Abstract
Twenty-eight specimens of Esophael squamous cell carcinoma (ESCC) were obtained by surgery procedures. The tissues were fixed in formalin and embedded in paraffin. In each case, all available hematoxylin and eosin stained sections were examined and a representative block was selected. The ages of these patients ranged from 40 to 93 years, with a mean age of 60 years. Results. The histological grade of tumors was 4 well-differentiated, 19 moderately differentiated and 5 poorly differentiated. Expression of Hsp27 and Hsp70 in ESCC was demonstrated in 23 (82,14%) and 26 (92,86%) cases, respectively. Adjacent normal mucosa was positive in 11 (39,29%) samples and 9 (32,15%) samples for Hsp27 and Hsp70, respectively. No relationship between the expression of Hsp27 and Hsp70 with the clinicopathological parameters, including gender, age, surgical margin, lymph node status and tumor differentiation. The median follow-up period was 60 months. Survival analysis of patients with ESCC showed no relationship with the expression of Hsp27 and Hsp70. Conclusion. Taken together, our results demonstrate that Hsp27 and Hsp70 are expressed in ESCC tissues, but they are not good prognostic factor for patients with ESCC.
Keywords: Esophageal carcinoma, HSP 27, HSP 70, prognosis, immunohistochemistry
Introduction
The aim of this research was to evaluate the expression of Hsp’s 27 and 70 in ESCC in order to compare the expression of these proteins in tumor tissues by immunohistochemistry.
The present study showed the expressions of Hsp27 and Hsp70 in ESCC. The limiting factor for a more accurate analysis was due to the small number of samples. We believe due to the genetic diversity in different regions such as America, Asia, Europe and Africa, tests of large samples are necessary in order to confirm the behavior of these proteins in ESCC.
Esophageal cancer (EC) is a neoplasm highly aggressive. In Brazil, according INCA (National Institute of Cancer), 1727 women and 6203 men died of esophageal carcinoma in 2013. The estimated incidence for 2016 was 10.810 new cases with 7.950 men and 2.860 women. The most common type of ESCC, which accounts for 96% of cases (INCA, 2016). Despite of the improvements in its detection as well as the treatment, the outcome in all patients diagnosed with esophageal cancer remains poor, with an overall 5-year survival of 18,4% (NIH, 2016).
Heat shock proteins (Hsp’s) are molecular chaperones that are evolutionary conserved, are ubiquitous and have multiple functions in cellular homeostasis. Hsp’s act as the primary cellular defense against damage to the proteome, initiating refolding of denatured proteins as well as regulating degradation after severe protein damage. They protect cells from various stresses such as hypoxia or ischemia, as well as sudden increases in temperature (Arrigo et al. 2007; Lelj-Garolla and Mauk, 2012). In addition, there are different types of Hsp’s interfering in several processes. Hsp27 and Hsp70 have been the most studied proteins from this group.
Hsp27 protein has a protective role during heat shock, other forms of environmental and pathophysiological stress. Evidence indicates that Hsp27 protein reveals a wide spectrum of functions including regulation of cell growth and differentiation (Hell-Pourmojib et al., 2002) and of cytoskeletal dynamics (Hirano et al., 2004), signal transduction (Park et al. 2003) and protection against apoptosis induced by different agents (Arrigo, 2000). Hsp27 are abundantly expressed in malignant cells, and have been accused of participating in oncogenesis or chemotherapy resistence (Garrido, 2006) presumably due to its capacity to disable apoptosis. In esophageal and gastric cancer, the high expression of Hsp27 has been associated with metastasis, prognosis and resistance to chemotherapy or radiation (Slotta-Huspenina, 2012).
Hsp70 is a stress inducible Hsp, which has been reported to play a role in therapy resistance (Galluzzi et al., 2009). In unstressed conditions of the cell, Hsp70 have been presented in low levels, in contrast, Hsp70 expression increases rapidly in response to various types of stresses (Daugaard et al., 2007). Moreover, increased expression of Hsp70 has been associated with malignant features and poor prognosis of patients with oral cancer (Tavassol et al., 2011). This evidence suggests that Hsp70 is a promising target in cancer treatment. Studies have shown a decrease HSP70 levels in some cultured tumor cell to induce cell death as well as interfering into cytotoxic agents. (Powers et al., 2008).
Patients under curative treatment for ESCC will eventually relapse, leading to death as a result of this cancer. The poor outcome of ESCC and its high incidence is important in order to understand the progression of the disease and, thus, to be able of identifying associated prognostic factors.
The aim of this research was to evaluate the expression of Hsp’s 27 and 70 in ESCC in order to compare the expression of these proteins in the tumor tissues. To our knowledge, this is the first study in which the concomitant expression of these immunomarkers has been demonstrated in these esophageal lesions in a Brazilian population.
Materials and Methods
A total of twenty-eight specimens of ESCC were obtained by surgery procedures. The tissues were fixed in formalin and embedded in paraffin according to standard procedures in Pathology Department, Federal University of São Paulo/Brazil. In each case, all available hematoxylin and eosin stained sections were examined and a representative block was selected after careful screening for quality of fixation and representative pattern of lesions. Ethical approval for this study was granted by the local Ethics Committee (The Resolution no 196 of National Health Council). This study included samples from 28 patients and their ages ranged from 40 to 93 years, with a mean age of 60 years.
Immunohistochemistry
Immunostaining was performed on sections of 3 μm mounted on 3-aminopropylotrimetoxy-silane (Sigma- Aldrich, St. Louis, MO, USA) coated slides. Briefly, sections were deparaffinized in xylene, rehydrated through graded ethanols, followed by blocking of endogenous peroxidase activity in 3% hydrogen peroxide for 20 minutes at room temperature. Antibody-binding epitopes were retrieved using steamer for 30 minin 10 mM sodium citrate buffer (pH 6,0). Sections were then incubated with mouse monoclonal antibody for Hsp27 (clone: 2B4, dilution 1:40, Novocastra Laboratories Ltd - Newcastle upon Tyne, UK), Hsp70 or rabbit polyclonal antibody for Hsp70 (K-20, dilution 1:200, Santa Cruz Biotechnology,
Inc.) overnight at 4°C in humid chamber. After washing twice with phosphate-buffered saline (PBS), pH 7.4, slides were incubated with biotinylated second-stage antibody for 30 minutes, followed by incubation with streptavidin-biotin-peroxidase complex (LSAB, Dako, Carpinteria, California, USA) for further 30 minutes, at room temperature. Staining as carried out using a solution 3-3’diaminobenzidine tetrahydrocloride (DAB- Dako, Carpinteria, California, USA). Washes with PBS were performed between each step. Nuclei were counterstained with Harris hematoxylin before mounting slides in Entellan (Sigma-Aldrich, St. Louis, MO, USA). Negative and positive controls were included in each batch of immunohistochemistry. Section of skin known to express high levels of Hsp27 and Hsp70 was included as positive control, while in negative control the primary antibody was omitted.
Expression was assessed based onthe intensity of cytoplasmic immunostaining and the percentage of stained tumour cells. The intensity was scored as 0 (negative), 1 (weak staining), 2 (moderate staining) or 3 (strong staining). The percentage of positive tumour cells was scored as 0 (none), 1 (1-10%), 2 (11–50%), 3 (51–80%) or 4 (>80%). Multiplication of the scores for intensity and percentage resulted in a semiquantitativeimmunoreactive score (IRS) ranging from 0 to 12. Two independent observers (RAN and CCFL) evaluated the tumor staining. Differences were discussed at a double-header microscope to achieve a final consensus (Sllota-Huspenina et al., 2013). IRS ≥4 was considered positive and value <4 was considered negative. Representative areas were photographed by a digital camera at 400× magnification.
Statistical analysis
Association between two categorical variables was tested using the chi-square test. The association between immunohistochemical findings was evaluated by Pearson’s correlation coefficient. All significance probabilities (p values) presented was the bilateral type and values less than 0.05 were considered statistically significant. The STATISTICS software was used for statistical analysis. The survival rates were calculated by using the Kaplan-Meier method for analysis of censored data. The statistical significance of differences in survival between the groups was calculated by using the log-rank test.
Results
Expression of Hsp27 and Hsp70 in ESCC was demonstrated in 23 (82,14%) and 26 (92,86%) cases, respectively. Adjacent normal mucosa was positive in 11 (39,29%) samples and 9 (32,15%) samples for Hsp27 and Hsp70, respectively (Table 1). Hsp27 and Hsp70 have both positive staining in 75% of cases (Table 2). Diffuse staining of Hsp27 and Hsp70 was predominantly identified in the cytoplasm of cancer cells (Figure 1), There is no expression in normal cells under physiological conditions.
Table 1.
Frequency of Protein Expression in Esophageal Squamous Cell Carcinoma and Adjacent Mucosa
| Protein | Total | Negative | Positive |
|---|---|---|---|
| n | n (%) | n (%) | |
| ESCC | |||
| Hsp27 | 28 | 5 (17.86) | 23 (82.14) |
| Hsp70 | 28 | 2 (7.14) | 26 (92.86) |
| Adjacent mucosa | |||
| Hsp27 | 28 | 17 (60.71) | 11 (39.29) |
| Hsp70 | 28 | 19 (67.85) | 9 (32.15) |
ESCC, esophagealsquamouscell carcinoma
Table 2.
Relation between Hsp27 and Hsp70 in Oesophagealsquamous Cell Carcinoma
| Hsp27TU | Total | p | |||||
|---|---|---|---|---|---|---|---|
| - | + | ||||||
| Hsp70TU | 0 | 0.00% | 2 | 7.2% | 2 | 7.10% | 0.468a |
| + | 5 | 17.80% | 21 | 75.0% | 26 | 92.90% | |
| Total | 5 | 17.80% | 23 | 56.3% | 28 | 100.00% | |
, not significant
Figure 1.
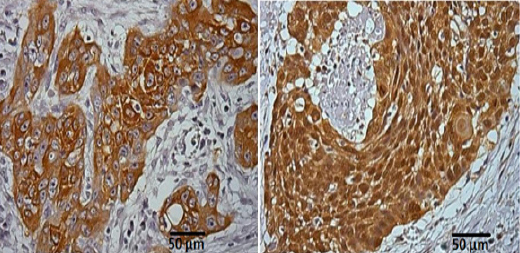
Immunostaining for Hsp27 and Hsp70 on ESCC.
(A). Section showing intense and diffuse staining of Hsp27 in cytoplasm of tumor cells. Membrane staining was also observed.x400.(B) Section showing intense and diffuse staining of Hsp70 in cytoplasm of tumor cells. Membranestainingwasalsoseen. x400 magnification
We did not find any relationship between the expression of Hsp27 and Hsp70 with the clinicopathological parameters, including gender, age, surgical margin, lymph node status and tumor differentiation. From all samples, 23 were men and 5were women with a mean age of 60 years. The histological grade of tumors was 4 well-differentiated, 19 moderately differentiated and 5 poorly differentiated. The median follow-up period was 60 months. Patients’ survival was classified as: alive without disease (N = 9), alive with disease (N = 3), death by cancer (N = 14), death by other causes (N = 0) and lost to follow-up (N = 2) (Table 3). Survival analysis of patients with ESCC showed relationship with the expression of Hsp27 and Hsp70 (Figure 2).
Table 3.
Relationship between Clinicopathological Parameters and Immunohistochemical Expression of Hsp27 and Hsp70 in the Esophageal Squamous Cell Carcinoma
| Hsp27 | Hsp70 | |||||
|---|---|---|---|---|---|---|
| Neg n (%) | Pos n (%) | p | Neg n (%) | Pos n (%) | p | |
| Gender (n=28) | NS | NS | ||||
| Male | 3 (10.71) | 20 (71.43) | 1 (3.57) | 22 (78.57) | ||
| Female | 2 (7.14) | 3 (10.72) | 1 (3.57) | 4 (14.29) | ||
| Age (n=28) | NS | NS | ||||
| ≥60 | 2 (7.14) | 10 (35.73) | 0 (0.00) | 12 (42.86) | ||
| <60 | 3 (10.71) | 13 (46.42) | 2 (7.14) | 14 (50.00) | ||
| Surgicalmargin (n=28) | NS | NS | ||||
| compromised | 0 (0.00) | 9 (32.10) | 1 (3.57) | 8 (28.6) | ||
| notcompromised | 5 (17.9) | 14 (50.00) | 1 (3.57) | 18 (64.30) | ||
| Lymph node status (n=20) | NS | NS | ||||
| Positive | 0 (0.00) | 7 (35.00) | 1 (5.00) | 6 (30.00) | ||
| Negative | 4 (20.00) | 9 (45.00) | 1 (5.00) | 12 (60.00) | ||
| Tumor differentiation(n=28) | NS | NS | ||||
| well-differentiated | 2 (7.14) | 2 (7.14) | 1 (3.57) | 3 (10.72) | ||
| moderatelydifferentiated | 2 (7.14) | 17 (60.71) | 2 (7.14) | 17 (60.71) | ||
| undifferentiated | 1 (3.57) | 4 (14.29) | 0 (0.00) | 5 (17.86) | ||
| Follow-up (n=28) | ||||||
| Live without disease | 5 (21.74) | 4 (17.39) | <0.05a | 3 (10.71) | 6 (21.42) | <0.05b |
| Live with disease | 2 (7.14) | 1 (3.57) | 3 (10.71) | 0 (0.00) | ||
| Death bycancer | 8 (34.78) | 6 (26.09) | 5 (17.85) | 9 (32.14) | ||
| Lost | 2 (7.14) | 0 (0.00) | 2 (7.14) | 0 (0.00) |
, Hsp27 expression exhibited a significantly better prognosis;
, Hsp70 expression exhibited a significantly better prognosis; NS, not significant
Figure 2.
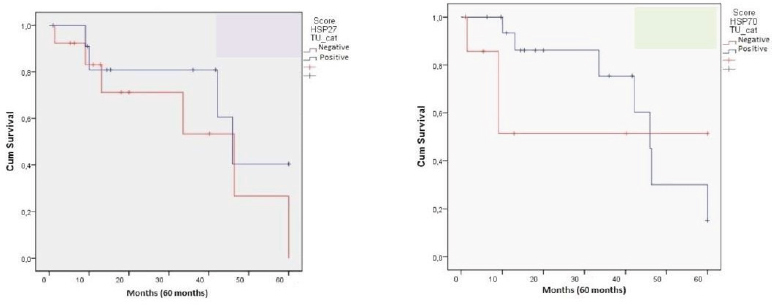
Kaplan-Meier Survival Curves of Patientes with ESCC According to Hsp27 and Hsp70 Expression
Discussion
Esophageal carcinogenesis is a complex multistep process. Several factors are postulated to play a role in the development of ESCC. Esophageal cancer is rare among young people and its rate increases with age, suggesting prolonged exposure to environmental carcinogens. The esophageal epithelium exposed to carcinogens can initiate a chronic inflammation process and develop dysplasia that may develop into cancer and finally metastases. Among the cancers of the gastrointestinal tract, ESCC has the worst prognosis and grows faster than other tumors of this tract. In this study, the incidence of ESCC was lower than in the past decade (4,6 male:1 female) and the mean age higher than the literature (Tercioti et al., 2011).
The mechanisms underlying the role of Hsp27 and Hsp70 in ESCC carcinogenesis are not clearly defined. Overexpression of Hsp27 contributes to the malignant progression including increased tumorigenicity, the resistance to the treatment and inhibition of apoptosis (Acunzo et al., 2012; Stope et al., 2012). On the other hand, Hsp70 can have their synthesis increased in response to cell damage, such as those induced by chemotherapy. Increased expression of this protein has been associated with cytoprotection against apoptosis (Brosina et al., 2007). In view of these considerations, these proteins represent molecular targets in intervention of ESCC in humans. One research group demonstrated in 2013 that Hsp27 was present in cervical intraepithelial lesions of Brazilian women. In cervical squamous cell carcinomas, expression of this protein was 98.11% (Dobo et al., 2013).
In this study, positive expression of Hsp27 and Hsp70 in ESCC was 82% and 92% and in adjacent normal mucosa was 40% and 32%, respectively. These results suggest no involvement of Hsp27 and Hsp70 in the defense of normal epithelia cells. These results suggest that both proteins when present in adjacent normal mucosa may protect it of common environmental insults, however, when present in the tumor tissue may protect it from chemotherapy or another treatment that aims eradicate it. Twenty-one tumor samples showed both Hsp27 and Hsp70 indicating that these proteins may also play a role after malignant transformation such as the protection of tumor progression. Despite this our results showed no significant correlation between Hsp27 and Hsp70 and gender, age, surgical margin, lymph node status and tumor differentiation. Hsp27 and Hsp70 were related with follow-up of patients.
Kawanishi et al., (1999) conducted a immunohistochemical study for Hsp 27 and Hsp 70 with 102 ESCC specimens. Normal squamous cells expressed both Hsp 27 and Hsp. In tumor tissue, expression of Hsp 27 was positive in 38%, reduced (+/-) in 52%, or negative in 10% and expression of Hsp 70 was positive in 14%, reduced in 56%, or negative in 30%. There was a strong correlation between the expression of Hsp 27 and Hsp 70 on this study. When compared with clinicopathologic features, expression of both Hsp 27 and Hsp 70 correlated negatively with lymph node metastases, but not with depth of invasion or histologic grade. The reduction of the Hsp’s was associated significantly with poor survival. Multivariate analysis revealed that negative Hsp 27 was the strongest prognostic factor among the clinicopathologic features. This study showed the expression of Hsp 27 and Hsp 70 was reduced in patients with ESCC and thus it should be considered as independent prognostic factor of this disease.
Immunohistochemical study was performed for Hsp’s 27 and 70 on 62 surgical specimens of ESCC (Nakajima et al., 2002). The expression of both Hsp’s 27 and 70 correlated inversely with depth of invasion and pathologic stage, and correlated positively with lymphocyte infiltration. Reduction of Hsp 70 expression was significantly correlated with poor prognosis. Patients with Hsp 27-negative tumors tended to have a poor prognosis compared with patients with Hsp 27-positive tumors. The authors suggested that Hsp’s 27 and 70 are significant prognostic factors for ESCC.
Expression of Hsp70 was analyzed by immunohistochemistry and correlated with TNM classification, vessel invasion, p53 expression, and clinical outcome after operation in tissue of 71 patients with ESCC. Overexpression of Hsp70 was related to sex, tumor configuration, lymph node metastasis, and lymphatic vessel invasion. Expression of p53 and Hsp70 were not correlated with each other. ESCC with Hsp70 expression exhibited a significantly better prognosis compared with Hsp70-negative ESCC in univariate analysis, but no significance was found in multivariate analysis. Noguchi et al., (2002) suggested that the examination of Hsp70 expression may be of use to assess clinical outcome after operation.
The sensitivity of tumors to chemotherapy and radiotherapy differs from one case to another. The presence of p53, p21, bax, bcl2, Hsp27 and Hsp70 was investigated in ESCC and the results were related to the response of the tumors to chemoradiotherapy and radiotherapy (Miyazaki, 2005). Tumors with p53-positive expression were less sensitive than p53-negative tumors. p21-positive patients, and Hsp27-negative and Hsp70-negative patients were all good responders. Multivariate analysis revealed that Hsp27 was the most reliable predictor of the effect of chemo-radiotherapy and radiotherapy among the four potential markers. p53-negative and Hsp70-negative patients had a more favorable prognosis than p53-positive and Hsp70-positive patients. The authors suggested that the expression of Hsp27 was a good predictor of the response of ESCC to chemo-radiotherapy and radiotherapy.
More recently in 2014, Xue et al., (2014) showed in vitro and in vivo assays that overexpression of Hsp27 in highly metastatic cells decreased their metastatic capacity. Also reported that the expression level of Hsp27 may be inversely correlated with the metastasis behavior of ESCC, and Hsp27 may play an important role in this progression. The researchers concluded that the Hsp27 may be a potential molecular target for the therapy and prognosis of patients with ESCC.
The present study showed the expressions of Hsp27 and Hsp70 in ESCC. The limiting factor for a more accurate analysis was due to the small number of samples. We believe due to the genetic diversity in different regions such as America, Asia, Europe and Africa, tests of large samples are necessary in order to confirm the behavior of these proteins in ESCC.
In view of these considerations, we conclude that Hsp27 and Hsp70 are expressed in ESCC tissues. These proteins may play an important role in the origin and development of ESCC and inhibitors of these proteins could reveal important therapeutic tool for controlling this one, which is extremely aggressive cancer. However, at present we cannot consider these proteins as a prognostic factor for patients with esophageal SCC.
Abbvreviations
CAPES, Personnel Improvement Coordination graduate; ESCC, Esophageal squamous cell carcinoma; FAPESP, Support Foundation Research of São Paulo; Hsp, Heat shock protein; INCA, National Institute of Cancer.
Declarations Ethics approval and consent to participate
This study was approved by the Federal University of São Paulo, an informed consent was waived. Research ethics committee of the Federal University of São Paulo.
Consent for publication
All authors read and approved the final manuscript.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by FAPESP and CAPES. The funding played on role in the design of the study, in the collection, analysis, and interpretation of data, and in writing the manuscript.
Author’s contributions
All authors made substantial contributions to conception and design, JN made the acquisition of data, RAN and CCFL made the analysis and interpretation of data; LBA and GM made the statistical analysis; TSG made the slides; and all the author´s were involved in drafting the manuscript or revising it critically for important intellectual content.
Acknowledgements
This work was supported by FAPESP, and CAPES. RAN is recipient of CNPQ (National council of development technology) fellowship.
References
- Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), alphaB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622–31. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. sHsp as novel regulators of programmed cell death and tumorigenicity. PatholBiol (Paris) 2000;48:280–8. [PubMed] [Google Scholar]
- Arrigo AP, Virot S, Chaufour S, et al. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2007;7:414–22. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- Brosina L, Von WerneBaes T, Zanoni C, Corrêa LP. Resistência ao fluorouracil estáassociada ao aumento do conteúdo da proteína Hsp70 em linhagens celulares de câncer de cólon. LUME. 2007;19:29–31. (in Portuguese) [Google Scholar]
- Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–10. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Dobo C, Stavale JN, Lima Fde O, et al. HSP27 is commonly expressed in cervical intraepithelial lesions of Brazilian women. Asian Pac J Cancer Prev. 2013;14:5007–10. doi: 10.7314/apjcp.2013.14.9.5007. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Giordanetto F, Kroemer G. Targeting HSP70 for cancer therapy. Mol Cell. 2009;36:176–7. doi: 10.1016/j.molcel.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, et al. Heat shockproteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Hell-Pourmojib M, Neuner P, Fischer H, et al. Differential expression of a novel gene in response to hsp27 and cell differentiation in human keratinocytes. J Invest Dermatol. 2002;119:154–9. doi: 10.1046/j.1523-1747.2002.01793.x. [DOI] [PubMed] [Google Scholar]
- Hirano S, Shelden EA, Gilmont RR. HSP27 regulates fibroblast adhesion, motility, and matrix contraction. Cell Stress Chaperones. 2004;9:29–37. doi: 10.1379/471.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional do Câncer INCA. Secretaria de Atenção àSaúde. Instituto Nacional do Câncer. Coordenação de Prevenção e Vigilância. Estimativa 2016. Rio de Janeiro: Incidência de câncer no Brasil; 2016. Ministério da Saúde. [Google Scholar]
- Kawanishi K, Shiozaki H, Doki Y, et al. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer. 1999;85:1649–57. doi: 10.1002/(sici)1097-0142(19990415)85:8<1649::aid-cncr2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lelj-Garolla B, Mauk AG. Roles of the N- and C-terminal sequences in Hsp27 self-association and chaperone activity. Protein Sci. 2012;21:122–33. doi: 10.1002/pro.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Kato H, Faried A, et al. Predictors of response to chemo-radiotherapy and radiotherapy for esophageal squamous cell carcinoma. Anticancer Res. 2005;25:2749–55. [PubMed] [Google Scholar]
- Nakajima M, Kuwano H, Miyazaki T, Masuda N, Kato H. Significant correlation between expression of heat shock proteins 27, 70 and lymphocyte infiltration in esophageal squamous cell carcinoma. italic>Cancer Lett. 2002;178:99–106. doi: 10.1016/s0304-3835(01)00825-4. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Takeno S, Shibata T, et al. Expression of heat shock protein 70 in grossly resected esophageal squamous cell carcinoma. Ann Thorac Surg. 2002;74:222–6. doi: 10.1016/s0003-4975(02)03641-x. [DOI] [PubMed] [Google Scholar]
- Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–8. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–62. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Slotta-Huspenina J, Berg D, Bauer K, et al. Evidence of prognostic relevant expression profiles of heat-shock proteins and glucose-regulated proteins in oesophageal adenocarcinomas. PloS One. 2012;27:e41420. doi: 10.1371/journal.pone.0041420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotta-Huspenina J, Wolff C, Drecoll E, et al. A specific expression profile of heat-shock proteins and glucose-regulated proteins is associated with response to neoadjuvant chemotherapy in oesophageal adenocarcinomas. Br J Cancer. 2013;9:370–8. doi: 10.1038/bjc.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stope MB, Schubert T, Staar D, et al. Effect of the heat shock protein HSP27 on androgen receptor expression and function in prostate cancer cells. World J Urol. 2012;30:327–31. doi: 10.1007/s00345-012-0843-z. [DOI] [PubMed] [Google Scholar]
- Surveillance, epidemiology, and end results program turning national cancer institute. SEER stat fact sheets: esophageal cancer. 2016. [retrieved november 9 2016]. available from: URL: http://seer.cancer.gov/statfacts/html/esoph.html .
- Tavassol F, Starke OF, Kokemüller H, et al. Prognostic significance of heat shock protein 70 (HSP70) in patients with oral cancer. Head Neck Oncol. 2011;23:3–10. doi: 10.1186/1758-3284-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercioti VJLRL, Neto JS, Campelo JB. Eficácia local e complicações da terapêutica neoadjuvanteno carcinoma epidermóide do esôfago: radioterapia versus radioterapia associada àquimioterapia. Rev Col Bras Cir. 2011;38:227–34. (in portuguese) [Google Scholar]
- Xue L, Yang L, Jin ZA, et al. Increased expression of HSP27 inhibits invasion and metastasis in human esophageal squamous cell carcinoma. italic>Tumour Biol. 2014;35:6999–7007. doi: 10.1007/s13277-014-1946-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.


