Abstract
Background:
The ketogenic diet is an effective non-pharmacologic treatment for medically resistant epilepsy. The aim of this study was to identify any predictors that may influence the response of ketogenic diet.
Methods:
A retrospective chart review for all patients with medically resistant epilepsy was performed at a tertiary care epilepsy center from 1996 to 2012. Patient- and diet-related variables were evaluated with respect to seizure reduction at 1, 3, 6, 9 and 12-month intervals and divided into four possible outcome classes.
Results:
Sixty-three patients met inclusion. Thirty-seven (59%) reported >50% seizure reduction at 3 months with 44% and 37% patients benefiting at 6-month and 12-month follow up, respectively. A trend toward significant seizure improvement was noted in 48% patients with seizure onset >1 year at 12-month (p = 0.09) interval and in 62% patients with >10 seizure/day at 6-month interval (p = 0.054). An ordinal logistic regression showed later age of seizure to have higher odds of favorable response at 1-month (p = 0.005) and 3-month (p = 0.013) follow up. Patients with non-fasting diet induction were more likely to have a favorable outcome at 6 months (p = 0.008) as do females (p = 0.037) and those treated with higher fat ratio diet (p = 0.034).
Conclusion:
Our study reports the effectiveness of ketogenic diet in children with medically resistant epilepsy. Later age of seizure onset, female gender, higher ketogenic diet ratio and non-fasting induction were associated with better odds of improved seizure outcome. A larger cohort is required to confirm these findings.
Keywords: Ketogenic diet, epilepsy, seizure, refractory seizures
Introduction
The ketogenic diet (KD) is an effective non-pharmacologic treatment for refractory childhood epilepsy used since the 1920s.1 The original diet protocol consisted of a high-fat, low-protein and low-carbohydrate diet. The hallmark features of KD treatment are the production of ketone bodies and reduced blood glucose levels2 though other hypotheses for its anti-epileptic actions have been proposed. These include changes in the nature and degree of energy metabolism in the brain, changes in neuronal cellular properties, changes in neurotransmitter function and synaptic transmission, and disease-modifying and anti-epileptogenic properties.2,3
KD is used in various forms including classic KD, medium-chain triglyceride (MCT) diet, long-chain triglyceride (LCT) diet, modified Atkins diet and low glycemic index diet (LGIT).4 It has shown to decrease seizure frequency by about 40%–50% from baseline in selected groups of patients5–9 and has a prolonged beneficial effect even after its discontinuation.10,11 The International Ketogenic Diet Study Group recommends that KD should be considered strongly in a child who fails two to three anticonvulsant medications, regardless of age or gender.12
Though KD is effective in a wide variety of clinical situations including epilepsy, it can be restrictive and cumbersome in implementation, frequently leading to its discontinuation. Recent insights into various mechanisms of action of KD have provided some information on favorable patient response,13 but predictors for successful outcome are less clearly defined. Age, duration of seizures, types of seizures, gender, electroencephalographic (EEG) patterns, age at epilepsy onset, age at diet initiation, time elapsed between epilepsy onset and diet initiation, success of KD at 3-month follow up and number of antiepileptic drugs (AEDs) used before diet initiation have been studied, but showed no predictive value on seizure outcome.8,9,14,15 Our objective in this study was to determine the possible predictors of the efficacy of the KD in children with medically resistant epilepsy.
Subjects and methods
This study is a retrospective chart review conducted by two independent pediatric neurologists and included all children treated with KD at the Women and Children’s Hospital of Buffalo, NY, USA, from 1996 until 2012. Patients with a diagnosis of medically resistant epilepsy (adequately treated with ⩾2 anti-epileptic medications in the past) and subsequently treated with dietary therapy were screened for inclusion. All patients were treated with classic KD at our center. Notes from physicians and the KD nutritionist during clinic visits were reviewed for collecting the data. Exclusion criteria included utilization of the diet for reasons other than epilepsy, diets other than classic KD, failure at diet induction and lack of proper documentation. A total of 91 patients were screened and 63 met the inclusion criteria. The University at Buffalo’s Institutional Review Board (IRB) approved the study protocol.
Study variables
Patient’s records were reviewed for demographics, gender, age of seizure onset, type of seizures, seizure frequency, development status, number of anti-epileptic medications used prior to KD, age of KD initiation, fasting or non-fasting induction, KD ratio and change in seizure frequency. Seizures were identified as per International League Against Epilepsy’s (ILAE) Proposal for Revised Terminology of Organization of Seizures and Epilepsies 201016 and were grouped into generalized and focal seizure types. Infantile spasms were grouped as generalized seizures. The ratio of KD and rate of induction were chosen by our nutritionists. All patients in this study were treated with classic KD based on a ratio of fat:carbohydrate and protein of 3:1 or 4:1. Diet was initiated typically starting at one-third strength (one-third formula and two-third water) on Day 1, advancing to two-third strength on Day 2 and full strength on Day 3. Blood glucose was monitored every 4 h at the diet initiation along with urine ketones every void. Daily serum beta-hydroxybutyrate levels were checked and liver function tests were done at the time of discharge. Adherence to KD, ketosis was monitored at home by urine dipstick ketone testing 2–3 times/day. Development status of the children was determined by the treating neurologist based on overall development, clinical assessment, and motor and cognitive status of the child. Seizure information was collected at baseline and 1, 3, 6, 9 and 12 months after starting the diet, or at any point within the above time frame if the diet was discontinued prior to 12 months.
Seizure Outcomes
The assessment of seizure control was based on percentage change in seizure frequency from the baseline as reported by caregivers during clinic visits. Baseline seizure frequency was recorded based on caregiver report and clinical documents over a 3-month period before starting the diet. Similarly, seizure frequency after KD initiation was noted from the clinic charts at follow-up visits. Seizure outcome was graded as follows: Class I: >90% seizure reduction, Class II: 50%–90% reduction, Class III: 25%–50% reduction, Class IV: <25% seizure reduction, no significant change or worsening. Favorable response was defined as >50% seizure reduction.
Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows (version 19; SPSS, Inc., Chicago, IL). Chi-square test (χ2) was used for non-parametric data and a p-value of <0.05 was considered significant. Dichotomous groups were created for data analysis: age of seizure onset (⩽1 vs >1 year), age of KD onset (⩽8 vs >8 years), seizure burden (0–10 vs >10 seizure/day) and cognitive delays (severe vs less severe delays). Ordinal regression analysis was performed using PLUM (Polytomous Universal Model) to study the effect of various individual predictor variables across the four possible class outcomes instead of looking at seizure outcomes as dichotomous only. Ordinal logistic regression is appropriate for looking at seizure outcome in this way because the true distance between class outcomes is not known reliably. This method is being used increasingly in medical research.17 Covariables included in the ordinal regression analysis included age at seizure onset, baseline seizure frequency, KD ratio, gender, type of seizure (generalized or partial), development status and fasting vs non-fasting induction.
Results
Clinical characteristics
A total of 91 patients were screened. Of the 63 patients who met inclusion criteria, 29 (46%) were males and 34 (54%) were females. Mean age at seizure onset was 13.6 months, (standard deviation (SD) = 19.6, age range = 0 month–9 years). Mean seizure frequency at baseline was 15.8 seizure/day (SD = 20.4). A total of 40 (63%) patients received KD orally, while 23 (37%) received it via a gastrostomy tube, with a mean KD ratio (fat:carbohydrate/protein) of 3.6:1 (range = 2:1–4:1). Generalized seizures were noted in the majority (59, 94%) of the patients. The mean number of anti-epileptic drugs used before diet initiation was 4.1 (SD = 1.43) (Table 1). In total, 28 patients were excluded after initial screening: 11 patients were excluded because of incomplete documentation and unavailability of records, 7 patients were treated with LGIT, 6 patients noted increase in seizure frequency in the diet induction period with parental refusal to continue and 1 patient was being treated for pyruvate dehydrogenase deficiency. One patient each failed diet induction (unable to reach initial target diet ratio) due to increased liver enzymes, recurrent vomiting and severe hypoglycemia on diet initiation and were excluded as these were not considered true representative of study population which aimed to study KD as an ongoing therapy.
Table 1.
Demographics and clinical characteristics.
| Total N = 63 | |
|---|---|
| Gender | |
| Male | 29 |
| Female | 34 |
| Seizure type | |
| Generalized seizures | 59 |
| Partial seizures | 4 |
| Age at seizure onset (year) | |
| ⩽1 | 42 |
| >1 | 21 |
| Diet induction | |
| Fasting | 13 |
| Non-fasting | 50 |
| Age at diet initiation (years) | |
| ⩽8 | 50 |
| >8 | 13 |
| Baseline seizure frequency (seizure/day) | |
| ⩽10 | 42 |
| >10 | 21 |
| Cognitive delays | |
| Mild/moderate delays/normal | 20 |
| Severe | 43 |
| No. of AED taken prior to KD | |
| ⩽4 | 43 |
| >4 | 20 |
| Route of KD | |
| Per oral | 40 |
| G tube | 23 |
AED: antiepileptic drug; KD: ketogenic diet.
Efficacy
Thirty-eight (60%) patients showed >50% reduction in their baseline seizure frequency at 1 month after starting the KD with 18 (29%) patients showing >90% seizure reduction. At the 3-month follow up, 37 (59%) patients continued to show >50% seizure reduction and 28 (44%) patients showed this outcome at 6-month interval (Table 2). After completing 12 months of KD treatment, 23 (37%) patients maintained efficacy with >50% reduction in their seizure frequency (Figure 1). Patients with generalized seizures had >50% seizure reduction in 33 (56%), 25 (42%) and 20 (34%) patients at 3-month, 6-month and 12-month intervals, respectively.
Table 2.
Seizure reduction in four outcome classes at 1-, 3-, 6-, 9- and 12-month follow up.
| Total |
Generalized |
Partial |
|
|---|---|---|---|
| 63 | 59 | 4 | |
| 1 month | |||
| >90% reduction (Class I) | 18 (29%) | 16 (27%) | 2 (50%) |
| 50%–90% reduction (Class II) | 20 (32%) | 18 (31%) | 2 (50%) |
| 25%–50% reduction (Class III) | 10 (16%) | 10 (17%) | 0 |
| <25% reduction/no imp (Class IV) | 10 (16%) | 10 (17%) | 0 |
| No data available | 5 (8%) | 5 (8%) | 0 |
| Continuing (n) | 63 (100%) | 59 (100%) | 4 (100%) |
| 3 months | |||
| >90% reduction (Class I) | 22 (35%) | 20 (34%) | 2 (50%) |
| 50%–90% reduction (Class II) | 15 (24%) | 13 22(%) | 2 (50%) |
| 25%–50% reduction (Class III) | 9 (14%) | 9 (15%) | 0 |
| <25% reduction/no imp (Class IV) | 10 (16%) | 10 (17%) | 0 |
| No data available | 3 (5%) | 3 (5%) | 0 |
| Continuing (n) | 59 (94%) | 55 (93%) | 4 (100%) |
| 6 months | |||
| >90% reduction (Class I) | 18 (29%) | 16 (27%) | 2 (50%) |
| 50%–90% reduction (Class II) | 10 (16%) | 9 (15%) | 1 (25%) |
| 25%–50% reduction (Class III) | 6 (10%) | 5 (8%) | 1 (25%) |
| <25% reduction/no imp (Class IV) | 7 (11%) | 7 (12%) | 0 |
| No data available | 5 (8%) | 5 (8%) | 0 |
| Continuing (n) | 46 (73%) | 42 (71%) | 4 (100%) |
| 9 months | |||
| >90% reduction (Class I) | 15 (24%) | 12 (20%) | 3 (75%) |
| 50%–90% reduction (Class II) | 4 (6%) | 4 (7%) | 0 |
| 25%–50% reduction (Class III) | 4 (6%) | 4 (7%) | 0 |
| <25% reduction/no imp (Class IV) | 3 (5%) | 2 (3%) | 0 |
| No data available | 11 (17%) | 11 (19%) | 0 |
| Continuing (n) | 37 (59%) | 34 (58%) | 3 (75%) |
| 12 months | |||
| >90% reduction (Class I) | 17 (27%) | 14 (24%) | 3 (75%) |
| 50%–90% reduction (Class II) | 6 (10%) | 6 (10%) | 0 |
| 25%–50% reduction (Class III) | 1 (2%) | 1 (2%) | 0 |
| <25% reduction/no imp (Class IV) | 3 (5%) | 3 (5%) | 0 |
| No data available | 8 (13%) | 8 (14%) | 0 |
| Continuing (n) | 35 (56%) | 32 (54%) | 3 (75%) |
Figure 1.
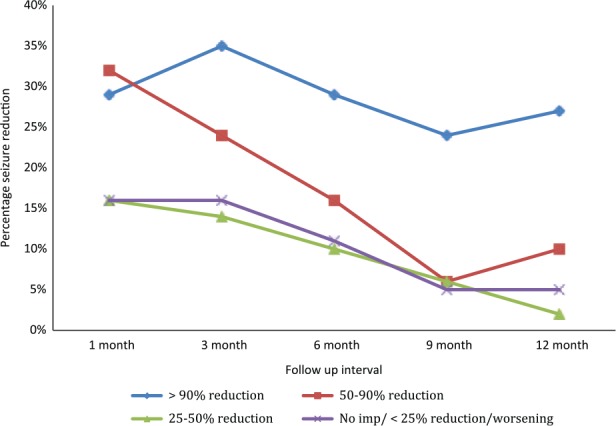
Total percentage seizure reduction from baseline in patients at 1-, 3-, 6-, 9- and 12-month follow up after initiation of the ketogenic diet.
Predictors of seizure outcome
Age of seizure onset
Patients were divided into two groups based on the age at seizure onset. Sixteen (38%) patients with the onset of seizures ⩽1 year of age reported >90% seizure reduction at the 1-month follow up, while 22 (52%) patients had >50% improvement at the 3-month interval (Table 3). In patients with seizure onset after 1 year of age, 15 (71%) patients reported >50% seizure improvement at 3 months and 10 (48%) patients continued to show this outcome at 6-month and 12-month follow up, respectively. This group also showed a trend toward significant seizure improvement at 12-month follow up as compared to younger seizure onset group (p = 0.09) (Table 3). Ordinal logistic regression analysis using age as a continuous variable revealed age at seizure onset as a significant predictor of seizure control at the 1-month (p = 0.005) and 3-month (p = 0.013) follow up with later age of seizure onset associated with higher chances of seizure reduction (Table 4).
Table 3.
Distribution of patients who had >50% seizure reduction with statistical significance.
| >50% seizure reduction |
|||||
|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 9 months | 12 months | |
| Gender | |||||
| Male | 14 (48%) | 16 (55%) | 10 (34%) | 7 (24%) | 9 (31%) |
| Female | 24 (71%) | 21 (62%) | 18 (53%) | 13 (38%) | 14 (41%) |
| Chi-square/p-value | 0.091 | 0.298 | 0.121 | 0.304 | 0.589 |
| Seizure type | |||||
| Generalized, n = 59 | 34 (58%) | 33 (56%) | 25 (42%) | 16 27%) | 20 (34%) |
| Focal, n = 4 | 4 (100%) | 4 (100%) | 3 (75%) | 3 (75%) | 3 (75%) |
| Chi-square/p-value | 0.132 | 0.136 | 0.761 | 0.299 | 0.443 |
| Age at seizure onset | |||||
| ⩽1 year, n = 42 | 28 (67%) | 22 (52%) | 18 (43%) | 12 (29%) | 13 (31%) |
| >1 year, n = 21 | 10 (48%) | 15 (71%) | 10 (48%) | 8 (38%) | 10 (48%) |
| Chi-square/p-value | 0.284 | 0.144 | 0.865 | 0.124 | 0.09 |
| Age at diet onset | |||||
| ⩽8 year, n = 50 | 30 (60%) | 32 (64%) | 21 (42%) | 16 (32%) | 4 (31%) |
| >8 year, n = 13 | 8 (62%) | 5 (38%) | 7 (54%) | 4 (31%) | 2 (15%) |
| Chi-square/p-value | 0.73 | 0.467 | 0.488 | 0.737 | 0.147 |
| Method of diet induction | |||||
| Non-fasting, n = 50 | 29 (58%) | 29 (58%) | 22 (44%) | 14 (28%) | 16 (32%) |
| Fasting, n = 13 | 9 (69%) | 8 (62%) | 6 (46%) | 6 (46%) | 7 (54%) |
| Chi-square/p-value | 0.206 | 0.960 | 0.105 | 0.200 | 0.826 |
| Cognitive delays | |||||
| Severe, n = 43 | 25 (58%) | 23 (53%) | 18 (42%) | 10 (23%) | 11 (26%) |
| Mild/moderate delays/normal, n = 20 | 13 (65%) | 14 (70%) | 10 (50%) | 10 (50%) | 12 (60%) |
| Chi-square/p-value | 0.47 | 0.388 | 0.523 | 0.326 | 0.315 |
| Seizure frequency | |||||
| 0–10 seizure/day, n = 42 | 24 (57%) | 21 (50%) | 15 (36%) | 13 (31%) | 16 (38%) |
| >10 seizure/day, n = 21 | 14 (67%) | 16 (76%) | 13 (62%) | 7 (33%) | 7 (33%) |
| Chi-square/p-value | 0.602 | 0.100 | 0.054 | 0.301 | 0.199 |
Table 4.
Significance of variables on ordinal logistic regression analysis.
| Ordinal regression (PLUM) p-value | |||||
|---|---|---|---|---|---|
| Variables | 1 month | 3 months | 6 months | 9 months | 12 months |
| Age at seizure onset | 0.005 (CI = −0.106, −0.019) | 0.013 (CI = −0.103, −0.012) | 0.495 (NS) | 0.278 (NS) | 0.177 (NS) |
| Seizure frequency baseline | 0.395 (NS) | 0.124 (NS) | 0.127 (NS) | 0.145 (NS) | 0.132 (NS) |
| KD ratio | 0.573 (NS) | 0.624 (NS) | 0.034 (CI = −2.704, −0.103) | 0.234 (NS) | 0.196 (NS) |
| Gender | 0.081 (NS) | 0.007 (CI = 0.419, 2.684) | 0.037 (CI = 0.084, 2.683) | 0.148 (NS) | 0.65 (NS) |
| Seizure type | 0.244 (NS) | 0.249 (NS) | 0.246 (NS) | – | – |
| Development status | 0.199 (NS) | 0.508 (NS) | 0.719 (NS) | 0.517 (NS) | 0.353 (NS) |
| Fasting/non-fasting | 0.795 (NS) | 0.127 (NS) | 0.008 (CI = 0.527, 3.463) | 0.075 (NS) | 0.971 (NS) |
Fasting/non-fasting diet induction
There were a total of 50 (79%) patients in the non-fasting group, while 13 (21%) patients had diet induction after a period of fasting. In the non-fasting group, 29 (58%) showed >50% seizure reduction each at the 1-month and 3-month follow up. After 6 months of follow up, 22 (44%) patients in the non-fasting group and 6 (46%) patients in the fasting group showed >50% seizure (NS) (Table 3). On regression analysis, patients in the non-fasting diet induction group showed significant chances of favorable outcome at 6 months (p = 0.008) while showing a positive trend toward higher odds of seizure control at 9-month follow up (p = 0.07) (Table 4).
Seizure frequency at baseline
Forty-two patients (67%) were noted to have 0–10 seizure/day, while 21 (33%) had >10 seizure/day at baseline before starting the KD. Among the patients with >10 seizure/day, 10 (47%) showed >90% reduction in their seizure frequency at 3 months, while >50% reduction in seizure frequency was seen in 16 (76%), 13 (62%) and 7 (33%) patients at 3, 6 and 12 months, respectively. Those with ⩽10 seizure/day showed >50% seizure reduction in 21 (50%), 15 (36%) and 16 (38%) patients at 3-, 6- and 12-month follow up, respectively (Table 3). A trend toward significant seizure improvement was seen in patients with higher seizure frequency at 6-month interval (p = 0.054) as compared to those with ⩽10 seizure/day (Table 3).
Gender
There were 29 male and 34 female patients in this study. Twenty-four (71%) females and 14 (48%) males showed >50% reduction in baseline seizure frequency 1-month after starting the KD (p = 0.091) with similar higher results for females at 3-, 6- and 12-month follow up (Table 3). Ordinal regression analysis showed that female patients had better odds of seizure control at 3-month (p = 0.007) and 6-month (p = 0.037) follow up as compared to their male counterparts (Table 4).
Age of diet initiation
A total of 50 patients started KD at ⩽8 years, whereas 13 patients started >8 years of age. Children in the younger age group showed a better response at the 3-month follow up and 32 (64%) patients reported more than 50% reduction in their seizure frequency as compared to 5 (38%) of those >8 years of age at the time diet initiation (NS). This effect was also seen at the 12-month interval with 19 (38%) patients in the younger age group reporting >50% seizure reduction as compared to 4 (31%) patients in the >8 year category.
Development status
Patients were grouped into mild, moderate and severely delayed cognitive function categories based on physician’s assessment and review of medical records at the initiation of KD. When patients with severe cognitive delays (N = 43) were compared to those with either mild to moderate delays or normal development (N = 20), the severely delayed group showed less efficacy to KD with 23 (53%) patients reporting >50% reduction in seizure frequency as compared to 14 (70%) patients with less severe impairment of cognition. A similar effect was noted at the 12-month interval with 11 (26%) and 12 (60%) patients in respective categories showing >50% seizure reduction. These results were statistically not significant (Table 3).
KD ratio
Children who were treated with a higher ratio showed better odds of improved seizure control (p = 0.034) at the 6-month interval as compared to children treated with lower ratio diet (Table 4). This significance was not sustained at 1-year follow up.
Discussion
In this study, we confirmed the efficacy of KD in patients with refractory epilepsy. We noted >50% reduction in seizure frequency in 59% of the patients at 3-month interval, while 44% and 37% patients showed these results at 6- and 12-month intervals, respectively, in line with previous reports.3,5,9,15,18 In addition, 27% patients had >90% reduction in the seizure frequency at 12-month follow up similar to previous observations.5 KD has been proven to show better efficacy in certain seizure types 6,13,19 with reports of better outcome in patients with generalized seizures6 or complex partial seizures being a negative prognostic factor.20 Yet another study found no difference between symptomatic generalized and symptomatic focal epilepsies in a randomized controlled trial.8 Our study cohort comprised generalized epilepsy patients predominantly; therefore, we were not able to comment on this difference.
Ordinal regression analysis was performed to study the effect of individual variables on four possible outcome classes (Table 4) while controlling for co-variables. Children with later age of epilepsy onset had better odds of successful outcome at 1-month and 3-month follow up though these odds were not sustained at later intervals. A recent study similarly did not find age of seizure onset to be significantly related to seizure outcome at 1 year.15 It may be hypothesized that children with early seizure onset represented severe spectrum of medically resistant and syndromic epilepsies that did not show a favorable response.
Fasting and non-fasting diet induction has also been a matter of research. In this study, patients with non-fasting diet induction showed significant better odds of seizure control at 6-months and a positive trend toward favorable outcome at 9-month follow up. Gradual non-fasting diet induction is tolerated well with fewer adverse effects,21,22 and a prospective randomized clinical trial21 showed that non-fasting patients had a better outcome at the 3-month follow up as compared to fasting patients with similar levels of ketosis, milder side effects, equal efficacy and higher patient responders. However, no difference between fasting and non-fasting groups is reported with regard to long-term outcome23 or time difference in achieving urinary ketosis is reported.22 This observation is particularly significant as non-fasting diet induction provides an opportunity to initiate diet in outpatient setting thereby avoiding hospitalization and improving patient compliance.
KD is usually started at a lower fat:carbohydrate ratio in most of the epilepsy centers in the United States and slowly titrated upwards to improve tolerability. Although there are patients who respond dramatically within days of initiating the KD, maximum efficacy is generally not achieved for several days or weeks after initiation, suggesting involvement of long-term adaptive metabolic and/or genetic mechanisms.3 We noted that patients treated with higher fat ratio diet had better odds of seizure control at the 6-month interval. Seo et al. also reported that more than 80% patients who failed to achieve seizure freedom on a lower ratio diet (3:1) showed marked improvement when treated with a higher fat ratio (4:1) without any significant difference in ketone levels in two groups.24 KD is also reported to show a more favorable response when started at an early age.5,6 In this study, children with younger age at KD onset showed better response with a higher number of children reporting >50% seizure reduction at 12-month follow up. In addition, a greater percentage of children with higher seizure frequency showed a favorable response after 6 months of dietary treatment. This was not found to be true in a previous study5 which failed to show any association between seizure frequency and outcome in patients treated with KD. A possible explanation for these results in our study is a more notable decline in seizure frequency in patients with higher baseline seizure frequency.
KD is used to treat refractory epilepsy patient across the cognitive spectrum. We noted worse, but statistically insignificant response to KD in patients with severe development delays compared to those with higher cognitive function. Patient’s intellect was not found to have predictive value on seizure outcome in a previous report though a higher number of patients with IQ ⩾ 70 showed seizure freedom on KD.6 Association of gender with seizure outcome was also noted in this study with female patients showing significantly better odds of favorable outcome at 3- and 6-month follow up. Gender was not found to be a predictor factor in earlier studies9,14,15 and causative association of gender with seizure outcome is unclear at this stage. It has been long known that interplay of genetic factors contributes a role in response with KD,13 and this may explain our findings although more specific research is needed is this regard.
In our study, 93% patients continued the diet at the 3-month interval, while 56% patients continued the KD beyond 1 year similar to a previous study5 that showed 83%, 71% and 55% diet continuation at 3-, 6- and 12-month intervals, respectively. A recent study also showed that KD response at 3 months was significantly related to success at 12 months15 and two-third children with ⩾50% seizure reduction at 3 months remained on the diet successfully after 1 year. One of the common reasons for discontinuing the diet is a lack of expected response. In concordance with previous reports,5 we found that those with >50% seizure reduction were more likely to continue the diet for a longer period. This may be related to treatment effect as patients expected to have significant seizure reduction or seizure freedom upon initiating the diet and discontinued it if desired results were not achieved, while those who showed marked improvement continued the diet.
Limitations
Limitations of this study include a retrospective, non-blinded design. A small sample size and dropouts during the study period possibly influenced the statistical results. Seizure outcomes were based on reports by caregivers who may have underestimated nocturnal seizures and runs the risk of introducing subjective errors. A seizure diary is a better and consistent way of documenting seizures. Patients with syndromic epilepsies were not classified separately, and hence we were not able to comment on this subgroup of patients. Development assessment was based on clinical examination, and formal assessment tools were not used. In addition, we were not able to report side effect profile of the patients due to inconsistent data.
Conclusion
This study confirms the effectiveness of KD as a non-pharmacological treatment in patients with medically resistant epilepsy. A higher fat ratio KD, non-fasting induction, female gender and later age of seizure onset were found to have better odds of seizure control till 6-month follow up. Patients with higher baseline seizure frequency and less severe development delays also showed a favorable response to KD, though not significant. More than half of the children continued the diet after 12 months successfully. Our report adds to the growing literature of studies with an attempt to define predictors that should be taken into consideration when implementing the KD. Additional prospective studies are needed to establish the predictors of successful response to KD treatment.
Acknowledgments
The authors would like to thank Dr Ahsan Moosa Naduvil Valappil, Pediatric Epileptologist at Cleveland Clinic Foundation, for his help in preparing and editing the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethics approval: Ethical approval for this study was obtained from the Children & Youth Institutional Review Board (initial approval no. DB#2477; reapproved study no. 414825-1).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Informed consent was not sought for this study because it was a retrospective chart review design study, and waiver for both written and verbal consent was obtained from the IRB.
References
- 1. Cross JH, Kluger G, Lagae L. Advancing the management of childhood epilepsies. Eur J Paediatr Neurol 2013; 17: 334–347. [DOI] [PubMed] [Google Scholar]
- 2. Masino SA, Rho JM. Mechanisms of ketogenic diet action. In: Noebels JL, Avoli M, Rogawski MA, et al. (eds) Jasper’s basic mechanisms of the epilepsies. 4th ed. Bethesda, MD: National Center for Biotechnology Information, 2012, pp. 1483–1515. [PubMed] [Google Scholar]
- 3. Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 2007; 48: 43–58. [DOI] [PubMed] [Google Scholar]
- 4. Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: an update for child neurologists. J Child Neurol 2009; 24: 979–988. [DOI] [PubMed] [Google Scholar]
- 5. Freeman JM, Vining EP, Pillas DJ, et al. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics 1998; 102: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 6. Maydell BV, Wyllie E, Akhtar N, et al. Efficacy of the ketogenic diet in focal versus generalized seizures. Pediatr Neurol 2001; 25: 208–212. [DOI] [PubMed] [Google Scholar]
- 7. Nangia S, Caraballo RH, Kang HC, et al. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Res 2012; 100: 252–257. [DOI] [PubMed] [Google Scholar]
- 8. Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol 2008; 7: 500–506. [DOI] [PubMed] [Google Scholar]
- 9. Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol 1998; 55: 1433–1437. [DOI] [PubMed] [Google Scholar]
- 10. Marsh EB, Freeman JM, Kossoff EH, et al. The outcome of children with intractable seizures: a 3- to 6-year follow-up of 67 children who remained on the ketogenic diet less than one year. Epilepsia 2006; 47: 425–430. [DOI] [PubMed] [Google Scholar]
- 11. Patel A, Pyzik PL, Turner Z, et al. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia 2010; 51: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 12. Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009; 50: 304–317. [DOI] [PubMed] [Google Scholar]
- 13. Hartman AL. Does the effectiveness of the ketogenic diet in different epilepsies yield insights into its mechanisms? Epilepsia 2008; 49 (Suppl. 8): 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassan AM, Keene DL, Whiting SE, et al. Ketogenic diet in the treatment of refractory epilepsy in childhood. Pediatr Neurol 1999; 21: 548–552. [DOI] [PubMed] [Google Scholar]
- 15. Vehmeijer FO, van der Louw EJ, Arts WF, et al. Can we predict efficacy of the ketogenic diet in children with refractory epilepsy? Eur J Paediatr Neurol 2015; 19: 701–705. [DOI] [PubMed] [Google Scholar]
- 16. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010; 51: 676–685. [DOI] [PubMed] [Google Scholar]
- 17. Bender R, Grouven U. Ordinal logistic regression in medical research. J R Coll Physicians Lond 1997; 31: 546–551. [PMC free article] [PubMed] [Google Scholar]
- 18. Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: a systematic review of efficacy. Pediatrics 2000; 105: E46. [DOI] [PubMed] [Google Scholar]
- 19. Thammongkol S, Vears DF, Bicknell-Royle J, et al. Efficacy of the ketogenic diet: which epilepsies respond? Epilepsia 2012; 53: e55–e59. [DOI] [PubMed] [Google Scholar]
- 20. Than KD, Kossoff EH, Rubenstein JE, et al. Can you predict an immediate, complete, and sustained response to the ketogenic diet? Epilepsia 2005; 46: 580–582. [DOI] [PubMed] [Google Scholar]
- 21. Bergqvist AG, Schall JI, Gallagher PR, et al. Fasting versus gradual initiation of the ketogenic diet: a prospective, randomized clinical trial of efficacy. Epilepsia 2005; 46: 1810–1819. [DOI] [PubMed] [Google Scholar]
- 22. Kim DW, Kang HC, Park JC, et al. Benefits of the nonfasting ketogenic diet compared with the initial fasting ketogenic diet. Pediatrics 2004; 114: 1627–1630. [DOI] [PubMed] [Google Scholar]
- 23. Kossoff EH, Laux LC, Blackford R, et al. When do seizures usually improve with the ketogenic diet? Epilepsia 2008; 49: 329–333. [DOI] [PubMed] [Google Scholar]
- 24. Seo JH, Lee YM, Lee JS, et al. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios–comparison of 3:1 with 4:1 diet. Epilepsia 2007; 48: 801–805. [DOI] [PubMed] [Google Scholar]


