Abstract
Effective axon regeneration is achieved mainly by precise regulation of gene expression after peripheral nerve injury. MicroRNAs play an important role in controlling axon regeneration owe to its key epigenetic function in regulating gene expression. Here, we reveal that microRNA-9 (miR-9) may be a new suppressor of axon regeneration and FoxP1 is the functional target of miR-9. High level of endogenous miR-9 in sensory neurons inhibited axon regeneration in vitro and in vivo. In addition, the regulatory effect of miR-9 was mediated by changes in FoxP1 levels. Full rescuing effect of axon regeneration was achieved by FoxP1 up-regulation. Most importantly, we showed that miR-9-FoxP1 might be a new signaling pathway to regulate mammalian axon regrowth. Moreover, we provided the first evidence that maintaining a higher level of FoxP1 in sensory neurons by the microRNA is necessary for efficient axon regeneration.
Keywords: MicroRNA-9, axon regeneration, peripheral nerve injury, electroporation
Introduction
The definition of neuropathic pain is “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.” At present, a large number of patients are suffering from neuropathic pain around the world, and there have no effective methods to treat these kinds of intractable pain,1 except trigeminal neuralgia which can effectively be treated by minimally invasive procedure.2–4 To date, the pathogenesis of peripheral nerve system (PNS) is still not fully understand; therefore, studying the mechanism of the axon regeneration after PNS injuries will contribute to the prevention and treatment of neuropathic pain.
Axon regeneration, the important process after PNS injuries, relies on coordinated gene expression in the neuronal soma, synthesized molecules transporting along the axon, and the assembly of the axon by the cytoskeletal and membrane machinery at the distal axon.5 Regulated gene expression offers the raw materials for axon assembly and controls the intrinsic axon regeneration ability. Thus, regulation of gene expression has been a crucial approach to promote axon regrowth after PNS injuries. However, our understanding of the molecular mechanisms via which gene expression is controlled during axon growth is very limited.
MicroRNAs (miRNAs) is emerging to be a novel cellular mechanism of gene regulation during development, especially in proliferating cells, such as cancer and stem cells.6,7 But the roles of miRNAs modification in postmitotic neurons during axon growth and regeneration are still much unknown. A few profiling studies have reported that the expression levels of many miRNAs s were changed in adult mouse sensory neurons after the peripheral nerve injury. Our recent study has revealed for the first time that miRNAs-26a and its target GSK3β played key roles on regulated gene expression during mammalian axon regeneration in vivo.8
A previous research showed microRNA-9 (miR-9) was an important regulator of motor neuron specification and columnar formation; the overlapping expression of miR-9 and its target FoxP1 further illuminated the importance of fine-tuning regulation by miRNAs s in motor neuron development; and miR-9 plays important roles in central nervous system,9,10 but its roles in peripheral nervous system were unknown. In this study, we used our new electroporation-transfecting approach to detect miR-9’s function in regulating mammalian axon regeneration in vitro and in vivo. We reported that miR-9 level decreased while FoxP1, miR-9’s potential target gene increased after peripheral nerve injury. Overexpression of miR-9 in adult mouse sensory neurons markedly impaired axon regeneration in vitro and in vivo; inhibition of FoxP1 alone also impaired axon regeneration. Moreover, FoxP1 expression controls the regulatory effect of miR-9 overexpression on axon regeneration. The up-regulation of FoxP1 completely rescued axon regeneration impaired by miR-9 overexpression. All in all, our study showed that miR-9-FoxP1 might be a new pathway in the regulation of axon regeneration-associated gene expression. In addition, we identified a new regulatory mechanism of FoxP1 signaling: a higher level of FoxP1 protein needed to be maintained by miRNAs in neurons to support efficient axon regeneration.
Materials and method
Animals and reagents
All animals in experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of China Medical University. Eight- to 12-week-old CF-1 mice were obtained from China Medical University animal facility. RNAoligos (siRNA and miRNAs mimics), mmu-miR-9-5 p mimics, and mouse FoxP1 siRNA were from Thermo Scientific Dharmacon (Chicago, IL, USA). The CMV promoter-EGFP (The pCMV-EGFP) and pcDNA3.1 FoxP1 plasmid were from Addgene (Cambridge, MA, USA). The actin antibody was from Sigma-Aldrich (St. Louis, MO, USA). The β III tubulin (TuJ1) was from Convance (Chantilly, VA, USA). Antibodies against FoxP1 were from Cell Signaling Technology (Beverly, MA, USA). All fluorescence secondary antibodies were from Invitrogen (Waltham, MA, USA).
Primary culture of adult mouse dorsal root ganglion (DRG) neurons
For in vitro regenerative axon growth assays, the sensory neurons of adult mouse were cultured by the protocols that published previously.11 Adult mice DRGs were harvest and digested by collagenase A (90 min) and trypsin-ethylene diamine tetraacetic acid (EDTA, 20 min) in sequence. The DRGs were triple washed with medium (minimum essential medium with L-glutamine, penicillin/streptomycin, and 10% fetal calf serum). The cells were then dissociated with a 1 ml pipette tip in culture medium. The neurons were centrifuged to remove the supernatant and plated at low density onto 24-well plates coated with PL10 solution (poly-D-lysin and laminin) and was grown in 5% fetal bovine serum (FBS) culture medium.
To transfect RNA oligos into dissociated DRG neurons, the neurons were centrifuged to remove the supernatant and then resuspended in 80–100 μl of Amaxa electroporation buffer (for mouse neuron) with siRNA and miRNAs mimics (0.2 nmol per transfection) and/or the enhanced green fluorescence protein (EGFP) (10 µg per transfection) plasmid. Suspended cells were then transferred into a 2.0-mm cuvette and electroporated with the Amaxa Nucleofector apparatus. After electroporation, cells were immediately mixed to prewarmed culture medium and plated on culture dishes. After 4 h, when neurons were fully attached to the cover slips, the medium was changed to remove the remnant electroporation buffer. For in vitro axon growth assay, the transfected neurons were cultured for three days. After three days culture, the adult DRG neurons were fixed with 4% paraformaldehyde (PFA) and then stained with anti-β III tubulin antibody.12
In vivo electroporation of adult DRG neurons and sciatic nerve crush
The in vivo electroporation of adult DRG neurons were performed according to previous researches.11,13 Briefly, DRGs of left L4 and L5 level were carefully exposed by Rongeur forceps. Plasmid or siRNA solutions together with EGFP (3–4 µg/μl) were injected into the DRG by means of capillary pipette driven by Picospritzer II (Parker Ins. Fairfield, NJ, USA). The live electroporation was performed using a custom-made tweezer-like electrode and an Electro Square Porator BTX ECM830 (five 15-ms pulses at 35 V with 950 ms interval). The incision sites were then sutured, and the mice were allowed to recover after surgery.
Two days post in vivo electroporation, the left side sciatic nerve was crushed by fine forceps, and the crush site was marked with nylon suture. Three days later, the mice were perfused with 4% PFA. The whole sciatic nerve were dissected out and further fixed in 4% PFA overnight at 4℃.
Measurement of axon growth
For measurement of axon growth in vitro, 4% PFA fixed neurons were washed with Phosphate buffered saline (PBS) and blocked in blocking buffer for 1 h (2% BSA, 0.1% Triton X-100, and 0.1% sodium azide in PBS).The fluorescence images of immunostained neurons were viewed and captured by Axio Vision 4.6 software (Carl Zeiss MicroImaging Inc. Thornwood, NY, USA) installed to inverted fluorescence microscope. Neurons with axons longer than twice the diameter of cell bodies were selected. We measured the longest axon of each neuron, and about 50–100 neurons per group were measured. Three independent experiments were performed at least.
For quantification of axon regeneration in vivo, the fluorescence images of the whole mount nerves were captured by means of the Axio Vision software. All whole length EGFP-labeled axons in the sciatic nerve were measured from proximal end (Crush site) to the distal end (Axonal tips). Fifteen axons were measured per mouse, and data from six mice for each group were utilized to compute the average axon length.
Quantification of mature miRNA
Expression of FoxP1 in vivo in DRG tissues was detected with qRT-PCR. The assay was performed according to the method mentioned previously. Briefly, total RNA was isolated with the TRizol reagent and then the reverse transcription and real-time PCR were performed. The FoxP1 primers sequences as following: Forward, 5′-GGTCTGAGACAAAAAGTAACGGA-3'; Reverse, 5′-CGCACTCTAGTAAGTGGTTGC-3'. Each assay was carried out in triplicate for each sample tested. Relative expression was calculated by the comparative Ct method (2−ΔΔCt).14
The miR-9 level of in vitro cultured DRG neurons or in vivo DRG tissues was tested with qRT-PCR three days after miR-9 mimics electroporation. The Hairpin-it miRNA and U6 snRNA were analyzed with Normalization RT-PCR Quantitation Kitby, a fast real-time PCR system (7900HT, ABI). Different samples were normalized to U6 expression.
Western blot analysis
DRG tissues or dissociated DRG neurons were harvested and lysed by using the radio immunoprecipitation assay (RIPA) buffer. To separate the extracted proteins, we run sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. Blocked membranes were incubated with primary antibodies and horseradish peroxidase (HRP)-linked secondary antibodies in sequence. The densities of protein bands from three independent experiments were quantified by utilizing the Image J software. The protein artificial unit was computed by normalizing to loading control actin.
Statistics analysis
Data are presented as mean ± SEM. Two-tailed Student’s t test was used to determine the statistical significance between different experimental groups, which was set at a value of P < 0.05.
Results
MiR-9 was significantly down-regulated after sciatic nerve axotomy
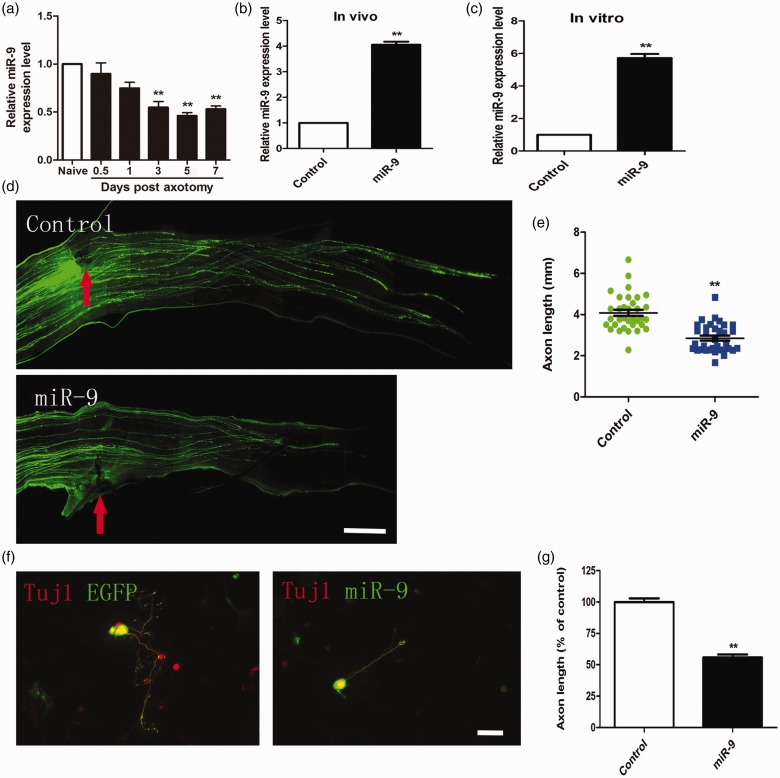
We first induced reactivating intrinsic axon regeneration using the DRG neurons, which regenerate dramatically during peripheral sciatic nerve axotomy and then examined the function of miR-9 in the regulation of axon regeneration.7 Using qRT-PCR technique, we found that miR-9 level remained largely unchanged from 12 h to one day after sciatic nerve injury. MiR-9 significantly decreased in DRG neurons from three to seven days after the peripheral axotomy (Figure 1(a)).
Figure 1.
MiR-9 overexpression inhibited axon regeneration in adult sensory neurons in vitro and in vivo. (a) qRT-PCR data indicating miR-9 levels in adult dorsal root ganglions after sciatic nerve injury. Note that miR-9 expression was significantly down-regulated from three to seven days after sciatic nerve axotomy (n = 3 for each condition). Error bars represent SEM. **P < 0.01. (b) and (c) Quantification of miR-9 mRNA level at three days after electroporation of miR-9 mimics in vivo and in vitro (n = 3 for each condition). Error bars represent SEM. **P < 0.01. (d) Representative images of EGFP-labeled regenerating axons in the whole-mount sciatic nerves. Red arrowheads mark the crush sites. Bar = 500 µm. (e) Scatter plot of average lengths of regenerating sciatic nerve axons (n = 6 mice for each condition). Error bars represent SEM. **P < 0.01. (f) Representative images of cultured adult mouse sensory neurons expressing EGFP (green), EGFP + miR-9 mimics (miR-9). All neurons were stained with Tuj1 (red). Scale bar = 100 µm. (g) Quantification of the average length of the longest axons (normalized to the average length of the control axons, n = 3). Error bars represent SEM. **P < 0.01.
MiR-9 overexpression suppresses axon regeneration in adult mouse sensory neurons in vitro and in vivo
To overexpress miR-9 and label transfected neurons, we cotransfected DRG neurons with the miR-9 mimics together with EGFP. Previous researches showed the transfection efficiency of electroporation mainly depended on plasmid size, and transfection efficiency of small RNAoligos (e.g., siRNA, miRNA mimics) was very high (>90%).11,16 In order to investigate the role of miR-9 in the regulation of axon regeneration in vivo, we used in vivo electroporation method to transfect both miR-9 mimics and EGFP into adult mouse DRG neurons.13,17 Two days later, the mice were subjected to a sciatic nerve crush procedure and sensory axon regeneration was assessed three days later. The result showed that overexpression of miR-9 mimics markedly elevated miR-9 level in adult DRGs (Figure 1(b)). MiR-9/EGFP-overexpressing neurons displayed impaired axon regeneration in vivo compared to those of control neurons expressing EGFP alone (Figure 1(d) and (e)).To investigate the effect of miR-9 overexpression in axon regrowth in vitro, the transfected neurons were cultured for three days and then regenerative axon was measured. The result showed that overexpression of miR-9 mimics markedly increased miR-9 mRNA levels in vitro (Figure 1(c)). Expression of miR-9 mimics markedly suppressed axon regeneration in adult sensory neurons (Figure 1(f) and (g)). Thus, we believe that miR-9 overexpression can inhibit axon regeneration both in vitro and in vivo.
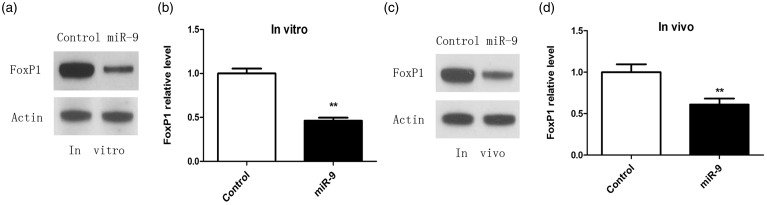
MiR-9 targets FoxP1 and down-regulation of FoxP1 impairs axon regeneration
To clarify the molecular mechanism of miR-9 in sensory axon regeneration, we first identified a reported target gene FoxP1.18,19 We up-regulated miR-9 level by transfecting miR-9 mimics into DRG neurons, and FoxP1 level was examined by Western blot analysis after three days in culture. The result showed that miR-9 overexpression significantly reduced the level of FoxP1 in cultured adult DRG neurons (Figure 2(a) and (b)). To determine if miR-9 regulated FoxP1 in sensory neurons in vivo, we used in vivo electroporation technique, which had allowed acute regulation of gene expression in DRG neurons of adult mice.13,20 In vivo overexpression of the miR-9 mimics markedly reduced endogenous FoxP1 (Figure 2(c) and (d)), indicating that miR-9 targets FoxP1 in adult DRG neurons in vivo. Taken together, these results demonstrated that FoxP1 might be a physiological target of miR-9 in adult DRG neurons during axon regeneration, and miR-9 could regulate expression of FoxP1 negatively.
Figure 2.
FoxP1 is target gene of miR-9 in adult sensory neurons during axon regeneration. (a) Representative Western blot images of FoxP1 in cultured adult dorsal root ganglion (DRG) neurons three days after transfection of miR-9 mimics. Overexpression of miR-9 leads to decreased FoxP1 protein level. (b) Quantification of FoxP1 level in vitro (normalized to actin, n = 3 for each condition). Error bars represent SEM. **P < 0.01. (c) Representative Western blot images of FoxP1 in cultured adult DRGs in vivo three days after electroporation of miR-9 mimics. (d) Quantification of FoxP1 level in vivo (normalized to actin, n = 3 for each condition). Error bars represent SEM. **P < 0.01.
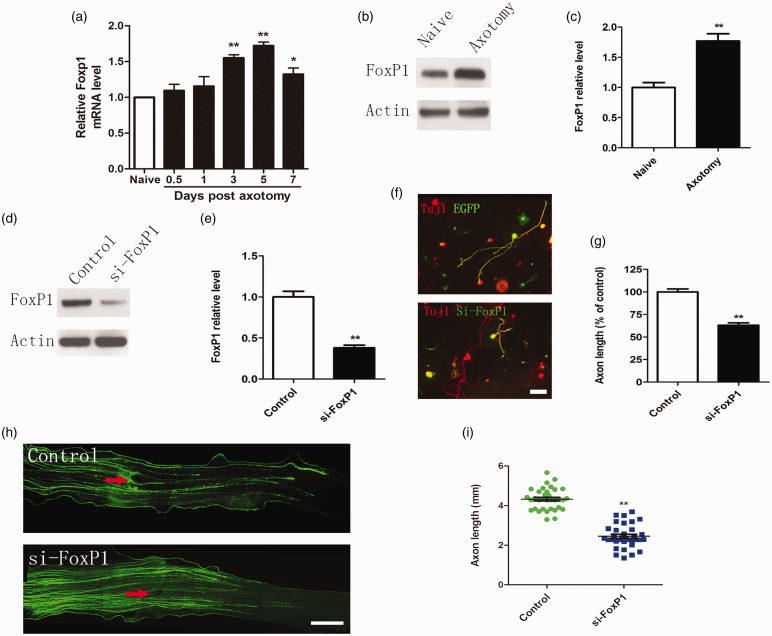
To determine the role of FoxP1 in the regulation of sensory axon regeneration, we examined the expression of FoxP1 in adult DRG neurons during peripheral axotomy-induced axon regeneration.
We found that mRNA level of FoxP1 remained largely unchanged from 12 h to one day after sciatic nerve injury. FoxP1 significantly increased from three to seven days after the peripheral axotomy. Protein levels of FoxP1 were markedly increased in adult DRGs seven days after the peripheral axotomy (Figure 3(a) to (c)). To determine whether FoxP1 regulated axon growth from adult DRG neurons, we knocked down endogenous FoxP1 with siRNAs (Figure 3(d) and (e)), we found that down-regulation of FoxP1 significantly impaired axon regeneration in vitro and in vivo (Figure 3(f) to (i)). This suggests that regulation of FoxP1 expression is necessary for axon regeneration.
Figure 3.
FoxP1 regulates sensory axon regeneration. (a) qRT-PCR data indicating Foxp1 levels in adult dorsal root ganglions (DRGs) after sciatic nerve injury. Note that FoxP1 expression was significantly up-regulated from three to seven days after sciatic nerve axotomy (n = 3 for each condition). Error bars represent SEM. *P < 0.05. **P < 0.01. (b) Representative Western blot images of FoxP1 in adult DRGs seven days after sciatic nerve axotomy. (c) Quantification of FoxP1 protein level (normalized to actin, n = 3 for each condition). Error bars represent SEM. **P < 0.01. (d) Representative Western blot images of FoxP1 in cultured adult sensory neurons three days after transfection of FoxP1 siRNA oligos (siFoxP1). Note that transfection of siFoxP1 markedly knocked down FoxP1 protein level. (e) Quantification of FoxP1 level (normalized to actin, n = 3 for each condition). Error bars represent SEM. **P < 0.01. (f) Representative images of cultured adult sensory neurons expressing EGFP, EGFP + FoxP1 siRNA (siFoxP1). All neurons were stained with anti-β III tubulin antibody. Red: Tuj1; Green: EGFP. Scale bar = 100 µm. (g) Quantification of the average length of the longest axons (normalized to the average length of the control axons, n = 3). Error bars represent SEM. **P < 0.01. (h) Representative images of EGFP-labeled regenerating axons in the whole-mount sciatic nerves. Red arrowheads mark the crush sites. Bar = 500 µm. (i) Scatter plot of average lengths of regenerating sciatic nerve axons (n = 6 mice for each condition). Error bars represent SEM. **P < 0.01.
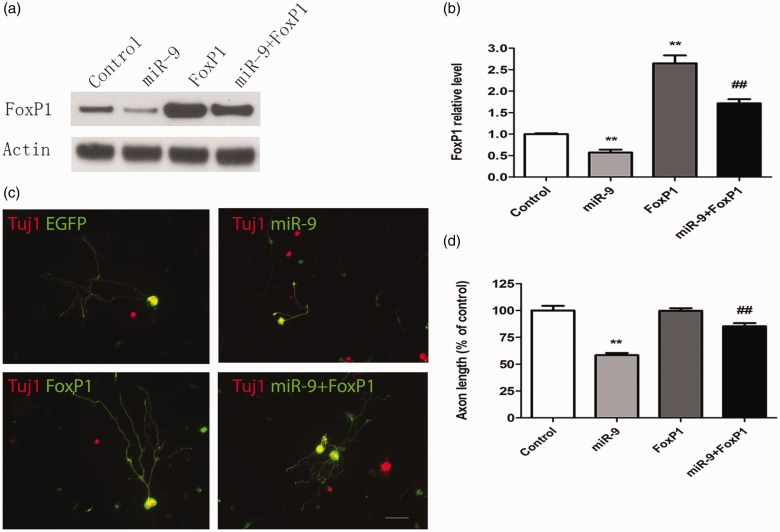
The regulatory effect of miR-9 on sensory axon regeneration is mediated by FoxP1
We found that the level of FoxP1 markedly decreased in sensory neurons transfected with miR-9 mimics. Moreover, such reduction could be fully rescued by treating the neurons with FoxP1 plasmid (Figure 4(a) and (b)). These results indicated that miR-9 could control the expression of FoxP1.To determine whether miR-9 impair sensory axon regeneration via controlling FoxP1 level, we performed rescue experiments in vitro to examine whether up-regulation of FoxP1 could reverse the inhibitory effect on axon growth by miR-9 mimics. Firstly, we cotransfected dissociated sensory neurons with miR-9 mimics, FoxP1 plasmid, and EGFP plasmid. Regenerative axon growth of the transfected neurons was analyzed after three days in culture. We found that up-regulation of FoxP1 fully rescued axon regeneration that was blocked by miR-9 mimics (Figure 4(c) and (d)). Together, these results indicated that FoxP1 might be the major target of miR-9 in adult mouse sensory neurons and participate in controlling axon regeneration.
Figure 4.
MiR-9 regulates axon regeneration by targeting FoxP1 expression. (a) Representative Western blot images of FoxP1 in cultured adult mouse sensory neurons with miR-9 mimics and/or FoxP1 plasmid. Note that expression of miR-9 leads to markedly decreased level of FoxP1, which can be fully reversed by expression of FoxP1. (b) Quantification of FoxP1 level from three independent experiments (normalized to actin, n = 3). Error bars represent SEM. **P < 0.01 indicates significant difference from Control; ##P < 0.01 indicates significant difference from miR-9-treated condition. (c) Representative images of cultured adult sensory neurons with EGFP, EGFP + miR-9, FoxP1, and FoxP1 + miR-9. Scale bar = 100 µm. (d) Quantification of the average length of the longest axons (normalized to the average length of the control axons, n = 3). Error bars represent SEM. **P < 0.01 indicates significant difference from Control; ##P < 0.01 indicates significant difference from miR-9-treated condition.
Discussion
During biological processes, epigenetic modification has recently been deemed as a pivotal approach to modulate gene expression.21 Our recent studies have further proved that the direct gene expression manipulation of miRNAs is of great significance in PNS.8,11 The advantage of the tuning regulation is to ensure optimized cellular protein expression through overlapping expression of miRNAs and their targets. In this study, we showed that miR-9 targets FoxP1 directly and regulates mammalian axon regeneration post injury.
Previous studies demonstrated that miR-9 expression-level modification influences the dendritic growth and synaptic transmission in the central nerve system. In addition, miR-9 not only regulates cortical axon branching and extension but also orchestrates motor neuron arrangement by tuning FoxP1 expression levels during spinal cord development.9,10,22 Recent studies have shown that FoxP1 manipulates the generation and axon guidance of spinal motor neurons.23,24 However, up to date, the role of miR-9 in the peripheral nervous system remains mysterious. In this study, we employ our well-established in vivo electroporation model to perform the gain–loss of function experiments of miR-9 in adult DRG. This new method gives us an opportunity to precisely assess the regenerating axon lengths in transfected neuron more efficiently and filters out those neurons failed to express the target gene.13 Here, we directly demonstrated that miR-9 overexpression impairs axon regrowth, and low expression level of miR-9 is critical for axon regeneration. Importantly, by clarifying the destroy effect of endogenous FoxP1 down-regulation on axon regeneration, we prove that FoxP1 may play a vital role in axon regeneration postperipheral nerve injuries.
Up-regulation of FoxP1 expression is able to rescue the axon regeneration that was completely destroyed by miR-9 overexpression. Moreover, we found that FoxP1 exists as a downstream target of miR-9 in the process of axon regeneration. Firstly, Western blot assay have shown that the level of FoxP1 was negatively modulated by the level of miR-9. FoxP1 level was clearly suppressed by miR-9 overexpression. In addition, FoxP1 up-regulation could completely rescue axon regrowth which was blocked by miR-9 overexpression. Previous studies in different system have shown that miR-9 targets FoxP1;18,19 however, we provided the evidence that miR-9 exists as a novel signaling controller and regulates mammalian axon regeneration by repressing FoxP1 expression.
Previous studies have shown that sciatic nerve crush induces chronic neuropathic pain,25–28 and axon regeneration plays a pivotal role in the maintenance of neuropathic pain.29,30 With the development of in vitro miRNAs synthesis and delivery technology, those small nucleotide sequences have been deemed as a promising approach for gene therapies.31,32 Here, our study illuminates that miR-9 regulation phenomenon in axon regeneration offers a new epigenetic pathway for peripheral nerve injury translation research.
Conclusion
In summary, high level of endogenous miR-9 in sensory neurons inhibited axon regeneration in vitro and in vivo. In addition, the regulatory effect of miR-9 was mediated by changes in FoxP1 levels. We showed that miR-9-FoxP1 might be a new signaling pathway to regulate mammalian axon regrowth. Moreover, we provided that maintaining a higher level of FoxP1 in sensory neurons by the miRNA is necessary for efficient axon regeneration.
Acknowledgments
The authors would like to thank the study participants for taking part in this study.
Author Contributions
JJ, YH, and BZ collected and analyzed the data; BZ, YS, and JZ helped breed the animals; JJ and YH made figures and tables; JJ, XW, and PY designed the experiment and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by National Natural Science Foundation of China (81500958; 31260233) and Provincial Natural Science Foundation of Liaoning (201602828).
References
- 1.Liang L, Zhao JY, Gu X, et al. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain 2016; 12: pii: 1744806916682242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao P, Hong T, Zhu YQ, et al. Efficacy and safety of continuous radiofrequency thermocoagulation plus pulsed radiofrequency for treatment of V1 trigeminal neuralgia. A prospective cohort study. Medicine (Baltimore) 2016; 95: e5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao P, Hong T, Wang ZB, et al. Treatment of bilateral idiopathic trigeminal neuralgia by radiofrequency thermocoagulation at different temperatures. Medicine (Baltimore) 2016; 95: e4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao P, Deng YY, Hong T, et al. Radiofrequency thermocoagulation for V2/V3 idiopathic trigeminal neuralgia: Effect of treatment temperatures on long-term clinical outcomes. Medicine (Baltimore) 2016; 95: e4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CM, Hur EM, Zhou FQ, et al. Coordinating gene expression and axon assembly to control axon growth: Potential role of GSK3 signaling. Front Mol Neurosci 2012; 5: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng L, He X, Wang Y, et al. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther 2014; 21: 37–43. [DOI] [PubMed] [Google Scholar]
- 7.Morgado AL, Rodrigues CM, Solá S. MicroRNA-145 regulates neural stem cell differentiation through the Sox2-Lin28/let-7 signaling pathway. Stem Cells 2016; 34: 1386–1395. [DOI] [PubMed] [Google Scholar]
- 8.Jiang JJ, Liu CM, Zhang BY, et al. MicroRNA-26a supports mammalian axon regeneration in vivo by suppressing GSK3β expression. Cell Death Dis 2015; 6: e1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dajas-Bailador F, Bonev B, Garcez P, et al. MicroRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci 2012; 15: 697–699. [DOI] [PubMed] [Google Scholar]
- 10.Otaegi G, Pollock A, Hong J, et al. MicroRNA miR-9 modifies motor neuron columns by a tuning regulation of FoxP1 levels in developing spinal cords. J Neurosci 2011; 31: 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CM, Wang RY, Saijilafu, et al. MicroRNA-138 and SIRT1 form a mutual negative feedback loop to regulate mammalian axon regeneration. Genes Dev 2013; 27: 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijilafu, Zhou FQ. Genetic study of axon regeneration with cultured adult dorsal root ganglion neurons. J Vis Exp 2012; 66: e4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saijilafu, Hur EM, Zhou FQ. Genetic dissection of axon regeneration via in vivo electroporation of adult mouse sensory neurons. Nat Commun 2011; 2: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 15.Zhou FQ, Walzer M, Wu YH, et al. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci 2006; 119: 2787–2796. [DOI] [PubMed] [Google Scholar]
- 16.Saijilafu, Hur EM, Liu CM, et al. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun 2013; 4: 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang BY, Saijilafu, Liu CM, et al. Akt-independent GSK3 inactivation downstream of PI3K signaling regulates mammalian axon regeneration. Biochem Biophys Res Commun 2014; 443: 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez GG, Volinia S, Croce CM, et al. Suppression of microRNA-9 by mutant EGFR signaling upregulates FOXP1 to enhance glioblastoma tumorigenicity. Cancer Res 2014; 74: 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otaegi G, Pollock A, Sun T. An optimized sponge for microRNA miR-9 affects spinal motor neuron development in vivo. Front Neurosci 2012; 5: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hur EM, Saijilafu, Lee BD, et al. GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev 2011; 25: 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweatt J. The emerging field of neuroepigenetics. Neuron 2013; 80: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giusti SA, Vogl AM, Brockmann MM. MicroRNA-9 controls dendritic development by targeting REST. Elife 2014; 3: e02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmesino E, Rousso DL, Kao TJ, et al. Foxp1 and lhx1 coordinate motor neuron migration with axon trajectory choice by gating reel in signalling. PLoS Biol 2010; 8: e1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams KL, Rousso DL, Umbach JA, et al. Foxp1-mediatedprogrammingof limb-innervating motor neurons from mouse and human embryonic stem cells. Nat Commun 2015; 6: 6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou Y, Xu F, Tang Z, et al. Distinct calcitonin gene-related peptide expression pattern in primary afferents contribute to different neuropathic symptoms following chronic constriction or crush injuries to the rat sciatic nerve. Mol Pain 2016; 12: 1744806916681566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buys MJ, Alphonso C. Novel use of perineural pregabalin infusion for analgesia in a rat neuropathic pain model. Anesth Analg 2014; 119: 481–488. [DOI] [PubMed] [Google Scholar]
- 27.Gibbons CR, Liu S, Zhang Y, et al. Involvement of brain opioid receptors in the anti-allodynic effect of hyperbaric oxygen in rats with sciatic nerve crush-induced neuropathic pain. Brain Res 2013; 1537: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emril DR, Wibowo S, Meliala L, et al. Cytidine 5′-diphosphocholine administration prevents peripheral neuropathic pain after sciatic nerve crush injury in rats. J Pain Res 2016; 9: 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quarta S, Baeumer BE, Scherbakov N, et al. Peripheral nerve regeneration and NGF-dependent neurite outgrowth of adult sensory neurons converge on STAT3 phosphorylation downstream of neuropoietic cytokine receptor gp130. J Neurosci 2014; 34: 13222–13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobianchi S, de Cruz J, Navarro X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp Neurol 2014; 255: 1–11. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison ER, Okun E, Mattson MP. The therapeutic potential of microRNAs in nervous system damage, degeneration, and repair. Neuromolecular Med 2009; 11: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu YW, Jiang JJ, Gao Y, et al. MicroRNA-210 promotes sensory axon regeneration of adult mice in vivo and in vitro. Neurosci Lett 2016; 622: 61–66. [DOI] [PubMed] [Google Scholar]