Abstract
Objective
To calculate the yield and cost per diagnosed tuberculosis (TB) case for three World Health Organization screening algorithms and one using the Chinese National TB program (NTP) TB suspect definitions, using data from a TB prevalence survey of people aged 65 years and over in China, 2013.
Methods
This was an analytic study using data from the above survey. Risk groups were defined and the prevalence of new TB cases in each group calculated. Costs of each screening component were used to give indicative costs per case detected. Yield, number needed to screen (NNS) and cost per case were used to assess the algorithms.
Findings
The prevalence survey identified 172 new TB cases in 34,250 participants. Prevalence varied greatly in different groups, from 131/100,000 to 4651/ 100,000. Two groups were chosen to compare the algorithms. The medium-risk group (living in a rural area: men, or previous TB case, or close contact or a BMI <18.5, or tobacco user) had appreciably higher cost per case (USD 221, 298 and 963) in the three algorithms than the high-risk group (all previous TB cases, all close contacts). (USD 72, 108 and 309) but detected two to four times more TB cases in the population. Using a Chest x-ray as the initial screening tool in the medium risk group cost the most (USD 963), and detected 67% of all the new cases. Using the NTP definition of TB suspects made little difference.
Conclusions
To “End TB”, many more TB cases have to be identified. Screening only the highest risk groups identified under 14% of the undetected cases,. To “End TB”, medium risk groups will need to be screened. Using a CXR for initial screening results in a much higher yield, at what should be an acceptable cost.
Introduction
Tuberculosis (TB) is still a major global health problem and has been identified in the Sustainable Development Goals as one of the major diseases to be eliminated by 2030. Recent estimates from World Health Organization (WHO) give the global prevalence of TB as 174/100,000 and the incidence as 133/100,000 [1].
Despite China having a much lower prevalence (89/100,000) and incidence (68/100,000), there were still 1,200,000 TB cases and 930,000 incident TB cases in 2015. This accounted for almost 10% of the estimated new cases worldwide [1]. China therefore has one of the highest burdens of TB globally.
Passive case finding (PCF) and treatment of diagnosed TB disease are currently the principal means globally and in China, of controlling transmission of Mycobacterium tuberculosis and reducing TB incidence [2,3]. The standard PCF approach has not been successful in detecting all cases and globally it has been estimated that nearly 37% of new TB cases are undiagnosed or not reported [1].
Active case finding (ACF) is believed to contribute to the earlier detection of persons with TB and an earlier initiation of treatment, and to result in better outcomes for individuals with reduced transmission in the community [4–6]. Almost all ACF interventions rely on sputum smear-microscopy as the basis for diagnosis; but there is also growing evidence that screening through the use of chest radiographs is both effective and cost-effective in high-burden settings [7,8].
The results of most ACF studies show a predictable rise in the number of TB cases identified [9,10]. However, the individual and community-level benefits from active screening for TB disease remain uncertain, and the benefits of earlier diagnosis on patient outcomes and on-going TB transmission have not yet been established [11].
WHO recently published operational guidelines on systematic screening for active tuberculosis [12,13]. These have been developed for use in settings with different diagnostic resources, and they acknowledge that the yield (number of new cases of PTB found by each screening algorithm) will vary depending on the prevalence of undiagnosed TB and will be greater in subgroups at higher risk of TB. The prioritization of high risk groups for screening should be based on potential benefits and harms, the feasibility of the initiative, the acceptability of the approach, the number needed to screen, and the cost of screening[12,13].
In China, the population of persons aged 65 years and over is rapidly expanding. They are at increased risk of TB due to a longer exposure to infection with Mycobacterium tuberculosis and declining immunity with age which allows latent infection to reactivate and cause disease[14,15]. Those 60 years and over have a high prevalence of TB (349/100,000). This is 2.6 times higher than those aged 45 to 59 [16]. Symptoms of TB may be non-specific or absent, and attendance at health facilities may be erratic [17].
The aim of our study was to compare four screening algorithms for TB in persons aged 65 years and over. Using data from a TB prevalence study conducted in China, we sought to identify the risk groups with the highest yield and determine the relative costs of the different algorithms.
Methods
Ethical considerations
The prevalence study was reviewed and approved by the Institutional Review Board of Chinese Center for Disease Control and Prevention before commencing data collection. This study using data collected in the prevalence study, was approved by the Ethics Advisory Group of the International Union against Tuberculosis and Lung Disease, Paris, France.
Study design
This was an analytic study based on data from a cross-sectional study.
Study setting
China has a population of 1.37 billion, of whom 10.1% are people aged 65 years or over, and a GDP per capita of $7590 [18]. The level of affluence and urbanization varies greatly across the country.
There is a National TB control program (NTP) which develops the national protocols for detection and treatment of TB. The diagnosis of TB, including microscopy and X-ray examination, first-line anti-TB drugs and DOTS are all offered free of cost.
Study population
All persons aged 65 years and over who were interviewed in the TB prevalence study were included in the study.
TB prevalence survey
A TB prevalence survey in adults was conducted in 2013, the results of which will be published in full elsewhere. Sample size was estimated using a method appropriate to estimate a single population proportion. The 369/100,000 prevalence of bacteria-positive PTB among elderly people (≥65 years) from the latest national tuberculosis prevalence survey was used as a reference. 95% confidence level and 0.2 allowable error were assumed. The formula was n = pq/(d/zα)2 (p = 369/100,000, q = 1-p, d = 0.2p,α = 0.05, Zα = 1.96,). A total of 25,931 elderly participants were requested. In consideration of 10% non-response, sample size should be 28,812.
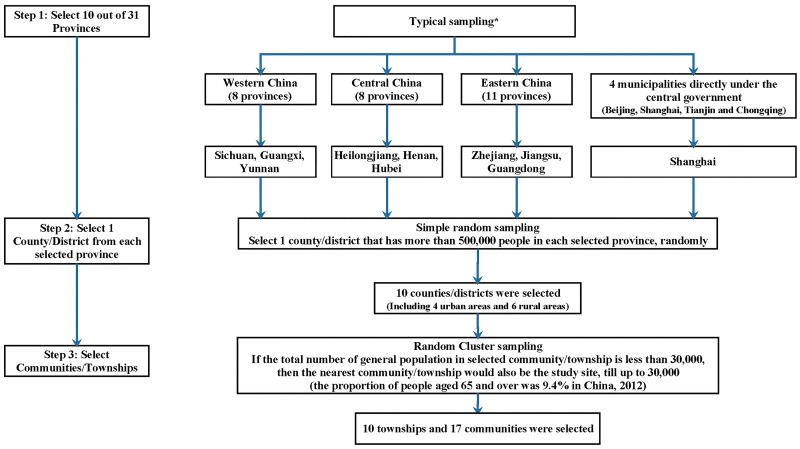
Multi-stage cluster sampling was used, and the procedure of sampling is shown in Fig 1. 27 study fields (10 townships and 17 communities) from 10 counties of 10 provinces were selected. The 10 counties are shown in Fig 2. The chosen number of townships or communities of each county depended on the population of aged people. Finally, 38,888 participants were included in the study.
Fig 1. The sampling procedure of the TB prevalence survey in China in 2013.
(*) 10 out of 31 provinces were selected, by considering the cooperative willingness, human resources and related abilities of each province.
Fig 2. The location of 10 sample counties in the TB prevalence survey in China in 2013.
Within this survey, each resident aged over 65 years was interviewed by trained staff face-to-face in their home using a standard questionnaire. Data on participants’ sex, age, marital status, education, past medical history, occupation, tobacco and alcohol use were collected. Their height and weight were used to calculate their Body Mass Index (BMI).
All participants were invited to have a full-size chest X-ray (CXR). Participants who had any suspected TB symptoms (cough for over 2 weeks or haemopytsis) or abnormal lung field shadows on CXR, were requested to submit 3 sputum samples (morning, night and spot sputum). The sputum samples were submitted and examined by smear microscopy for acid-fast bacilli and were assessed for mycobacterial culture using the solid Löwenstein—Jensen medium.
The cost of each component of the screening process is shown in Table 1. The cost of the household visits was based on the additional daily allowance, agreed nationally for work relating to infectious diseases, divided by the average number of households visited in a day. The price of a CXR, sputum smear and culture was the average market price paid.
Table 1. Cost of each component of active TB case-finding in this study in China in 2013.
| Contents | Cost per unit (USD) |
|---|---|
| Household primary screening by village health workers | 0.15 |
| Chest X-ray | 9.0 |
| Sputum smear | 3.9 |
| Sputum culture | 4.8 |
Pulmonary TB was diagnosed as sputum smear-positive and/or culture positive, or it was diagnosed on CXR based on a decision by a group of clinical doctors and radiologists [16]. Quality checks were done according to the National Guidelines [19].
The data collected in the survey were double entered using an online input system developed by a local software company.
TB screening algorithms
WHO has published three different algorithms for use depending on the risk groups and the diagnostic resources available [12]. These WHO algorithms (A1, A1b, A2 and A3) are shown in Table 2. Algorithm A1b is similar to A1 but is altered to reflect national policy in China which is to screen those with cough for more than 2 weeks and/or haemoptysis, rather than cough alone.
Table 2. Algorithms to screen the population for TB aged 65 or over in the different high risk group in this study, based on the WHO recommendations.
| Algorithms | Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 |
|---|---|---|---|---|
| WHO A1 | Interview | CXR | Smear | Culture |
| If cough lasting > 2 weeks, then | If positive, then | If positive = TB | If positive = TB | |
| If negative, then | If negative, then possible clinical diagnosis with CXR | |||
| WHO A1b | Interview | CXR | Smear | Culture |
| If cough lasting > 2 weeks &/or haemoptysis, then | If positive, then | If positive = TB | If positive = TB | |
| If negative, then | If negative, then possible clinical diagnosis with CXR | |||
| WHO A2 | Interview | CXR | Smear | Culture |
| If any TB symptoms(cough of any duration, haemoptysis, weight loss, fever, night sweats), then | If positive, then | If positive = TB | If positive = TB | |
| If negative, then | If negative, then possible clinical diagnosis with CXR | |||
| WHO A3 | CXR | Smear | Culture | NA |
| If positive, then | If positive = TB | If positive = TB | ||
| If negative, then | If negative, then possible clinical diagnosis with CXR |
The high risk groups [12] for TB identified from the literature are shown in Table 3.
Table 3. Definition of high risk factors for TB used in this study.
| Previous TB cases: registered in TB Management Information System, and finished treatment or cured. |
| HIV/AIDS: registered in local CDC database. |
| Known Diabetes: recorded on the Citizen Health Management Files as diagnosed with Diabetes, plus those using medicine to control Blood glucose by self-report. |
| Close Contacts: living with new active PTB case for at least 7 days in the three months before diagnosis. |
| BMI<18.5: Weight (kg)/Height2 (m2) <18.5. |
| Tobacco use: ever smoked tobacco by self-report. |
| Drinking history: drinking more than one unit (21 grams pure alcohol) per week. |
Data analysis
We obtained the point prevalence (number of missing TB cases detected/ population screened) data from the TB prevalence study and transferred this into our electronic database. For each algorithm and for different high risk groups, we calculated the yield of TB screening.
Yield = number new TB cases (smear-positive PTB, culture-positive PTB and active TB)
Number needed to screen to detect one case (NNS) = total number screened / number of cases identified
The costs of each of the algorithms were applied to the number of TB cases diagnosed to give indicative costs per case of TB detected: this was done by dividing the relative total cost of the tests in the algorithm by the number of new cases of TB identified.
All tests were performed using SAS 9.3 (SAS Institute Inc., USA).
Results
From the TB prevalence survey, there were 38,888 eligible people aged 65 years and over in the ten sample areas. Demographic characteristics are shown in Table 4.
Table 4. Demographic characteristics of the population aged 65 or over in the sample population in China in 2013.
| Characteristics | No. | % |
|---|---|---|
| Total | 38,888 | 100.0 |
| Sex | ||
| Male | 18,005 | 46.3 |
| Female | 20,883 | 53.7 |
| Age group | ||
| 65–74 | 24,102 | 62.0 |
| 75–84 | 12,193 | 31.3 |
| 85- | 2,593 | 6.7 |
| Place of residence | ||
| Urban | 13,533 | 34.8 |
| Rural | 25,355 | 65.2 |
Nineteen people were excluded as they were known TB cases under treatment. 4,619 refused to participate. 34,250 (88.1%) agreed to participate in this study. Of those who agreed to participate 33,510 (97.8%) had a chest X-ray, and 1,534 submitted sputum for smear and culture.
The number of people diagnosed with TB by smear and/or culture is shown in Table 5. There were 172 new TB cases, of which 116 were diagnosed only by CXR and clinical diagnosis.
Table 5. Total number of new TB cases found in the study population in China in 2013 and how diagnosed, smear positive TB and/or culture positive TB, or CXR and clinical alone.
| culture | Total | |||
|---|---|---|---|---|
| + | - | |||
| Smear | + | 23 | 8 | 31 |
| - | 25 | 116 | 141 | |
| Total | 48 | 124 | 172 | |
The number, yield, and prevalence of new TB cases, in each risk group is shown in Table 6. The prevalence of new TB cases in males was 3 times higher than in females. The prevalence rates in “previous TB” and “close contacts” were very high, 3,698 and 3,192/100,000 respectively. Also, the groups “BMI<18.5” and “Tobacco use” had high prevalence of new TB cases. For all risk groups except “BMI<18.5”, the new TB case prevalence in “rural areas” was 2 to 3 times higher than that in “urban areas”
Table 6. Number in each risk group, number of new TB cases diagnosed and, prevalence of new TB cases, in the prevalence survey China, 2013.
| Groups | Total | Urban areas | Rural areas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. in risk group | No. of TB diagnosed | Prevalence of new TB cases (1/100,000) | No. in risk group | No. of TB diagnosed | Prevalence of new TB cases (1/100,000) | No. in risk group | No. of TB diagnosed | Prevalence of new TB cases (1/100,000) | |
| All aged 65 and over | 34250 | 172 | 502 | 12932 | 34 | 263 | 21318 | 138 | 647 |
| Previous TB | 595 | 22 | 3698 | 251 | 6 | 2390 | 344 | 16 | 4651 |
| Close contacts | 94 | 3 | 3192 | 20 | 0 | 0 | 74 | 3 | 4054 |
| BMI<18.5 | 3632 | 39 | 1074 | 931 | 9 | 967 | 2701 | 30 | 1111 |
| Tobacco use | 6763 | 55 | 813 | 2168 | 12 | 554 | 4595 | 43 | 936 |
| Male | 16044 | 129 | 804 | 6044 | 25 | 414 | 10000 | 104 | 1040 |
| Alcohol use | 6543 | 40 | 611 | 1907 | 6 | 315 | 4636 | 34 | 733 |
| Diabetes | 2400 | 14 | 583 | 1306 | 3 | 230 | 1094 | 11 | 1006 |
| Female | 18206 | 43 | 236 | 6888 | 9 | 131 | 11318 | 34 | 300 |
| HIV/AIDS | 1 | 0 | 0 | 0 | 0 | — | 1 | 0 | 0 |
Two specimen groups at increased risk of TB were identified to run the WHO algorithms. Group 1 “medium risk” was a group of 12,006, with a prevalence (between 936/100,000 and 4,651/100,000). This medium risk group comprised those living in a “rural area”, who were “men” or a “previous TB case”, or were a “close TB contact” or a “BMI <18.5” or “tobacco users”.
Group 2 “high risk” totalled 668 people, comprised the groups with the highest prevalence (over 3,000/100,000), which were all “previous TB cases” and all “close TB contacts”. (Table 7)
Table 7. Risk group 1 and 2, and yield, and prevalence of new TB cases, for each group.
| Groups | No. in risk group | No. of new TB cases diagnosed | Prevalence of new TB cases (per 100,000) |
|---|---|---|---|
| medium risk group 1* | 12006 | 119 | 991 |
| high risk group 2** | 688 | 25 | 3,634 |
*Group 1 medium risk: Living in a rural area and male, or previous TB, or close contacts, or BMI<18.5, or tobacco use
**Group 2 high risk: previous TB or close contacts.
The numbers of each tests used in the screening algorithm, number of new TB diagnosed and relative cost per case for each group are shown in Table 8.
Table 8. Number of tests to be taken, yield and cost per case for each algorithm of the medium risk group 1, high risk group 2 and all aged 65 and over.
| Algorithms | Group 1* | Group 2** | All aged 65 and over | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of CXR | No. of Smear | No. of Culture | No. of new TB diagnosed | NNS | Cost per case (USD) | No. of CXR | No. of Smear | No. of Culture | No. of new TB diagnosed | NNS | Cost per case (USD) | No. of CXR | No. of Smear | No. of Culture | No. of new TB diagnosed | NNS | Cost per case (USD) | |
| WHO A1 | 366 | 74 | 62 | 25 | 481 | 221 | 59 | 31 | 25 | 12 | 58 | 72 | 611 | 103 | 90 | 31 | 1,105 | 330 |
| WHO A1b | 386 | 79 | 67 | 26 | 462 | 221 | 61 | 32 | 26 | 12 | 58 | 74 | 643 | 110 | 97 | 32 | 1,071 | 331 |
| WHO A2 | 683 | 107 | 94 | 29 | 414 | 298 | 97 | 41 | 35 | 12 | 58 | 108 | 1313 | 153 | 138 | 37 | 926 | 458 |
| WHO A3 | 11953 | 551 | 531 | 116 | 104 | 963 | 677 | 160 | 154 | 24 | 29 | 309 | 33510 | 989 | 959 | 164 | 209 | 1,881 |
*Group 1 medium risk: Living in a rural area and male, or previous TB, or close contacts, or BMI<18.5, or tobacco use.
**Group 2 high risk: previous TB or close contacts.
The yield for algorithms WHO 1, 1b and 2 increased slightly from 15% (25/172) to 17% (29/172) in the medium risk group with cost per case increasing from $221 to $298 and was unchanged in the high risk group, 7% (12/172) at a cost of between $72 and $108 per case. For all aged 65 and over, algorithms WHO 1, 1b and 2 found 18% (31/172) to 22% (37/172) at a cost of between $330 and $458 per case.
WHO A3 diagnosed 67% (116/172), 14% (24/172) and 95% (164/172) of new TB cases respectively in two risk groups and all aged 65 and over, which were two and five times as many new TB cases as the other three algorithms, but the cost per case was three to five times higher at $963, $309 and $1,881 per case respectively.
Discussion
This is the first study that has applied the WHO TB algorithms to population data of a country. The study discussed the yield and relative cost per case when each algorithm was applied on a population aged 65 years and over.
The prevalence survey demonstrates the difficulty of screening 100% of a population. Only 88% agreed to participate and of those a small proportion did not attend to have a CXR.
Using the WHO algorithms, the NNS and the cost per case detected, varies depending on the prevalence in risk groups and which algorithm is used. But the lower cost per case detected may leave up to 93% of new cases undetected.
Using CXR as the first screening test as in algorithm WHO A3 in the medium risk group detects a much higher proportion of the new TB cases in the whole population (116/ 172) than the other algorithms (25 to 29/172) at a cost of $963 per case.
The strengths of this study were that it used a large dataset collected as part of a carefully designed and implemented survey, which used the current TB diagnostic protocols and tests in China for diagnosis. Using real data to model the WHO algorithms showed how they work in practice. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [20] and sound ethics principles for the conduct and reporting of this study [21].
The study had a few limitations. The costing data that was collected was basic and was only indicative of the relative costs of the different groups being screened. Only current occupation was recorded and most participants were retired, thus risk groups based on previous occupation could not be identified. The original prevalence survey identified 172 new cases of TB and this has been used in this study. This is likely be incorrect for several reasons 1) the reported sensitivity of CXR as a screening tool is 87% and the specificity 89% [12]. 2) There will also be false positives included particularly as 67% of the new diagnoses were not confirmed by smear or culture. 3) Only 88% of the target population was screened. In our study, no modeling of screening of people living with HIV was possible, as in the data there was only one person who was living with HIV.
Screening the high risk group only, of “previous TB cases” and “close contacts”, gives a low cost per case. Studies from Africa and Cambodia also confirmed it was cost-effective to implement ACF among close contacts but the proportion of undiagnosed cases detected in the population is only 7% [22,23]. If the End TB target is to be achieved this is not effective.
It is being argued that the Stop TB proposal of ACF costing $350 per case [24] is too low to enable enough TB cases to be identified to reduce the prevalence in the community. It has been suggested that $1000 per case is more realistic [24] and is more similar to the cost and benefit of ART.
Use of WHO algorithm 3 in China would detect 67% of the new cases in the elderly community, at a crude cost of under $1000. The actual cost will be higher, but if the screening was set up as a large scale program the cost per test may well markedly reduce, for example with increased use of digital CXR [25].
The National Project of Basic Public Health Service launched by Ministry of Health in 2011 has made it easier for China to implement ACF [26]. In this project, all elderly people have an annual interview and physical examination, and the information recorded in the citizen health management file. This means that high risk groups as in Table 2 can be identified from routine data, and ACF can be combined with the annual physical examination. A pilot study in China, which integrated TB screening into annual health examinations for the rural elderly, and targeted diabetes patients and close contacts, had a significant yield. But no TB case was identified from close contacts alone [27].
China NTP used a different definition of TB symptoms from those in the WHO algorithm in that it uses “cough for 2 weeks or more, and haemoptysis”. This was used in the algorithm WHO A1b. The study results found there was little difference in the number of cases detected from using cough alone. Three more cases were identified when weight loss, fever and night sweats were added in algorithm 2. This shows that it is not necessary to change the nationally agreed TB symptoms used for screening.
Algorithm WHO A1 and WHO A2, using symptoms as the initial screening, will miss many undiagnosed TB cases when implemented in China, but it can still be used in some resource-limited areas, such as Western China.
This study has implications for other TB high burden countries which are also resource-limited, such as India and Indonesia. Choosing the optimal ACF strategy depends on the TB prevalence, economics, and human resources, etc. and it needs to fit with local health policies and available technology. This study has shown how the yield varies greatly and higher costs may need to be accepted in order to have an impact on the burden of TB.
To achieve the ambitious targets of ending the TB epidemic by 2035, ACF screening has to be implemented more widely.
Conclusions
WHO recommends that indiscriminate mass screening should be avoided, and the prioritization of risk groups for screening should be based on the prevalence of new cases [12]. Knowing the expected prevalence of TB in risk groups enables appropriate targeting of screening and in China risk groups can be identified from routine data. The cost per diagnosed case, and NNS increases as the prevalence reduces. However if just the highest risk groups are screened, only between 7 and 14% of the undetected cases will be found, depending on the algorithm used. To “End TB”, appropriate medium risk groups will need to be screened. To obtain the highest yield, a CXR should be used for initial screening, as in WHO Algorithm 3.
Acknowledgments
We thank the tireless contributions of staff in the provincial CDCs, local CDCs, and other related health care workers in undertaking the primary study. The study sites are located in Jiangsu Province, Zhejiang Province, Guangdong Province and Shanghai of eastern China, Henan Province, Heilongjiang Province and Hubei Province of central China, and Sichuan Province, Guangxi Zhuang Autonomous Region and Yunnan Province of western China.
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins sans Frontières (MSF/Doctors Without Borders). The specific SORT IT programme which resulted in this publication was jointly developed and implemented by: The Union South-East Asia Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; The Union, Mandalay, Myanmar; the Operational Research Unit (LUXOR), MSF Brussels Operational Center, Luxembourg; Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India; Department of Community Medicine, Government T.D Medical College, Alappuzha, India; College of Life and Environmental Sciences, Exeter University, UK; Velammal Medical College Hospital & Research Institute, Madurai, India; and Institute of Medicine, University of Chester, UK.
Data Availability
Data cannot be publicly shared due to restrictions imposed by The Institutional Review Board of Chinese Center for Disease Control and Prevention. In the signed Informed Consent Form of the primary study, there is a Confidentiality Clause saying for each participant: The investigation records and results of health examination will be kept secret. Without your agreement, no individual-level data can be publicly shared. Data requests may be sent to corresponding author Dr. Jun Cheng (chengjun@chinatb.org). According to the management requirements of the IRB of China CDC, data requests should be sent to the corresponding authors, then the authors apply to the IRB for data sharing to interested readers.
Funding Statement
The primary study, “Study on TB epidemic and intervention mode” was funded by The National Twelfth Five-year Mega-Scientific Projects of Infectious Diseases in China (Grant Number: 2013ZX10003-004-001). The training programme was funded by the Department for International Development (DFID), UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. Geneva: World Health Organization. 2015 (WHO/HTM/TB/2015.22). ISBN 978 92 4 156505 9
- 2.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005; 9(11): 1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 3.Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375(9728): 1814–1829. 10.1016/S0140-6736(10)60483-7 [DOI] [PubMed] [Google Scholar]
- 4.Soares ECC, Vollmer WM, Cavalcante SC, Pacheco AG, Saraceni V, Silva JS, et al. Tuberculosis control in a socially vulnerable area: A community intervention beyond DOT in a Brazilian favela. Int J Tuberc Lung Dis. 2013;17(12): 1581–1586. 10.5588/ijtld.13.0152 [DOI] [PubMed] [Google Scholar]
- 5.den Boon S, Verver S, Lombard CJ, Bateman ED, Irusen EM, Enarson DA, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect. 2008;136(10): 1342–1349. 10.1017/S0950268807000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris RC, Grandjean L, Martin LJ, Miller AJP, Nkang J-EN, Allen V, et al. The effect of early versus late treatment initiation after diagnosis on the outcomes of patients treated for multidrug-resistant tuberculosis: a systematic review. BMC Infect Dis. 2016;16(1): 193 10.1186/s12879-016-1524-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen DTM, Bang ND, Hung NQ, Beasley RP, Hwang LY, Graviss EA. Yield of chest radiograph in tuberculosis screening for HIV-infected persons at a district-level HIV clinic. Int J Tuberc Lung Dis. 2016;20(2): 211–217. 10.5588/ijtld.15.0705 [DOI] [PubMed] [Google Scholar]
- 8.Paquette K, Cheng MP, Kadatz MJ, Cook VJ, Chen W, Johnston JC. Chest radiography for active tuberculosis case finding in the homeless: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(10): 1231–1236. 10.5588/ijtld.14.0105 [DOI] [PubMed] [Google Scholar]
- 9.Yuen CM, Amanullah F, Dharmadhikari A, Nardell EA, Seddon JA, Vasilyeva I, et al. Turning off the tap: Stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet. 2015;386(10010): 2334–2343. 10.1016/S0140-6736(15)00322-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yimer S, Holm-Hansen C, Yimaldu T, Bjune G. Evaluating an active case-fi nding strategy to identify smear-positive tuberculosis in rural Ethiopia. Int J Tuberc Lung Dis. 2009;13(11): 1399–1404. [PubMed] [Google Scholar]
- 11.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, Schaap A, et al. The benefits to communities and individuals of screening for active tuberculosis disease: A systematic review. Int J Tuberc Lung Dis. 2013;17(4): 432–446. 10.5588/ijtld.12.0743 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. Geneva: World Health Organization; 2013. (WHO/HTM/TB/2013.04). ISBN 978 92 4 154860 1 [PubMed] [Google Scholar]
- 13.World Health Organization. Systematic screening for active tuberculosis: an operational guide. Geneva: World Health Organization; 2015. (WHO/HTM/TB/2015.16). ISBN 978 92 4 154917 2. [Google Scholar]
- 14.Meyer KC. Aging. Proc Am Thorac Soc. 2005;2: 433–439. 10.1513/pats.200508-081JS [DOI] [PubMed] [Google Scholar]
- 15.Narayanan PR. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in South India: A 15-year follow-up. Int J Tuberc Lung Dis. 2003;7: 1083–1091. [PubMed] [Google Scholar]
- 16.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990–2010: a longitudinal analysis of national survey data. Lancet. 2014;383(9934): 2057–2064. 10.1016/S0140-6736(13)62639-2 [DOI] [PubMed] [Google Scholar]
- 17.Technical Guidance Group of the Fifth National TB Epidemiological Survey the Office of the Fifth National TB Epidemiological Survey. The report of the Fifth National TB Epidemiological Survey in 2010 (in Chinese). Chinese J Antituberc. 2012;34: 485–508.
- 18.National Bureau of Statistics of China. Statistical Communiqué of the People’s Republic of China on the 2015 National Economic and Social Development. 2016 Feb 29 [cited 12 Dec 2016]. Available from: http://www.stats.gov.cn/tjsj/zxfb/201602/t20160229_1323991.html
- 19.Department of disease control, Chinese Ministry of Health. Guidelines for implementing the national tuberculosis control program in China (2008). Beijing: Beijing Union Medical College Press; 2009(ISBN 978-7-81136-190-2) [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596): 1453–1457. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 21.Edginton M, Enarson D, Zachariah R, Reid T, Satyanarayana S, Bissell K, et al. Why ethics is indispensable for good-quality operational research. Public Health Action. 2012;2(1): 21–22. 10.5588/pha.12.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav RP, Nishikiori N, Satha P, Eang MT, Lubell Y. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighborhood contacts in Cambodia. Am J Trop Med Hyg. 2014;90(5): 866–872. 10.4269/ajtmh.13-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekandi JN, Dobbin K, Oloya J, Okwera A, Whalen CC, Corso PS. Cost-effectiveness analysis of community Active Case Finding and Household Contact Investigation for tuberculosis case detection in urban Africa. PLoS One. 2015;10(2): e0117009 10.1371/journal.pone.0117009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. 2014;12: 216 10.1186/s12916-014-0216-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Check TB. Key differences analog vs digital radiography [Internet]. 2016 [cited 12 Dec 2016]. Available from: http://www.checktb.com/index.php?option=com_content&view=article&id=189%3Aanaloguevsdigital&catid=44%3Adigital-imaging&Itemid=135&lang=en
- 26.Cheng J, Wang L, Zhang H, Xia Y. Diagnostic value of symptom screening for pulmonary tuberculosis in China. PLoS One. 2015; 10(5): e0127725 10.1371/journal.pone.0127725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XL, Li SG, Li HT, Li GX, Guo XY, Wang Y, et al. Integrating tuberculosis screening into annual health examinations for the rural elderly improves case detection. Int J Tuberc Lung Dis. 2015;19(7): 787–791. 10.5588/ijtld.14.0617 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cannot be publicly shared due to restrictions imposed by The Institutional Review Board of Chinese Center for Disease Control and Prevention. In the signed Informed Consent Form of the primary study, there is a Confidentiality Clause saying for each participant: The investigation records and results of health examination will be kept secret. Without your agreement, no individual-level data can be publicly shared. Data requests may be sent to corresponding author Dr. Jun Cheng (chengjun@chinatb.org). According to the management requirements of the IRB of China CDC, data requests should be sent to the corresponding authors, then the authors apply to the IRB for data sharing to interested readers.