.8%. The prepared agar and 2xDMEM / RPMI + 10% FBS were heated to 40°C in a water bath, after which the two solutions were mixed in equal amounts. The mixture was kept at 37°C for cooling. Subsequently, 1 ml of this mixture was taken into a 6-well petri dish. The cultured cells were removed by trypsinization and counted to hematocytometrically count at least 2500 cells per petride. The top agar was prepared to be 0.6% in the same manner as the basal agar. Harvested cells and stem cells sorted by flow cytometry were added in appropriate numbers to the top agar.1 ml of the mixture was transferred to wells to form agar. Petri dishes were then incubated at 37°C for 10-14 days. A top agar medium was changed every 4-5 days. Finally, colony formation was observed by staining wells with Crystal violet.
Quantitative real-time polymerase chain reaction
Total RNA isolation was carried out by the phenol-chloroform extraction method, and cDNAs were synthesized byRevertAid First Strand cDNA Synthesis kit according to the manufacturer’s instructions. Quantitative RT-PCR analysis was performed in triplicate with LightCycler 480 instrument (Roche Diagnostics,). GeNorm analysis (PrimerDesign Ltd, Southampton UK) was performed to decide a housekeeping gene that not affectedbythe treatment of cells. Housekeeping genes including Ubiquitin C (UBC), Eukaryotic initiation factor 4A2 (eIF4A2), 18S ribosomal RNA, Glyceraldehyde 3-Phosphate dehydrogenase (GAPDH), Beta-actin (ACTB), β2 Microglobulin (B2 M), Glucose-6 phosphate dehydrogenase(G6PDH) were tested for consistent expression, following the treatment of cells. Eventually, Glucose-6 phosphate dehydrogenase (G6PDH) was chosen as a stably expressed gene for normalization. RealTime Ready Assay’s primers and probes (Roche Diagnostics GmbH, Mannheim Germany) was used as primers and probes for gene expression analysis. Oct4, Sox2, Nanog, Bax, Bcl2, p53 gene expression analysis wascarried out by this assay. Primer and probe optimizations were done. Quantitative real-time PCR (RT-qPCR) was performed in triplicates. The comparative CT method was used to calculate the relative quantification of gene expression.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 software (GraphPadSoftwareInc., La Jolla, CA, USA). Comparisons between treated and theuntreatedgroup were carried out by using paired t-test, one-way ANOVA test, and Tukey-Kramer post-test. Data were expressed as means ± SEM. Fold changes in gene expression were calculated using the comparative CT method. The Relative Expression Software Tool (REST 2009, Qiagen) was used for these calculationsP <0.05 was considered statistically significant.
Results
Fentanyl inhibited cell proliferation in human MCF-7 cells
To analyze the effects of fentanyl on cell viability, MCF-7 human breast adenocarcinoma cells treated with various concentrations of fentanyl (10, 15, and 20 μM) for 24, 48 and 72 h and cell viability was evaluated by MTT assay. Results indicated that fentanyl significantly inhibited the growth of MCF-7 cells in a dose -and time-dependent manner (Figure 1). Results show that fentanyl exhibits clearly an anti-proliferative effect on MCF-7 cells. The IC50 dose of fentanyl was determinedas 20 μM for 24 hby using the result of this analysis.
Figure 1.
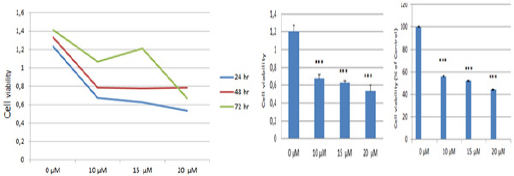
Fentanyl Inhibited Cell Proliferation in MCF-7 Human Breast Adenocarcinoma Cells. Cell viability was analyzed by MTT assay on MCF-7 cells after incubation with various concentrations of Fentanyl for 24, 48 h and 72 hr. Values are present as means ± standard deviation of six independent experiments. The differences between mean values were assessed by the One-Way ANOVA. **P < 0.01, ***P < 0.001 against the control
Examination of apoptosis by AnnexinV-FITC/PI-Flow Cytometer
MCF-7 and HEK-293 cells were treated with 20 µM Fentanyl for 24 hr and stained with annexin V-FITC/PI, and then analyzed by flow cytometer. Theearlyapoptotic stage was used for the evaluation of results (Figure 2). Results were obtained by comparing fentanyl-treatedand untreated groups. Results indicated that Fentanyl treatment significantly increases apoptosis in MCF-7 cells (p<0.001; Figure A) while decreases in HEK-293 cells (p<0.001; Figure B).
Figure 2.
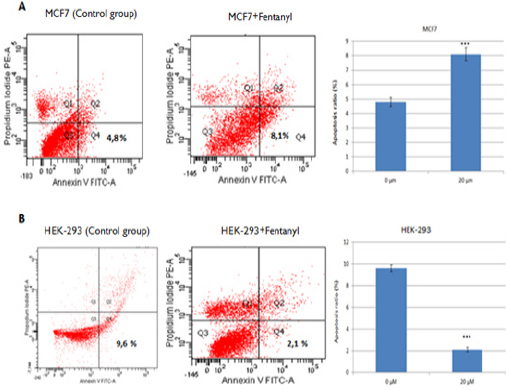
Fentanyl-Induced Apoptosis of MCF-7 and HEK-293 Cells. MCF7 and HEK-293 cells treated with 20 μM Fentanyl for 24 hr. Apoptosis analysis by annexin V–FITC/PI double staining of (A) MCF-7 and (B) HEK-293 cells treated with 20 μMFentanyl for 24 hours. Apoptosis ratios represent the percentage of cells in the early apoptosis stage (PI-negative/annexin V–FITC-positive). Data displays the mean ± standard deviation of three independent experiments. **P<0.01; ***P<0.001. Abbreviations: FITC, fluorescein isothiocyanate; PI, propidium iodide; Q, quadrant
Examination of the effect of Fentanyl on cancer stem cells
To investigate the effect of Fentanyl on cancer cell subpopulations, surface markers were evaluated in MCF-7 and HEK-293 cells. Results were obtained by comparing fentanyl-treatedand untreated groups of MCF-7 and HEK-293 cells (Figure 3). Fentanyl treatment significantly decreased the CD44+/CD24− subpopulation in MCF-7 cells (p<0.01; Figure 3A), and significantlyincreased the SSEA-4 (+)/SSEA-1(-) phenotype inHEK-293 cells(p<0.001; Figure 3B). These results indicate that fentanyl might be inducing a transition of MCF-7 cells to differentiated cancer cells.
Figure 3.
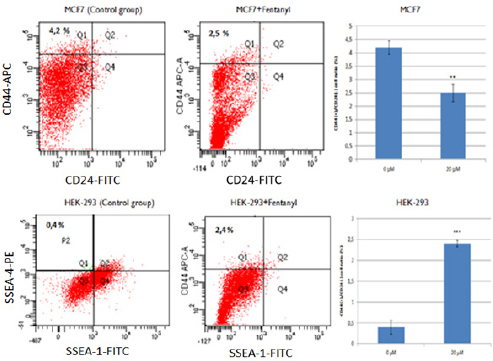
Fentanyl Reduced the Amount of CSCs in the MCF-7 Cells and Increased the Number of Stem Cells in the HEK-293. (A) Results of the CD44+/CD24−cells in the Fentanyl untreated and treated MCF-7. (B) Representation of SSEA-4 and SSEA-1 cells in the Fentanyl untreated (B) and treated HEK293 cells by flow cytometry. Abbreviations: FITC, fluorescein isothiocyanate; APC-A, Allophycocyanin A, PE; Phycoerythrin; Q, quadrant
Gene expression analysis
Expression analysis of genes associatedwith apoptosis and stem cells carried out by qPCR analysisin stem cells that sorted from treated and untreated groups of MCF-7 and HEK293 cells. We evaluatedBax, Bcl2, p53 genes to analyze apoptosis and Oct4, Sox2, Nanoggenes as stem cell marker. As shown in Figure 4, fentanyl treatment decreased the expression of Bax, Bcl2, Oct4, Sox, and Nanog genes in MCF7 cancer stem cells. These results demonstrate that fentanyl reduces the expression of genes which are considered as stem cell markers. However, fentanyl decreases both the expressions of Bax gene with pro-apoptotic effect and Bcl2 gene with anti-apoptotic effect. In HEK293 stem cells, fentanyl decreased Sox2, Nanog, Bax genes, while increased Oct4gene. However, Bcl2 expression was not detected in the fentanyl-treated HEK293 stem cells. This result suggests that the expression of Bcl2mayinhibitby fentanyl. p53 gene expression was also not detected both in MCF7 cancer stem cells and HEK-293 stem cells.
Figure 4.
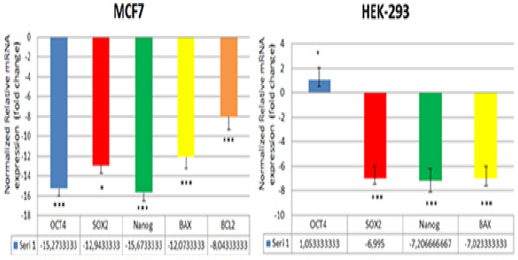
qPCR Analysis of Genes Related to Apoptosis and Stem Cells; BAX, BCL2, andOCT4, SOX2, Nanog in MCF-7 and HEK-293 after Treatment with 20 µM Fentanyl for 24 Hours as Triplicate. BCL2 could not be compared between the two groups, it has not been detected in the HEK-293 control group. The experiment was accomplished in triplicates and the data are expressed as fold change. Error bars indicate the standard error of the mean. *P<0.05;**P<0.01;** p< 0.001.
Discussion
Fentanyl is often used in cancer patients because of its analgesic effects. A few anesthetic and analgesic medications, which including fentanyl, have been thought to be responsible for the recurrence and metastatic rate of malignancies (Bovill, 2010; Lennon et al., 2012). It has been reported (Qin et al., 2012) that the fentanyl inhibits growing of gastric cancer cells by down-regulation of NF-κB and up-regulation of PTEN. As suggested that (Lennon et al., 2014) the mu opioid receptors (MOR) play an important function in the epithelial-mesenchymaltransition changes that were formed during lung cancer progression Recently, Zhang et al. demonstrated that fentanyl inhibits the colorectal tumor growth and HCT-116 cell invasion via downregulationof β-catenin and miR-182 expression (Zhang et al., 2015). According to our findings, antitumor activity of fentanyl is depending on the cell type. Results obtained from the examination of apoptosis by Annexin-V-FITC/PI flow cytometry showed that fentanyl treatment significantly increased the apoptosis in MCF-7 cells while decreased apoptosis in HEK293 cells. Consistent with this result, fentanyl treatment decreased the CD44+/CD24− subpopulation in MCF-7 cells and increased the SSEA-4(+)/SSEA1(-) phenotype subpopulation intheHEK293 cells. These results indicate that fentanyl might be inducing a transition of MCF-7 derived stem cells to differentiated cancer cells.
As discussed above, chemotherapy is themaintreatment for cancer patients and many cancers develop resistance to chemotherapeutics. A cause of failure of conventional treatment is considered to be dormant or resistant cancer cells (Greaves and Maley 2012; Ajani et al., 2015). It has been suggested that a subpopulation of cancer cells with a number of stem cell features, so-called”cancer stem cells” (CSCs), also called as cancer-initiatingcells (CICs), tumor-propagatingcells (TPCs), and tumor-initiatingcells(TICs), may have responsibilities in cancer recurrence and resistance to treatment (Kaur et al., 2014). To determine the effect of fentanyl on the cancer stem cells, stem cells sorted by Flow cytometry and subjected to qPCR analysis after the fentanyl treatment. Results showed the reduced expression of genes; Oct4, Sox2 and Nanog, which are considered as stem cell markers in MCF-7 cancer stem cells. Like as stem cell markers, Bax and Bcl2 gene expressions were decreased in the fentanyl-treatedgroup too. Results of our study demonstrated that fentanyl is significantly reducing the number of cancer stem cells in the MCF-7 cell population while increasing the number of stem cells in the HEK293 population. This contradiction in HEK293 cells should be investigated with further studies. However, results of flow cytometryanalysis in MCF-7 are consistent with gene expression outcomes. These results indicate that fentanyl is inhibiting apoptosis and reducing the number of cancer stem cells in MCF-7 human breast adenocarcinoma cells.
In conclusion, our study supports the antitumor role of fentanyl; inducingcells to apoptosis and reducing the number of cancer stem cells in MCF-7 human breast adenocarcinoma cells. Fentanyl can be thought as a treatment option besides being a painkiller. Also, it is important to clarify the impact mechanisms of associated pathways.
No competing financial interests exist.
References
- Ajani JA, Song S, Hochster HS, Steinberg IB. Cancer stem cells: the promise and the potential. Semin Oncol. 2015;42:3–17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Australian institute of health and welfare (AIHW) Australian cancer incidence and mortality (ACIM) books: All cancers combined. 2015. [Last accessed April 28, 2016 28.04.2016]. Available at http://www.aihw.gov.au/acim-books/
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bovill JG. Surgery for cancer: does anesthesia matter? Anesth Analg. 2010;110:1524–6. doi: 10.1213/ANE.0b013e3181d8d183. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Roberts TG. Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- Darwish M, Messina J. Clinical pharmacology of buccal tablet for the treatment of breakthrough pain. Exp Rev Clin. Pharmacol. 2008;1:39–47. doi: 10.1586/17512433.1.1.39. [DOI] [PubMed] [Google Scholar]
- Davies A, Sitte T, Elsner F, et al. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage. 2011;41:358–6. doi: 10.1016/j.jpainsymman.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Davis MP. Fentanyl for breakthrough pain: a systematic review. Exp Rev Neurotherapeut. 2011;11:1197–6. doi: 10.1586/ern.11.63. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grape S, Schug SA, Lauer S, Schug BS. Formulations of fentanyl for the management of pain. Drugs. 2010;70:57–2. doi: 10.2165/11531740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–3. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco MT, Corli O, Montanari M, et al. Writing protocol committee;Cancer pain outcome research study group (CPOR SG) investigators. Epidemiology and pattern of care of breakthrough cancer pain in a longitudinal sample of cancer patients: results from the cancer pain outcome research study group. Clin J Pain. 2011;27:9–18. doi: 10.1097/AJP.0b013e3181edc250. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–9. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–4. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–1. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Singh G, Kaur K. Cancer stem cells: an insight and future perspective. J Cancer Res Ther. 2014;10:846–2. doi: 10.4103/0973-1482.139264. [DOI] [PubMed] [Google Scholar]
- Kress HG, Ororiska A, Kaczmarek Z, et al. Efficacy and tolerability of intranasal fentanyl spray 50 to 200 mg for breakthrough pain in patients with cancer: a phase III, multinational, randomized, double-blind, placebocontrolled, crossover trial with a l-month, open-label extension treatment period. Clin Ther. 2009;31:1177–1. doi: 10.1016/j.clinthera.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Lennon FE, Mirzapoiazova T, Mambetsariev B, et al. The Mu opioid receptor promotes opioid and growth factor induced proliferation, migration and epithelial mesenchymal transition (EMT) in human lung cancer. PloS One. 2014;9:e91577. doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon FE, Moss J, Singleton PA. The mu-opioid receptor in cancer progression: is there a direct effect? Anesthesiology. 2012;116:940–5. doi: 10.1097/ALN.0b013e31824b9512. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Cancer pain curr. Opin Support Palliat Care. 2013;7:139–3. doi: 10.1097/SPC.0b013e3283610433. [DOI] [PubMed] [Google Scholar]
- Qin Y, Li L, Chen J. Fentanyl inhibits progression of human gastric cancer MGC-803 cells by NF-kappaBdownregulation and PTEN upregulation in vitro. Oncol Res. 2012;20:61–9. doi: 10.3727/096504012x13473664562501. [DOI] [PubMed] [Google Scholar]
- Rauck R, North J, Gever LN, Tagarro I, Finn AL. Fentanyl buccal soluble film (FBSF) for breakthrough pain in patients with cancer: a randomized, double-blind, placebo controlled study. Ann Oncol. 2010;21:1308–4. doi: 10.1093/annonc/mdp541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauck R, Tark M, Reyes E, et al. Efficacy and long-term tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain. Curr Med Res Opin. 2009;25:2877–5. doi: 10.1185/03007990903368310. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2011;414:105–1. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Sakariassen PQ, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–2. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by Aldehyde Dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin- based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–1. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- Taylor D, Galan V, Weinstein SM, et al. Fentanyl pectin nasal spray 043 study group. Fentanyl pectin nasal spray in breakthrough cancer pain. J Support Oncol. 2010;8:184–90. [PubMed] [Google Scholar]
- Taylor DR. Fentanyl buccal tablet: rapid relief from breakthrough pain. Exp Opin Pharmacother. 2007;8:3043–1. doi: 10.1517/14656566.8.17.3043. [DOI] [PubMed] [Google Scholar]
- The national institute for occupational safety and health (NIOSH) FENTANYL: incapacitating agent, centers for disease control and prevention. 2014. [Last accessed April 28 2016]. available at http://www.cdc.gov/niosh/ershdb/EmergencyResponseCard_29750022.html .
- Zhang XL, Chen ML, Zhou SL. Fentanyl inhibits proliferation and invasion of colorectal cancer via β-catenin. Int J ClinExpPathol. 2015;8:227–5. [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Zhang H, Damelin M, et al. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806–23. doi: 10.1038/nrd2137. [DOI] [PubMed] [Google Scholar]


